Abstract
Behçet’s syndrome (BS) is a complex vasculitis, characterised by peculiar histological, pathogenetic and clinical features. Superficial venous thrombosis (SVT) and deep vein thrombosis (DVT) are the most frequent vascular involvements, affecting altogether 15–40% of BS patients. Atypical thrombosis is also an important clinical feature of BS, involving the vascular districts of the inferior and superior vena cava, suprahepatic veins with Budd–Chiari syndrome, portal vein, cerebral sinuses and right ventricle. On the other hand, arterial involvement, although affecting only 3–5% of patients, represents a unique feature of BS, with aneurysms potentially affecting peripheral, visceral and pulmonary arteries. Vascular events in BS are promoted by inflammation, with neutrophils playing a key role in the pathogenesis of thrombotic events; in turn, coagulative components such as fibrinogen, thrombin, factor Xa and factor VIIa amplify the inflammatory cascade. Understanding the contribution of inflammatory and coagulation components in the pathogenesis of BS vascular events is crucial to define the most effective therapeutic strategy. Control of vascular thrombosis is achieved with immunosuppressants drugs rather than anticoagulants. In particular, use of azathioprine and cyclosporine in association with low-dose corticosteroids should be considered in DVT and SVT cases, while treatment with cyclophosphamide together with anti-TNF-α agents can be effectively used in arterial involvement. More recently, the anti-TNF-α drugs have also been reported as a valid alternative for the treatment also of venous events, especially DVT. An exception to the use of anticoagulant in BS could be represented by cerebral veins thrombosis. In this review, we will depict the main characteristics of the vascular involvement in BS, briefly describing histological and pathogenetic features, while focusing on the clinical and therapeutical approaches of the vascular manifestations of BS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behçet’s syndrome (BS) is a complex and intriguing vasculitis, with a peculiar vascular involvement. Indeed, BS is the model of inflammation-induced thrombosis, and new pathogenetic concepts have been raised regarding vascular involvement in patients affected by BS [1].
To date Behçet is considered a syndrome, since it includes a spectrum of different clinical phenotypes, instead of a single entity. Among many different phenotypes, the “vascular cluster” or “angio-Behçet” identifies a specific group of patients who suffer from recurrent inflammatory thrombosis involving the venous and, more rarely, the arterial vascular tree [2].
Different epidemiological studies confirmed that the most frequent type of vascular involvement in BS is superficial thrombosis, followed by deep vein occlusions, and more rarely, by aneurysm formation on the arterial side [2].
In this review, we will briefly describe the main histological, pathogenetic and clinical features of the vascular involvement in BS. Whereupon, we will focus on the clinical studies describing the different therapeutic approaches (mainly with immunosuppressants) of the more important kind of vascular manifestations.
Classification and histological features
The clinical importance of vascular thrombosis in BS is underlined by the more recent classification criteria, including for the first time in the scoring system a specific item for the vascular involvement. These new classification criteria allowed the clinicians to easily classify patients as having BS with predominant “vascular” clinical events, even in the absence of oral aphthosis [3].
Moreover, BS is classified, together with Cogan’s syndrome, as a “variable vessels” vasculitis, a term that underlies its atypical histological and clinical features [4]. Indeed, several important clinical and histological differences exist between BS and other systemic vasculitides: (a) contemporary involvement of both arteries and veins of all sizes in BS (venous in general more frequently affected than arteries); (b) no clearly reported increased risk of atherosclerosis compared to other vascular inflammatory diseases; (c) unique tendency for aneurysm formation in particular in the pulmonary arterial circulation; (d) usual absence of granulomatous inflammatory lesions in vessel wall, with elastic fibres usually spared.
The infiltrates in BS are predominantly constituted of neutrophils and lymphocytes. Unlike the histology of other systemic vasculitides, in BS, these cells are localised more around the vessels than inside the wall. This histologic “perivascular” pattern of BS, probably more similar to neutrophilic dermatosis than to classical systemic vasculitides, has been demonstrated in several districts, and in particular, in mucosal and ocular inflammatory lesions, as well as even in pulmonary aneurysms [5,6,7]. Similarly, the histopathology of a skin pathergy test at different time points reveals perivascular infiltrates of neutrophils and lymphocytes, however, without the typical features of a “true” vasculitis [8].
Pathophysiology of vascular inflammation in Behçet’s syndrome
BS is considered a very complex disease in which environmental, geographical, genetic, and immunological factors together with microbiome alterations, contribute to disease onset and persistence or recurrence of symptoms over time [9,10,11,12,13].
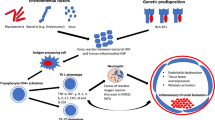
Regarding the subset of BS patients with vascular manifestations, during the past few years, new light has been shed on the correlation between inflammation and thrombosis. Cytokines such as IL1, IL6, IL17, TNF-α and CXCL8 (previously known as IL8) are now considered major players for BS pathogenesis. Moreover, it is now recognised that inflammation, as for many chronic inflammatory conditions, promotes thrombotic events through endothelial dysfunction, platelets hyperactivation and increased tissue factor (TF) expression. On the other hand, coagulative components such as fibrinogen, thrombin, factor Xa and factor VIIa are potentially able to interact with specific receptors (integrins, PARs 1-3-4 and PARs 2, respectively) on the immune system cells, and to amplify the inflammatory cascade [14, 15].
Neutrophil hyperfunction in BS was originally proposed in 1975, when increased chemotaxis was demonstrated, and more recently, new data have linked neutrophils to BS inflammatory organ damage [16]. Serum levels of CXCL8 and G-CSF, chemokines typically involved with neutrophils recruitment and activations, are indeed usually high in the active phase of BS [17]. Moreover, intense neutrophils perivascular infiltrates have also been described in pulmonary arteries complicated with rupture and haemoptysis [18].
The link between neutrophil-mediated inflammation and organ damage in Behçet’s syndrome is described in Fig. 1.
A specific mechanism of programmed cell death (NETosis) has been recently described for neutrophils. This mechanism indicates the external release of structures containing adenylsated chromatin and known as Neutrophils Extracellular Traps (NETs). NETs together with neutrophils granules, contribute to the entrapment and killing of extracellular microorganisms. This physiological mechanism can be potentially altered and hyperactivated in some autoimmune diseases, and in particular, in small vessels vasculitides, maintaining inflammation and determining vascular damage [19].
Our group recently demonstrated for the first time in BS patients a crucial role of neutrophils in the pathogenesis of thrombotic events. In particular, our study shows in Behçet’s patients, fibrinogen post-translational modifications (carbonylation) and reduced fibrin susceptibility to plasmin lysis, due to increased leukocyte oxidative stress and ROS generation, mainly derived by neutrophils. These findings support a strong rational basis to understand why the thrombus of BS is less responsive to anticoagulation therapy and often more to immunosuppressive drugs or to biological agents [20].
Main clinical features of vascular manifestations in Behçet’s syndrome
Superficial venous thrombosis (SVT) and deep vein thrombosis (DVT) are the most frequent types of vascular involvement, affecting altogether up to 15–40% of patients with BS. Thrombophlebitis is usually diagnosed by direct physical examination; however, Doppler ultrasound can be a useful tool to confirm the diagnosis in doubtful or uncertain cases [21].
DVT in BS may involve both the superior and inferior limbs, with post thrombotic syndrome being a later complication for the most severe cases. Moreover, thrombosis of atypical sites is also an important clinical feature of BS. In particular, the following vascular districts have been described to be potentially involved: inferior and superior vena cava, suprahepatic veins with Budd–Chiari syndrome, portal vein, cerebral sinuses and right ventricle [22].
On the other hand, arterial involvement, although far less common and affecting only a minority of the patients, is considered a unique vascular feature of BS. Indeed, this condition is perhaps the only chronic inflammatory disease causing aneurysms potentially affecting both peripheral, visceral and pulmonary arteries. The true prevalence of aneurysms in BS is probably underestimated, since autopsy studies showed significantly more lesions than usually clinically detected [23].
Another peculiar BS feature is the co-occurrence of venous and arterial manifestations, starting in the latter usually before the venous ones. Venous thrombosis is often detected together with aneurysms and pseudo-aneurysms. In rare cases, pulsatile masses from peripheral arteries are also recognised as a complication of bigger aneurysms. Hence, BS should always be included and kept in mind in the differential diagnosis of diffuse or localised aneurysms, in particular, when these finding are not explained by more common causes (Table 1).
Haemoptysis and cough are the main symptoms when haemorrhage complicates rupture of aneurysmatic pulmonary arteries. However, clinically silent aneurysms are often accidentally detected on lung X-ray or CT scan as vascular masses. Screening for such silent aneurysms is mandatory whenever anticoagulation is considered in Behçet patients, since such therapy significantly increases the risk of rupture and mortal haemorrhage [24].
The presence of arterial involvement in BS is a very important risk factor affecting both morbidity and on the long-term mortality, in particular when pulmonary aneurysms occur [25]. Moreover, about 80% of the patients with pulmonary arterial involvement were found to have concomitant venous thrombosis which consists mostly of lower-extremity DVT (66%), underlining the fact that probably the same inflammatory process is responsible for events both on the venous and the arterial side of the vascular tree. The contemporary occurrence of arterial pulmonary aneurysms and peripheral venous thrombosis is the hallmark of the so called Hughes–Stovin syndrome, nowadays considered by some authors as a clinical variant of vascular Behçet [26, 27].
Treatment of vascular involvement
Most appropriate management of vascular involvement in BS should be tailored on specific vessel disease [28].
While the role of immunosuppressive therapy is well acknowledged, a controversy exists regarding the value of anticoagulant treatment, and the usefulness of anticoagulation in different vascular manifestations related to BS is still unclear. Furthermore, the availably of an increasing number of immunomodulating drugs targeting different inflammatory mediators poses a concern on the choice of the most appropriate immunosuppressant agent [29].
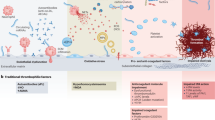
In the following paragraphs, we summarise current literature evidence on the pharmacological management of the most frequent venous and arterial involvements (Fig. 2, Supplementary Table 1 and 2).
Venous involvement
Deep and superficial venous thrombosis in typical sites
In DVT, use of immunosuppressants is pivotal for the control of recurrences, whereas concomitant use of anticoagulation therapy is not associated with additional positive effects in reducing the risk of DVT relapse [30, 31]. In fact, occurrence of venous thrombotic events in BS is thought to be related to a sustained systemic inflammation rather than to a specific thrombophilic state [28]. Benefits of steroid or immunosuppressant therapy also override those of anticoagulants also in superficial venous thrombosis (SVT) [32, 33].
According to the 2018 EULAR recommendations, there are no definite data for preferring one immunosuppressant drug over another. However, evidence suggests the choice of azathioprine, cyclophosphamide (CYC) or cyclosporine-A [28].
Recently, the anti-TNF-α adalimumab (ADA), alone or in combination with other immunosuppressants, proved proves to be more effective than DMARDs alone in inducing clinical and ultrasound resolution of venous thrombosis. Moreover, ADA allows a more pronounced steroid tapering, while no additional benefits from anticoagulation were shown [33]. According to the 2018 EULAR recommendations, use of anti-TNF-α should be considered in patients with refractory thrombosis, while the addition of anticoagulants should be weighted on the risk of bleeding as well as of potentially coexistent pulmonary artery aneurysms [28].
Deep vein thrombosis in atypical sites
Vena cava thrombosis
An exception to the use of anticoagulants is perhaps represented by vena cava thrombosis (VCT). In a retrospective chart review of 29 patients by Hamzaoui et al. [34], treatment of VCT was based on anticoagulants, and concomitant treatment with corticosteroids and immunosuppressants (mainly CYC) was prescribed in 22 subjects. Good outcome was reported in 12 patients, whereas in four cases, extension of VCT occurred.
The use of CYC as a preference treatment in vena cava thrombosis is supported also by the last EULAR recommendations, that suggest CYC for patients with extensive thrombosis of large vessels such as vena cava, in view of its potential adverse events [28].
Budd–Chiari syndrome
In atypical thrombosis such as in the Budd–Chiari syndrome (BCS) in BS subjects, lack of use of immunosuppressant treatment in the first 6 months following the onset of symptomatic liver disease has been associated with poor prognosis, whereas anticoagulant or thrombolytic therapy does not seem to reduce mortality [35]. As for the specific contribution of anti-TNF biologics such as infliximab in BS-related BCS, evidence from the literature is still unclear [36].
Intracardiac thrombosis
In a series of eight cases reported by Ben Ghorbel et al. [37], treatment of intracardiac thrombosis (ICT) was mainly based on anticoagulants, colchicine, and prednisone, and concomitant immunosuppressive therapy with azathioprine (AZA) and/or CYC was used in five cases. ICT resolution was obtained in five cases and clinical remission in four. The effectiveness of the triple association of anticoagulants, immunosuppressants and corticosteroids in ICT is also confirmed in a retrospective study by Wang et al. [38]. Based on this study, use of this combination either as first line treatment or following surgery accounts for no ICT reoccurrence. When administered as first-line, these therapies accounted for complete resolution of ICT in 78% of cases.
Central nervous system venous thrombosis
Anticoagulation is also required in the management of central nervous system venous thrombosis (CNSVT) [39]. In a recent study by Uluduz et al. [40], 108 subjects with central venous sinus thrombosis (CVST) were initially treated with pulsed steroid and low-molecular-weight heparin; after the first month of treatment, this therapy was replaced with warfarin. Warfarin alone was also used as an effective maintenance treatment. According to a retrospective observational study by Saadoun et al. [41], up to 90% of patients responded to anticoagulant treatment without severe haemorrhagic complications. Furthermore, concomitant use of immunosuppressants did not influence the risk of sequalae or relapses as compared to use of anticoagulants alone. In a recent study by Shi et al. [42], co-treatment with corticosteroids, immunosuppressants, anticoagulants and dehydration accounted for remission of CVST in more than 85% of subjects.
Arterial involvement
In arterial lesions, use of immunosuppressants should always be taken into account, since they have been positively associated with the achievement of complete remission, as well as with lower risk of post-operative complications. In these patients, benefit from the concomitant use of anticoagulants is mainly ascribable to the reduction in the risk of post-operative thrombosis.
Pulmonary artery involvement
Immunosuppressants are the mainstay of treatment in pulmonary artery involvement (PAI) [43, 44], whereas use of anticoagulants in this condition is negligible.
In subjects refractory to immunosuppressants, embolisation, lobectomy, cavitectomy, and decortication can be considered [43, 45, 46].
In addition, when therapy with conventional immunosuppressants is ineffective, anti-TNF-α agents (mainly infliximab) can represent a life-saving treatment [47]. Nevertheless, doubts related to the occurrence of PAI during anti-TNF-α treatment, as well as safety concerns, have been reported [47].
As for pulmonary artery aneurysm (PAA), pharmacological treatment is mainly based on immunosuppressants, namely CYC or AZA, alone or in combination with corticosteroids [24, 48]. Based on a retrospective study by Hamuryudan et al. [24] such a treatment accounts for a 5-year overall survival rate of 62% (even assuming that all subjects lost during follow-up had died). In an observational study by Uzun et al. [49], five PAA patients were treated with colchicine and corticosteroids, plus CYC and embolisation. Despite treatment, mortality rate was high, and four out of five patients died.
Use of CYC in association with high-dose corticosteroids in PAA is strongly suggested by the 2018 EULAR recommendations, while use of anti-TNF-α should be considered for refractory cases [28].
Pseudoaneurysm
In patients with pseudoaneurysm, corticosteroids and immunosuppressants, prednisone alone or in combination with AZA seems a pivotal pre-surgery therapy, before endovascular treatment [50, 51]. In the days after surgery, successful use of hydrocortisone plus CYC has been reported. The use of oral immunosuppressants therapy following surgical intervention has been associated with successful prevention of pseudoaneurysm recurrences [50].
Conclusions
BS can be considered a “neutrophilic perivasculitis”, characterised by peculiar vascular manifestations. Indeed, BS can involve both arterial and venous vessels, of any size and in any body district, often simultaneously [4]. Behçet is not a single unique entity, but rather a syndrome with different clinical phenotypes. In this context, patients with a predominant vascular involvement belong to the “vascular cluster.” [52]. The vascular involvement in BS is one of the most important in terms of morbidity and mortality, however, improvement in the treatment has occurred [28].
In general, control of vascular thrombosis is achieved with immunosuppressants drugs rather than anticoagulants. The latter indeed seems not effective in patients with BS in preventing recurrent thrombotic events, which are thought to be caused by an inflammatory process rather than a thrombophilic state. An exception to the use of anticoagulant in BS appears to be due to cerebral veins thrombosis, since some data seem to indicate a partial benefit of such therapy. However in selected BS patients for whom anticoagulation seems a useful treatment to add to immunosuppressants, it is strongly recommended to perform a CT lung scan before starting such therapy, in order to rule out the presence of occult pulmonary artery aneurysms, and to prevent the consequent risk of rupture and bleeding.
With regard to immunosuppressive therapy, the use of AZA and cyclosporine in association with low-dose corticosteroids should be considered in DVT and SVT cases, while treatment with CYC can be effectively prescribed for arterial involvement [28]. More recently, anti-TNF-α agents have been increasingly reported as an alternative treatment for acute and long-term management of vascular Behçet [28, 33], while proving effective also in the control of general BS manifestations [53,54,55,56,57,58].
References
Silvestri E, Emmi G, Prisco D (2013) Vascular Behçet’s disease: new insights in the management of thrombosis. Expert Rev Cardiovasc Ther 11:1583–1585. https://doi.org/10.1586/14779072.2013.836449
Yazici H, Ugurlu S, Seyahi E (2012) Behçet syndrome: is it one condition? Clin Rev Allergy Immunol 43:275–280. https://doi.org/10.1007/s12016-012-8319-x
International Team for the Revision of the International Criteria for Behçet’s Disease (ITR-ICBD) F, Assaad-Khalil S, Calamia KT et al (2014) The International Criteria for Behçet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol 28:338–347. https://doi.org/10.1111/jdv.12107
Jennette JC, Falk RJ, Bacon PA et al (2013) 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum 65:1–11. https://doi.org/10.1002/art.37715
McDonald DR, Lee C, Fowler RA, Abuhaleeqa K (2007) Behcet’s disease. CMAJ 176:1273–1274. https://doi.org/10.1503/cmaj.061136
Boyd SR, Young S, Lightman S (2001) Immunopathology of the noninfectious posterior and intermediate uveitides. Surv Ophthalmol 46:209–233
Hirohata S, Kikuchi H (2009) Histopathology of the ruptured pulmonary artery aneurysm in a patient with Behçet’s disease. Clin Exp Rheumatol 27:S91–S95
Ergun T, Gürbüz O, Harvell J et al (1998) The histopathology of pathergy: a chronologic study of skin hyperreactivity in Behçet’s disease. Int J Dermatol 37:929–933
Verity DH, Wallace GR, Vaughan RW, Stanford MR (2003) Behçet’s disease: from Hippocrates to the third millennium. Br J Ophthalmol 87:1175–1183
Emmi G, Silvestri E, Squatrito D et al (2014) Behçet’s syndrome pathophysiology and potential therapeutic targets. Intern Emerg Med 9:257–265. https://doi.org/10.1007/s11739-013-1036-5
Consolandi C, Turroni S, Emmi G et al (2015) Behçet’s syndrome patients exhibit specific microbiome signature. Autoimmun Rev 14:269–276. https://doi.org/10.1016/j.autrev.2014.11.009
Aldinucci A, Bonechi E, Biagioli T et al (2018) CSF/serum matrix metallopeptidase-9 ratio discriminates neuro Behçet from multiple sclerosis. Ann Clin Transl Neurol 5:493–498. https://doi.org/10.1002/acn3.538
Emmi G, Silvestri E, Della Bella C et al (2016) Cytotoxic Th1 and Th17 cells infiltrate the intestinal mucosa of Behcet patients and exhibit high levels of TNF-α in early phases of the disease. Medicine 95:e5516. https://doi.org/10.1097/MD.0000000000005516 (Baltimore)
Aksu K, Donmez A, Keser G (2012) Inflammation-induced thrombosis: mechanisms, disease associations and management. Curr Pharm Des 18:1478–1493
Emmi G, Silvestri E, Squatrito D et al (2015) Thrombosis in vasculitis: from pathogenesis to treatment. Thromb J 13:15. https://doi.org/10.1186/s12959-015-0047-z
Matsumura N, Mizushima Y (1975) Leucocyte movement and colchicine treatment in Behcet’s disease. Lancet 2:813 (London, England)
Kawakami T, Ohashi S, Kawa Y et al (2004) Elevated serum granulocyte colony-stimulating factor levels in patients with active phase of sweet syndrome and patients with active behcet disease: implication in neutrophil apoptosis dysfunction. Arch Dermatol 140:570–574. https://doi.org/10.1001/archderm.140.5.570
Erkan F, Gül A, Tasali E (2001) Pulmonary manifestations of Behçet’s disease. Thorax 56:572–578
Mantovani A, Cassatella MA, Costantini C, Jaillon S (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11:519–531. https://doi.org/10.1038/nri3024
Becatti M, Emmi G, Silvestri E et al (2016) Neutrophil activation promotes fibrinogen oxidation and thrombus formation in Behçet disease. Circulation 133:302–311. https://doi.org/10.1161/CIRCULATIONAHA.115.017738
Nasr H, Scriven JM (2015) Superficial thrombophlebitis (superficial venous thrombosis). BMJ 350:h2039. https://doi.org/10.1136/bmj.h2039
Sarr SA, Fall PD, Mboup MC et al (2015) Superior vena cava syndrome revealing a Behçet’s disease. Thromb J 13:7. https://doi.org/10.1186/s12959-015-0039-z
Lakhanpal S, Tani K, Lie JT et al (1985) Pathologic features of Behçet’s syndrome: a review of Japanese autopsy registry data. Hum Pathol 16:790–795
Hamuryudan V, Er T, Seyahi E et al (2004) Pulmonary artery aneurysms in Behçet syndrome. Am J Med 117:867–870. https://doi.org/10.1016/j.amjmed.2004.05.027
Saadoun D, Wechsler B, Desseaux K et al (2010) Mortality in Behçet’s disease. Arthritis Rheum 62:2806–2812. https://doi.org/10.1002/art.27568
Hughes JP, Stovin PG (1959) Segmental pulmonary artery aneurysms with peripheral venous thrombosis. Br J Dis Chest 53:19–27
Khalid U, Saleem T (2011) Hughes–Stovin syndrome. Orphanet J Rare Dis 6:15. https://doi.org/10.1186/1750-1172-6-15
Hatemi G, Christensen R, Bang D et al (2018) 2018 update of the EULAR recommendations for the management of Behçet’s syndrome. Ann Rheum Dis 77:808–818. https://doi.org/10.1136/annrheumdis-2018-213225
Vitale A, Rigante D, Lopalco G et al (2016) New therapeutic solutions for Behçet’s syndrome. Expert Opin Investig Drugs 25:827–840. https://doi.org/10.1080/13543784.2016.1181751
Ahn JK, Lee YS, Jeon CH et al (2008) Treatment of venous thrombosis associated with Behcet’s disease: immunosuppressive therapy alone versus immunosuppressive therapy plus anticoagulation. Clin Rheumatol 27:201–205. https://doi.org/10.1007/s10067-007-0685-z
Alibaz-Oner F, Karadeniz A, Ylmaz S et al (2015) Behçet disease with vascular involvement. Medicine 94:e494. https://doi.org/10.1097/MD.0000000000000494 (Baltimore)
Desbois AC, Wechsler B, Resche-Rigon M et al (2012) Immunosuppressants reduce venous thrombosis relapse in Behçet’s disease. Arthritis Rheum 64:2753–2760. https://doi.org/10.1002/art.34450
Emmi G, Vitale A, Silvestri E et al (2018) Adalimumab-based treatment versus DMARDs for venous thrombosis in Behçet syndrome. A retrospective study of 70 patients with vascular involvement. Arthritis Rheumatol 70:1500–1507. https://doi.org/10.1002/art.40531
Hamzaoui A, Fatima J, Thouraya BS et al (2014) Vena cava thrombosis in Behçet’s disease. Anadolu Kardiyol Derg 14:292–293. https://doi.org/10.5152/akd.2014.5042
Seyahi E, Caglar E, Ugurlu S et al (2015) An outcome survey of 43 patients with Budd–Chiari syndrome due to Behçet’s syndrome followed up at a single, dedicated center. Semin Arthritis Rheum 44:602–609. https://doi.org/10.1016/j.semarthrit.2014.10.014
Seyahi E, Hamuryudan V, Hatemi G et al (2007) Infliximab in the treatment of hepatic vein thrombosis (Budd–Chiari syndrome) in three patients with Behcet’s syndrome. Rheumatology 46:1213–1214. https://doi.org/10.1093/rheumatology/kem103 (Oxford)
Ben Ghorbel I, Belfeki N, Houman MH (2016) Intracardiac thrombus in Behçet’s disease. Reumatismo 68:148–153. https://doi.org/10.4081/reumatismo.2016.887
Wang H, Guo X, Tian Z et al (2016) Intracardiac thrombus in patients with Behcet’s disease: clinical correlates, imaging features, and outcome: a retrospective, single-center experience. Clin Rheumatol 35:2501–2507. https://doi.org/10.1007/s10067-015-3161-1
Prisco D, Silvestri E, Di Scala G, Emmi G (2018) Behçet’s disease as a cause of cerebral sinus vein thrombosis: an emerging role. Rheumatology. https://doi.org/10.1093/rheumatology/key279 (Oxford)
Uluduz D, Midi I, Duman T et al (2018) Behçet’s disease as a causative factor of cerebral venous sinus thrombosis: subgroup analysis of data from the VENOST study. Rheumatology 55:464. https://doi.org/10.1093/rheumatology/key153 (Oxford)
Saadoun D, Wechsler B, Resche-Rigon M et al (2009) Cerebral venous thrombosis in Behçet’s disease. Arthritis Rheum 61:518–526. https://doi.org/10.1002/art.24393
Shi J, Huang X, Li G et al (2018) Cerebral venous sinus thrombosis in Behçet’s disease: a retrospective case-control study. Clin Rheumatol 37:51–57. https://doi.org/10.1007/s10067-017-3718-2
Seyahi E, Melikoglu M, Akman C et al (2012) Pulmonary artery involvement and associated lung disease in Behçet disease: a series of 47 patients. Medicine 91:35–48. https://doi.org/10.1097/MD.0b013e318242ff37 (Baltimore)
Zhang X, Dai H, Ma Z et al (2015) Pulmonary involvement in patients with Behçet’s disease: report of 15 cases. Clin Respir J 9:414–422. https://doi.org/10.1111/crj.12153
Rossi GM, Emmi G, Vaglio A (2018) Hemoptysis in Behçet’s syndrome: from bedside to bench? Intern Emerg Med 13:467–469. https://doi.org/10.1007/s11739-018-1863-5
Voiriot G, Parrot A, Antoine M et al (2018) Transcatheter embolotherapy of pulmonary artery aneurysms as emergency treatment of hemoptysis in Behcet patients: experience of a referral center and a review of the literature. Intern Emerg Med 13:491–500. https://doi.org/10.1007/s11739-018-1817-y
Hamuryudan V, Seyahi E, Ugurlu S et al (2015) Pulmonary artery involvement in Behçet׳s syndrome: effects of anti-Tnf treatment. Semin Arthritis Rheum 45:369–373. https://doi.org/10.1016/j.semarthrit.2015.06.008
Hamuryudan V, Yurdakul S, Moral F et al (1994) Pulmonary arterial aneurysms in Behçet’s syndrome: a report of 24 cases. Rheumatology 33:48–51. https://doi.org/10.1093/rheumatology/33.1.48
Uzun O, Erkan L, Akpolat I et al (2008) Pulmonary involvement in Behçet’s disease. Respiration 75:310–321. https://doi.org/10.1159/000101954
Balcioglu O, Ertugay S, Bozkaya H et al (2015) Endovascular repair and adjunctive immunosuppressive therapy of aortic involvement in Behçet’s disease. Eur J Vasc Endovasc Surg 50:593–598. https://doi.org/10.1016/j.ejvs.2015.07.011
Kwon Koo B, Shim W-H, Yoon Y-S et al (2003) Endovascular therapy combined with immunosuppressive treatment for pseudoaneurysms in patients with Behçet’s disease. J Endovasc Ther 10:75–80. https://doi.org/10.1177/152660280301000116
Seyahi E (2016) Behçet’s disease: how to diagnose and treat vascular involvement. Best Pract Res Clin Rheumatol 30:279–295. https://doi.org/10.1016/j.berh.2016.08.002
Vitale A, Emmi G, Lopalco G et al (2017) Adalimumab effectiveness in Behçet’s disease: short and long-term data from a multicenter retrospective observational study. Clin Rheumatol 36:451–455. https://doi.org/10.1007/s10067-016-3417-4
Fabiani C, Vitale A, Rigante D et al (2018) Predictors of sustained clinical response in patients with Behçet’s disease-related uveitis treated with infliximab and adalimumab. Clin Rheumatol 37:1715–1720. https://doi.org/10.1007/s10067-018-4092-4
Fabiani C, Sota J, Vitale A et al (2018) Cumulative retention rate of adalimumab in patients with Behçet’s disease-related uveitis: a 4-year follow-up study. Br J Ophthalmol 102:637–641. https://doi.org/10.1136/bjophthalmol-2017-310733
Fabiani C, Vitale A, Emmi G et al (2017) Efficacy and safety of adalimumab in Behçet’s disease-related uveitis: a multicenter retrospective observational study. Clin Rheumatol 36:183–189. https://doi.org/10.1007/s10067-016-3480-x
Lopalco G, Emmi G, Gentileschi S et al (2017) Certolizumab Pegol treatment in Behcet’s disease with different organ involvement: a multicenter retrospective observational study. Mod Rheumatol 27:1031–1035. https://doi.org/10.1080/14397595.2017.1285857
Vitale A, Emmi G, Lopalco G et al (2018) Correction to: long-term efficacy and safety of golimumab in the treatment of multirefractory Behçet’s disease. Clin Rheumatol 5:123. https://doi.org/10.1007/s10067-018-4302-0
Acknowledgements
This study was not funded. The authors wish to thank Stefano Salvati and Javier Hernández Plasencia for their help in preparing Fig. 2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Statement of human and animal rights
This study does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent is not applicable to this study (review).
Additional information
This article is part of the topical collection “Behcet Disease”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Emmi, G., Bettiol, A., Silvestri, E. et al. Vascular Behçet’s syndrome: an update. Intern Emerg Med 14, 645–652 (2019). https://doi.org/10.1007/s11739-018-1991-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-018-1991-y