Abstract
Almost one third of patients with Behçet's syndrome (BS) display vascular involvement. However, data regarding the prevalence and management of venous thromboembolism (VTE) in BS are scanty. We assessed the differential characteristics between patients with and without VTE and the factors associated with VTE incidence. A case–control study in a cohort of patients with BS was performed. 57 patients were included (56.1% women) with a mean follow-up of 10.56 (± 10.7) years. Mean age at diagnosis of BS and diagnosis of the first VTE episode was 34.7 (± 12.1) and 31.2 (± 8.9) years, respectively. Erythema nodosum (OR 4.6, CI 95% 1.2–18.1) and fever (OR 8.2, CI 95% 1.6–42.1) were associated with a higher risk of VTE. 26 episodes of VTE were registered in 12/57 (21%) patients. 83.3% of patients were not diagnosed with BS when the first episode of VTE occurred and, among them, the episode of VTE led to the diagnosis of BS in 40% of cases. Half of patients had at least one VTE recurrence. The absence of immunosuppressive treatment was associated with a higher risk of developing a first episode of VTE (OR 20 CI 95% 19.2–166.6). All patients were treated with anticoagulation and 75% were treated with immunosuppressants after the first VTE event. The diagnosis of VTE usually precedes that of BS, with a high frequency of VTE recurrence. Erythema nodosum and fever were associated with a higher risk of VTE, while the immunosuppressants showed a protective role for the development of VTE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
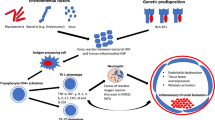
Behçet's syndrome (BS) is a chronic multisystemic disorder with a characteristic geographical distribution along the silk route [1], characterized by skin-mucosa lesions. It may also involve the eyes, blood vessels, joints, gastrointestinal system, and central nervous system [2]. The pathogenesis of BS is very complex and it is represented by a genetic predisposition (mainly HLA dependent and specifically HLA B*51 which is the major susceptibility genetic factor), activation of innate and adaptive immunity by several pathogens, with consequent interaction of both T lymphocytes (mainly Th1 and Th17 phenotype) and activated neutrophils, all of which are responsible for tissue damage in BS [3].
There are some difficulties in developing universal classification criteria. First, because of its geographical variation in prevalence and disease expression [1]. BS has an episodic course that may resemble auto-inflammatory diseases, although it has some differences since it is not monogenic and rarely occurs during childhood. Moreover, it does not fit into a “vasculitis disease”, because BS affects veins and arteries, with tendency to aneurysm formation and the absence of granulomatous inflammatory lessions within vessel wall. For all these reasons, BS is classified as a variable vessel vasculitis, and different studies have attempted to categorize BS into several phenotypes (or "clusters") depending on predominant symptoms. There is a “vascular cluster” [4, 5] that presents approximately in a third of the patients and is one of the major causes of mortality and morbidity in BS [4, 6, 7]. Vascular involvement occurs early during the course of the disease and is seen mainly among men. BS may affect blood vessels of different sizes and types, including arteries (stenosis, aneurysms, thrombosis, bleeding) and more commonly veins (thrombosis) [2, 8]. The pathogenesis of thrombosis in BS is unclear, and in the past few years, some studies have shown the relationship between neutrophils and thrombosis. An Italian study demonstrated the important role of neutrophils in the pathogenesis of thrombosis in BS, through NADPH oxidase (specifically NOX2), by producing an excess of reactive oxygen species (ROS) that are able to modify the secondary structure of fibrinogen being less susceptible to plasmin-induced lysis [5, 9,10,11]. A specific mechanism of programmed cell death for neutrophils has also recently been described [5, 12]. These findings help to understand why the thrombus in BS is less responsive to anticoagulant therapy. Therefore, immunosuppressants play an important role, although there are no controlled studies for the management of vascular BS [5, 13]. Pulmonary embolism is rare, because thrombi in the affected veins of the lower extremities are strongly adherent [2, 14]. Due to the concern about bleeding-associated mortality (that is more frequent in case of pulmonary artery aneurysms) [15] as well as the fact that anticoagulation has not demonstrated to prevent venous thrombosis recurrences, anticoagulants are not usually recommended for the treatment of venous thrombosis in BS, even though the evidence is scarce [13].
To better characterize venous thromboembolism (VTE) in the setting of BS and its related management, we performed a case–control study in a cohort of patients with BS to assess the differential characteristics between patients with and without VTE and the factors that could be associated with the recurrences of venous thrombosis.
Material and methods
Type of study
Case–control study to assess the differential characteristics between patients with BS with or without VTE and the factors that could be associated with VTE recurrence.
Study population
Consecutive patients with diagnosis of BS between 2006 and 2018 in a tertiary hospital in Madrid were included. BS was defined according to the International Study Group for Behçet’s Disease (ISGBD) [16] diagnostic criteria and the International Team for the Revision of the International Criteria for Behçet's Disease (ITR-ICBD) diagnostic criteria [17]. All patients underwent serological study for the screening of other autoimmune diseases.
Follow-up and variables
Retrospective analysis of the medical records of the patients was performed. Information about epidemiological characteristics, symptoms, diagnostic tests, evolution and anticoagulant and immunosuppressant treatment was obtained from all patients. Deep venous thrombosis of lower extremities (DVTLE) and superficial venous thrombosis (SVT) were diagnosed by Doppler ultrasound. Inferior cava vein (ICV) thrombosis and suprahepatic vein thrombosis were diagnosed by contrast-enhanced computed tomography (CECT). Pulmonary embolism was diagnosed by computed tomography pulmonary angiography (CTPA). Intracardiac thrombosis was diagnosed by transthoracic echocardiogram and/or transesophageal echocardiogram when indicated. Intracranial thrombosis was diagnosed by cranial computed tomography. The diagnostic delay was defined as the delay between the first medical visit and the diagnosis of the BS. The presenting symptom is defined as the patient’s main complaint when first consulted.
Statistical analysis
Qualitative variables are presented by the frequency distribution. Quantitative variables are presented by the mean and the standard deviation in case they have a normal distribution or median and the 25th (P25) and 75th (P75) percentiles if they have a non-normal distribution. Chi-square test or Fisher’s exact test were used for comparison between qualitative variables, depending on the sample size. T-Student and Mann–Whitney tests were used for mean comparison between two groups when distribution was parametric and non-parametric, respectively. A univariate logistic regression analysis was performed to obtain odds ratio of clinical and epidemiological features for the development of VTE. A value of p < 0.05 was accepted as significant.
Ethic and risks
This study was carried out following the international ethical recommendations for conducting research in humans in the latest revision of the Declaration of Helsinki, as well as those established in the Good Clinical Practice Guidelines and in the current legislation. Informed consent was not requested, because it was a retrospective study. However, there is no personal information that allows patient identification. The study was approved by the Institutional Ethics Committee (03/2019).
Results
Total study sample
Seventy-six medical records of patients diagnosed with BS were found. Nineteen patients were excluded due to incomplete information on their medical records. Therefore, the study included 57 patients (56.1% women). The mean age at diagnosis of BS was 34.7 (± 12.1) years. ISGBD and ITR-ICBD diagnostic criteria were fulfilled by 73.7% (42/57) and 98.2% (56/57) of patients, respectively. The mean time delay of diagnosis was 14.7 (± 32.1) months. Among study cohort, 85.9% of patients were Spaniards, 5.3% were Latin Americans, 5.3% were Africans, one patient was Portuguese and one patient was Turkish. A previous family history of BS was found in 3.5% of the patients, whereas 8.8% had family history of autoimmune diseases. The main complaints were genital ulcers, oral ulcers, eye lesions, vascular involvement, neurological involvement and fever. HLA (human leukocyte antigen) study was performed in 33.3% (19/57) patients. They were classified as B51+ (31.5%, 6/19), A26+ (5.2%, 1/19) and the remainder was “negative for BS-associated HLA type”. Baseline and clinical characteristics of the patients with and without VTE are listed in Table 1. Seven percent (4/57) of patients had arterial involvement. Three of the patients with arterial involvement also presented VTE.
The most frequent background treatments found in the entire sample were 87.7% colchicine, 91.2% corticoids and 45.6% azathioprine. Other immunosuppressive treatments were infliximab (21.4%), methotrexate (19.3%), cyclosporine (15.8%), rituximab (14%), cyclophosphamide (10.5%), etanercept (10.5%) and adalimumab (10.5%).
The mean follow-up for the entire sample was 10.56 (± 10.7) years. Two patients died from neurologic BS involvement and other patient died from gastrointestinal bleeding.
Patients with BD and VTE
Twenty-six episodes of VTE were registered in 12/57 (21%) patients with a mean age at diagnosis of the first VTE episode of 31.2 (± 8.9) years. A univariate logistic regression showed that erythema nodosum (OR 4.6, CI 95% 1.2–18.1) and fever (OR 8.2, CI 95% 1.6–42.1) were associated with a higher risk of VTE (Table 1). The absence of immunosuppressive treatment was associated with a higher risk of developing a first episode of VTE (OR 20 CI 95% 19.2–166.6). Clinical characteristics of the patients with VTE are listed in Table 2. The 15.4% (4/26) of VTE episodes presented thrombosis in more than one level. At diagnosis of VTE, mean C-reactive protein was 6.68 (± 5.8) mg/dL, mean D-dimer was 1371.8 (± 616.8) ng/mL, mean erythrocyte sedimentation rate was 28 (± 25.4) mm first hour and mean creatinine was 0.91 (± 0.21) mg/dL.
Ninety-two percent of the first VTE events did not present other provoking factors. Eighty three percent (10/12) of patients were not diagnosed with BS when the first episode of VTE occurred, with a mean time from the first VTE episode to BS diagnosis of 3.5 (± 6.4) months. Interestingly, at the time of the first VTE episode, 9/10 patients undiagnosed with BS already fulfilled both ISGBD and ITR-ICBD criteria. Among them, the episode of VTE led to the diagnosis of BS in 40% (4/10) of cases. Among the two patients that were diagnosed with BS before the first VTE episode occurred, the mean time from the BS diagnosis to the first VTE episode was 20.1 (± 22.4) months. All thrombophilia studies that were performed (5/12) were negative. No patient had antiphospholipid syndrome. Fifty percent (6/12) of patients had one VTE recurrence, while 5/12 patients had two relapses, 2/12 patients had three relapses and one patient had four relapses.
Only one patient (8.3%) was receiving immunosuppressants before the first episode of VTE. All patients were treated with anticoagulation after the first episode of VTE. 78.5% (7/8) were treated with low molecular weight heparin or intravenous heparin during the acute phase and acenocoumarol afterwards. One (12.5%) patient was treated with rivaroxaban. Mean duration of anticoagulation after the first VTE episode was 8.4 months (data available in 5/12 patients). Seventy-five percent (9/12) of patients were treated with immunosuppressants after the first episode of VTE. A univariate logistic regression among patients with a first VTE episode showed that immunosuppressive treatment (OR 0.40, CI 95% 0.02–6.16) and anticoagulation (50% of patients, CI 95% 21.1–78.9%) did not affect the risk of developing a recurrence of VTE. Before a recurrence, 7.1% (1/14) of patients were receiving only immunosuppressants, 21.4% (3/14) were receiving only anticoagulation and 71.5% (10/14) were receiving both treatments. After a recurrence, 100% (11/11) were treated with anticoagulation and 91% (10/11) were treated with both.
The mean duration of anticoagulation per VTE episode was 2.3 (± 3.8) years. Only one episode of minor bleeding (metrorrhagia) was registered during follow-up. Twenty-five percent (3/12) of patients developed post-thrombotic syndrome and no cases of chronic thromboembolic pulmonary hypertension or death were registered among patients with VTE and BS. One patient had infectious complications due to immunosuppressants.
Discussion
The evidence regarding the association between VTE and BS is based on a low number of case series. VTE is a common finding in BS and patients often suffer from recurrent VTE. According to our study, treatment with anticoagulants and immunosuppressant drugs was safe, with low rate of side effects and no death recorded.
BS is classified as a systemic vasculitis associated with significant morbidity and mortality, particularly in males with early age of onset [8]. Our sample showed a distribution by sex similar to that found in the Spanish Registry of Behçet’s Disease (REGEB) (56.1% and 52.2% were women, respectively) [18].
Venous thromboembolism (VTE) occurs early during the course of the disease, with the majority of the patients (75%) presenting their first vascular event within 5 years of the disease onset [2, 4]. In our study, the first VTE event led to the diagnosis of BS in 40% of patients, a proportion much higher than that found in other similar studies, including the Spanish registry [18]. Recently published case series of patients with BS and VTE are listed in Table 3.
The diagnosis of BS is mainly clinical with no specific laboratory tests. However, the typical clinical characteristics often do not appear simultaneously, which hampers and may delay the diagnosis of the disease. The International Study Group for Behçet’s Disease (ISGBD) diagnostic criteria published in 1990 remain the most widely used and well-accepted criteria among experts in BS [17]. The International Criteria for Behçet’s Disease (ICBD) were developed in 2006 and included vascular involvement as a diagnostic criterion [17].
Vascular involvement is present in up to 40% of the patients with BS [5, 6, 18,19,20,21,22,23]. Our sample showed a similar prevalence of vascular involvement (21% of VTE, 23% when arterial involvement is included) than that found in the Spanish Registry [18]. Some studies included confusingly both arterial and venous involvement as vascular involvement [21,22,23], unlike the Spanish Registry [18] and our study, that have focused only on VTE. These differences between the classification in the different studies might limit the comparison between studies and the extraction of conclusions.
Vascular involvement is much more frequent in males (between 70% and 92%) [4]. The highest frequency of males among the patients with BS and vascular involvement has been found in Turkey [23] and China [21] (91.8% and 82.7%, respectively). Our study results were similar to those found in the Spanish Registry (66.7% and 70.3%, respectively).
According to the published studies, lower extremities venous thrombosis (LEVT) is the most common type of vascular involvement in BS, forming 70% of all vascular events [2]. Regarding the distribution of venous thrombosis, it should be pointed out that our study showed a higher frequency of intracardiac thrombosis (11.5%) and Budd–Chiari syndrome (11.5%), and a lower frequency of cerebral venous thrombosis and retinal vein thrombosis in comparison with other case series (Table 3). One case of Budd–Chiari syndrome and one case of intracardiac thrombosis in patients with BS have been published as case reports [24, 25].
Vascular involvement is frequently associated with fever, elevated acute phase response, and constitutional symptoms; usually, these could be accompanied by organ-specific symptoms [1]. In our study, fever and erythema nodosum showed a higher risk of VTE among patients with BS (Table 1), similar to Spanish registry [18].
It has been shown that approximately 30–40% of patients with vascular BS have recurrent vascular events in follow-up [8, 19, 20, 23]. The Spanish registry [18] showed 19.4% of recurrences. Our study showed that 50% of patients had vascular recurrence, being the highest rate reported to date in the literature. The mean of episodes of VTE per patient with VTE in our study was higher than the one showed in the Spanish registry (2.2 vs 1.17) and the other case series (Table 3). Some retrospective studies have shown that treatment with immunosuppressants reduces the relapse of thrombosis when compared with no treatment (HR 0.27 [95% CI 0.14–0.52, p < 0.001]) [20]. Our study results also showed a risk reduction of a first VTE episode in patients receiving immunosuppressants. However, immunosuppressive treatment after a first VTE episode did not affect the risk of VTE recurrence. Maybe, this lack of statistically significance was due to the small sample size (no. of patients = 12). Some retrospective studies did not find additional benefit for the prevention of thrombosis recurrences when anticoagulation was added to immunosuppressants [19, 20, 26]. EULAR [13] (European League Against Rheumatism) recommendations for the management of acute VTE in BS are glucocorticoids and immunosuppressives such as azathioprine, cyclophosphamide or cyclosporine-A (IIIC). Monoclonal anti-TNF antibodies could be considered in refractory venous thrombosis. Anticoagulants may be added in refractory patients, provided the risk of bleeding in general is low and coexistent pulmonary artery aneurysms are ruled out (IIIC) [13]. Nevertheless, there are not randomized controlled trials that have demonstrated the efficacy of these therapies in the setting of VTE in patients with BS. Despite that, current clinical practice often shows some discrepancies with these recommendations. A study consisting in a survey to rheumatologists from Turkey, Israel and the USA that were asked about the kind of treatment they would give to patients with BS and major vessel thrombosis demonstrated that the therapeutic approach differs significantly among them. More than 87% of the Israeli and American rheumatologists would give anticoagulation at the time of diagnosis for the cases of venous thrombosis compared with only 40–44% of the Turkish physicians. The study concluded that the different prevalence of the disease in these countries may explain this difference [27]. However, great caution is required with respect to bleeding in anticoagulated patients with BS. This is especially important since arterial aneurysms are closely associated with DVT in BS. Patients need to be scrutinised for aneurysms when starting anticoagulants and physicians should be alert about the risk of developing aneurysms during the course of treatment since almost all BS patients with aneurysms have a history of VTE. In such cases, anticoagulation therapy could increase the risk of aneurysmal rupture [6, 26, 28]. In our study, none of the patients that underwent a thoracic CT were found to have pulmonary aneurysms; however, it is notable that they were not ruled out in those patients with only DVT that were treated with anticoagulation. In our study, there were no episodes of major bleeding associated with anticoagulation. This may lead to the assumption that anticoagulation might be safe in patients with BS that experience VTE.
Our study owes several limitations. First of all it is a retrospective analysis of a case series, which may bring some further potential limitations. Since the limited number of patients and events a regression analysis was not feasible and then not performed. As our hospital is a referred tertiary hospital, which may result in more severe cases enrolled in our study, we cannot exclude the possibility of selection bias in this study population. Some of the statistical significant differences associated to VTE could be due to this fact. Despite the fact that BS is a rare disease, the limited number of patients analyzed may have prevented from finding statistical significant differences. Moreover, conclusions are only limited to a Spanish population and not especially generalizable to other populations. In addition, discrepancies between published studies could be secondary to genetic background and differences in study designs or variable definitions.
In conclusion, most patients with BS and VTE were young males and the first episode of VTE triggered the diagnosis of BS in 40% of patients. Erythema nodosum and fever were associated with a higher risk of VTE. The immunosuppressive treatment showed a protective role for the development of a first VTE episode. VTE recurrences affected half of the patients. Currently, the management of VTE in BS is based on anticoagulants and immunosuppressants, with scarce side effects. However, current recommendations for its management only include anticoagulation in refractory patients with low bleeding risk.
Abbreviations
- BS:
-
Behçet’s syndrome
- VTE:
-
Venous thromboembolism
- ISGBD:
-
International Study Group for Behçet’s Disease
- ITR-ICBD:
-
International Team for the Revision of the International Criteria for Behçet's Disease
- DVTLE:
-
Deep venous thrombosis of lower extremities
- SVT:
-
Superficial venous thrombosis
- ICV:
-
Inferior cava vein
- CECT:
-
Contrast-enhanced computed tomography (CECT)
- CTPA:
-
Computed tomography pulmonary angiography (CTPA)
- HLA:
-
Human leukocyte antigen
- ICBD:
-
International criteria for BD
- LEVT:
-
Lower extremities venous thrombosis
- DVT:
-
Deep venous thrombosis
References
Yazici H, Seyahi E, Hatemi G, Yazici Y (2018) Behçet syndrome: a contemporary view. Nat Rev Rheumatol 14:107–119 (Erratum in Nat Rev Rheumatol. 2018 14:119)
Seyahi E (2016) Behçet’s disease: how to diagnose and treat vascular involvement. Best Pract Res Clin Rheumatol [Internet] 30(2):279–295
Emmi G, Silvestri E, Squatrito D, Prisco D, Emmi L (2014) Behçet’s syndrome pathophysiology and potential therapeutic targets. Intern Emerg Med 9:257–265
Seyahi E (2019) Phenotypes in Behçet’s syndrome. Intern Emerg Med 14(5):677–689
Emmi G, Bettiol A, Silvestri E, Di G, Matteo S, Claudia B et al (2018) Vascular Behçet’s syndrome: an update. Intern Emerg Med 14(5):645–652
Kural-Seyahi E, Fresko I, Seyahi N, Ozyazgan Y, Mat C, Hamuryudan V et al (2003) The long-term mortality and morbidity of Behçet syndrome a 2-decade outcome survey of 387 patients followed at a dedicated center. Medicine (Baltimore) 82:60–76
Fabiani C, Vitale A, Orlando I, Sota J, Capozzoli M, Franceschini R et al (2017) Quality of life impairment in Behçet’s disease and realationship with disease activity: a prospective study. Intern Emerg Med 12:947–955
Zeidan MJ, Saadoun D, Garrido M, Klatzmann D, Six A, Cacoub P (2016) Behçet's disease physiopathology: a contemporary review. Auto Immun Highlights 7:4
Emmi G, Becatti M, Bettiol A, Hatemi G, Prisco D, Fiorillo C (2019) Behçet’s syndrome as a model of thrombo-inflammation: the role of neutrophils. Front Immunol 10:1085
Becatti M, Emmi G, Silvestri E, Bruschi G, Ciucciarelli L, Squatrito D et al (2016) Neutrophil activation promotes fibrinogen oxidation and thrombus formation in Behçet disease. Circulation 133:302–311
Becatti M, Emmi G, Bettiol A, Silvestri E, Scala G, Taddei N et al (2019) Behçet's syndrome as a tool to dissect the mechanisms of thrombo-inflammation: clinical and pathogenetic aspects. Clin Exp Imunol. 195(3):322–333
Mantovani A, Cassatella MA, Constatini C, Jaillon S (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Inmunol 11:519–531
Hatemi G, Christensen R, Bang D, Bodaghi B, Celik AF, Fortune F et al (2018) 2018 update of the EULAR recommendations for the management of Behçet’s syndrome. Ann Rheum Dis 77(6):808–818
Fei Y, Li X, Lin S, Song X, Wu O, Zhu Y et al (2013) Major vascular involvement in Behçet's disease: a retrospective study of 796 patients. Clin Rheumatol 32:845–852
Seyahi E, Yazici H (2015) Behçet’s syndrome : pulmonary vascular disease. Curr Opin Rheumatol 27:18–23
International Study Group for Behçet’s Disease (1990) Criteria for diagnosis of Behçet’s disease. Lancet 335:1078–1080
Davatchi F, Assaad-Khalil S, Calamia KT, Crook JE, Sadeghi-Abdollahi B, Schirmer M et al (2014) International Team for the Revision of the International Criteria for Behçet's Disease (ITR-ICBD). The International Criteria for Behçet's Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol 28:338–347
Rodríguez-Carballeira M, Solans R, Larrañaga JR, García-Hernández FJ, Ríos-Fernández R, Nieto J et al (2018) Venous thrombosis and relapses in patients with Behçet’s disease. Descriptive analysis from Spanish network of Behçet’s disease (REGEB cohort). Autoimmune Diseases Study Group (GEAS). Clin Exp Rheumatol 36((6 Suppl 115)):40–44
Alibaz-Oner F, Karadeniz A, Yılmaz S, Balkarlı A, Kimyon G, Yazıcı A et al (2015) Behçet disease with vascular involvement: effects of different therapeutic regimens on the incidence of new relapses. Med Baltim 94:e494
Desbois AC, Wechsler B, Resche-Rigon M, Piette JC, Huong Dle T, Amoura Z et al (2012) Immunosuppressants reduce venous thrombosis relapse in Behçet's disease. Arthritis Rheum 64:2753–2760
Wu X, Li G, Huang X, Wang L, Liu W, Zhao Y et al (2014) Behçet’s disease complicated with thrombosis a report of 93 Chinese cases. Medicine (Baltimore) 93(28):e263
Tohmé A, Aoun N, El-Rassi B, Ghayad E (2003) Vascular manifestations of Behçet’s disease. Eighteen cases among 140 patients. Joint Bone Spine 70:384–389
Tascilar K, Melikoglu M, Ugurlu S, Sut N, Caglar E, Yazici H et al (2018e) Vascular involvement in Behçet’s syndrome: a retrospective analysis of associations and the time course. Rheumatol Oxf 53:2018e22
Galeano-Valle F, Demelo-Rodriguez P, Álvarez-Sala-Walther L, Pinilla-Llorente B, Echenagusia-Boyra MJ, Rodriguez-Abella H et al (2018) Intracardiac thrombosis in Behçet’s Disease successfully treated with immunosuppressive agents: a case of vascular pathergy phenomenon. Intractable Rare Dis Res 7(1):54–57
Oblitas CM, Galeano-Valle F, Toledo-Samaniego N, Pinilla-Llorente B, Del Toro-Cervera J, Álvarez-Luque A et al (2019) Budd-Chiari syndrome in Behçet's disease successfully managed with immunosuppressive and anticoagulant therapy: a case report and literature review. Intractable Rare Dis Res 8(1):60–66
Ahn JK, Lee YS, Jeon CH, Koh EM, Cha HS (2008) Treatment of venous thrombosis associated with Behcet’s disease: immunosuppressive therapy alone versus immunosuppressive therapy plus anticoagulation. Clin Rheumatol 27:201–205
Tayer-Shifman OE, Seyahi E, Nowatzky J, Ben-Chetrit E (2012) Major vessel thrombosis in Behçet’s disease: the dilemma of anticoagulant therapy—the approach of rheumatologists from different countries. Clin Exp Rheumatol 30:735–740
Hamuryudan V, Er T, Seyahi E, Akman C, Tüzün H, Fresko I et al (2004) Pulmonary artery aneurysm in Behçet syndrome. Am J Medd 117:867–870
Funding
This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conception and design: NT-S, FG-V. Administrative support: NT-S, FG-V. Provision of study materials or patients: NT-S, FG-V. Collection and assembly of data: NT-S, FG-V. Data analysis and interpretation: NT-S, FG-V, PD-R. Manuscript writing: NT-S, FG-V. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no conflict of interest.
Statement of human and animal rights
This study was carried out following the international ethical recommendations for conducting research in humans in the latest revision of the Declaration of Helsinki, as well as those established in the Good Clinical Practice Guidelines and in the current legislation. The study was approved by the Institutional Ethics Committee (03/2019).
Informed consent
Informed consent was not requested because it was a retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toledo-Samaniego, N., Galeano-Valle, F., Pinilla-Llorente, B. et al. Clinical features and management of venous thromboembolism in patients with Behçet’s syndrome: a single-center case–control study. Intern Emerg Med 15, 635–644 (2020). https://doi.org/10.1007/s11739-019-02237-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-019-02237-7