Abstract
Endovascular treatment (EVT) has been widely used for treating acute ischemic stroke (AIS). However, the safety and efficacy of treating AIS with tirofiban combined with EVT remain controversial. Therefore, we conducted a meta-analysis to evaluate this treatment. Randomized controlled trials and cohort studies that compared treatment with tirofiban combined with EVT and EVT alone were included in our meta-analysis. Those published from inception to March 31, 2020, were searched using the PubMed, Web of Science, Embase, and Cochrane Library databases. Safety was assessed based on symptomatic intracranial hemorrhage (sICH) incidence and 3-month mortality. Efficacy was assessed based on modified Rankin Scale (mRS) scores at 3 months post-EVT and recanalization rates. Data were analyzed using either the random-effects or fixed-effects model based on the heterogeneity of studies. In total, one RCT, six prospective studies, and four retrospective studies (2387 AIS cases) were assessed. Our meta-analysis showed that tirofiban combined with EVT did not increase sICH risk (RR, 1.06; 95%CI, 0.79 to 1.42; P = 0.72) and 3-month mortality (RR, 0.87; 95%CI, 0.74 to 1.04; P = 0.12). Recanalization rates were not significantly different between patients treated with tirofiban combined with EVT and those treated with EVT alone (RR, 1.04; 95%CI, 1.00 to 1.08; P = 0.07), but tirofiban combined with EVT was significantly associated with favorable functional outcomes (mRS score, 0–2) in AIS patients (RR, 1.13; 95%CI, 1.02 to 1.25; P = 0.02). Tirofiban combined with EVT appears to be safe and potentially effective in treating AIS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute ischemic stroke (AIS) is one of the most common causes of disability and death worldwide [1]. Intravenous thrombolysis is considered the most effective therapy for AIS patients within 4.5 h, but endovascular treatment (EVT) may be superior in terms of achieving large artery revascularization, especially beyond the time window of intravenous thrombolysis [2]. However, EVT frequently leads to endothelial injuries, and the following platelet aggregation may cause thromboembolic complications and early reocclusion [3].
Tirofiban, a non-peptide platelet glycoprotein (GP) IIb/IIIa receptor inhibitor with a short half-life, can potently inhibit the final pathway of platelet activation and subsequent thrombus formation [4]. Currently, tirofiban has been widely used for AIS patients treated with EVT in order to improve clinical outcomes. However, there has been no consensus regarding the safety and efficacy of tirofiban in AIS patients treated with EVT. Therefore, we conducted a meta-analysis to evaluate the safety and efficacy of tirofiban combined with EVT in treating AIS patients by comparing it with EVT alone.
Methods
Ethics
This meta-analysis adhered to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analysis [5].
Search strategy
A systematic literature search was independently performed by two authors (Yingying Sun and Meiqi Wang) using the PubMed, Web of Science, Embase, and Cochrane Library databases. Literature published from inception to March 31, 2020, were searched. The following key words were used for finding relevant studies from the databases: “stroke,” “cerebrovascular accident,” “apoplexy,” “brain vascular accident,” “endovascular therapy,” “endovascular procedures,” “endovascular techniques,” “thrombectomy,” “recanalization,” “tirofiban,” “Aggrastat,” “MK 383,” and “L 700462,” After identifying all potentially relevant articles, we removed duplicate articles with Endnote X9 reference management software. The two authors independently assessed the title, abstract, and full text of each article identified by the literature search for inclusion. Moreover, we reviewed the reference lists of the retrieved articles to identify any omitted studies.
Study selection
The inclusion criteria for articles were as follows: (1) studies that compared patients with AIS who were treated with EVT combined with tirofiban to those treated with EVT alone; (2) randomized controlled trials (RCTs) and cohort studies; (3) studies that reported on at least one of the following outcomes: symptomatic intracranial hemorrhage (sICH), mortality, modified Rankin Scale (mRS) score at 3 months post-EVT, and recanalization rate; and (4) studies published in English. The exclusion criteria were as follows: (1) duplicated articles and studies with populations that came from duplicate databases; (2) single-arm trials, editorials, letters to the editor, conference abstracts and posters, review articles, case reports, and animal experimental studies; and (3) articles in which relevant data could not be extracted.
Outcomes
The safety outcomes we assessed were sICH incidence and mortality at 3 months post-EVT. sICH was defined according to the European Cooperative Acute Stroke Study III definition [6]. The efficacy outcomes we assessed were mRS score at 3 months post-EVT and recanalization rate. mRS scores ranged from 0 (no symptoms) to 6 (death) [7]. A favorable functional outcome was defined as an mRS score of 0–2. Recanalization was defined as a Tissue Thrombolysis in Cerebral Ischemia (TICI) score of ≥ 2b, as determined via angiogram scans or magnetic resonance imaging.
Data extraction and quality assessment
Data from studies were independently extracted and assessed by two authors (Yingying Sun and Meiqi Wang) in accordance with the inclusion criteria mentioned above. Disagreements were solved by consensus. The following information was extracted from eligible studies: name of the first author, year of publication, study country, study design, study center, sample size, occlusion location, therapeutic strategies, rate of bridging therapy, and general information on the use of tirofiban. The quality of cohort studies was assessed with the Newcastle-Ottawa Scale (NOS) [8]. NOS scores ranged from 0 to 9 and were assessed based on the following three factors: selection, comparability, and outcome. Cohort studies with an NOS score of ≥ 7 and RCTs were considered high in quality.
Statistical analysis
All meta-analyses were performed using Review Manager for Windows version 5.2 and STATA 12.0. Risk ratios (RRs) were calculated for dichotomous variables, and all results are reported with 95% confidence intervals (95%CIs). We assessed statistical heterogeneity between studies using chi-square tests, with a P value of < 0.1 considered statistically significant. Heterogeneity was quantified using I2 values; an I2 value of ≥ 50% indicated heterogeneity [9]. If heterogeneity among studies was detected, we used the random-effects model for meta-analyses. If not, we used the fixed-effects model. Data were presented as forest plots, with a P value of < 0.05 considered statistically significant. A sensitivity analysis was conducted through leave-one-out cross validation to assess the stability of meta-analysis results. Publication bias was assessed by funnel plot symmetry [10].
Results
Search results and study characteristics
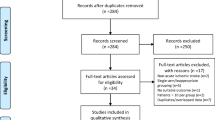
The initial literature search yielded a total of 898 studies. After assessing these studies, 11 studies, which included 2387 AIS cases, met the inclusion criteria and were included in the final analysis. The literature search and screening process are described in Fig. 1. Our meta-analysis included one RCT [11], six prospective cohort studies [12,13,14,15,16,17], and four retrospective cohort studies [18,19,20,21]. Study characteristics and quality assessment results are shown in Table 1. General information on the use of tirofiban is shown in Table 2. All included studies were considered high in quality.
Safety and efficacy outcomes
sICH incidence
Of the 2387 AIS patients, 2382 from the 11 studies were included in the safety analysis regarding sICH incidence (5 patients lost to follow-up in the original studies). There was no significant difference in the incidence of sICH between patients treated with tirofiban combined with EVT and those treated with EVT alone (RR, 1.06; 95%CI, 0.79 to 1.42; P = 0.72) (Fig. 2). There was no significant heterogeneity between these studies (I2 = 21%, P = 0.24).
3-month mortality
All 2387 patients were included in the safety analysis regarding mortality at 3 months post-EVT. There was no significant difference in the rates of mortality between patients treated with tirofiban combined with EVT and those treated with EVT alone (RR, 0.87; 95%CI, 0.74 to 1.04; P = 0.12) (Fig. 3). There was no significant heterogeneity between these studies (I2 = 0%, P = 0.53).
Favorable functional outcomes
In total, 10 studies reported that 2161 patients exhibited favorable functional outcomes at 3 months post-EVT. The meta-analysis showed that tirofiban combined with EVT was significantly associated with favorable functional outcomes (RR, 1.13; 95%CI, 1.02 to 1.25; P = 0.02) (Fig. 4). There was no significant heterogeneity between these studies (I2 = 13%; P = 0.32).
Recanalization rate
In total, nine studies reported that 2031 patients exhibited recanalization after EVT. There was no significant difference in recanalization rates between patients treated with tirofiban combined with EVT and those treated with EVT alone (RR, 1.04; 95%CI, 1.00 to 1.08; P = 0.07) (Fig. 5). There was no significant heterogeneity between these studies (I2 = 34%; P = 0.14).
Sensitivity analysis and publication bias
The results of the sensitivity analysis are shown in Supply 1-4. The sensitivity analysis showed that after removing the study reported by Wu et al. [14], patients treated with tirofiban and EVT had a lower rate of mortality at 3 months post-EVT than those treated with EVT alone (Supply 2). Furthermore, after removing the studies reported by Pan et al. [15] and Sun et al. [16], the sensitivity analysis showed that there was no significant difference in terms of the incidence of favorable functional outcomes at 3 months post-EVT between patients treated with tirofiban and EVT and those treated with EVT alone (Supply 3). Then, after removing the study reported by Zhao et al. [13], the sensitivity analysis showed that patients treated with tirofiban and EVT had higher recanalization rates than those treated with EVT alone (Supply 4). The other sensitivity analysis results were consistent with those of the primary analysis.
With regard to the funnel plot analysis, the shape of the funnel plot did not indicate obvious asymmetry upon visual inspection (Supply 5-8).
Discussion
Ours is the first meta-analysis to evaluate the safety and efficacy of tirofiban combined with EVT in treating patients with AIS. We found that tirofiban combined with EVT did not increase the risk of sICH and 3-month mortality. Moreover, there was no significant difference in recanalization rates between patients treated with tirofiban combined with EVT and those treated with EVT alone. However, tirofiban combined with EVT was more likely to achieve favorable functional outcomes.
The safety and efficacy of treating AIS with tirofiban therapy remain controversial. In a previous meta-analysis, researchers suggested that treating AIS with tirofiban did not increase the risk of sICH and mortality and did not provide any obvious improvements in terms of functional outcomes [22]. Simultaneously, the study by Zhou et al. [23] found that, for patients with AIS who underwent intravenous thrombolysis, tirofiban therapy may be safe, but its role in improving functional outcomes was unclear. Compared with previous studies, our meta-analysis included more recently published studies relatively and we further found that tirofiban combined with EVT increased the incidence of favorable functional outcomes and did not increase the risk of sICH and mortality in treating AIS patients. Additionally, while our meta-analysis demonstrates that tirofiban combined with EVT can be safe and effective in treating AIS patients, several studies included in our meta-analysis have indicated the opposite. For instance, Kellert et al. [12] showed a higher risk of fatal ICH and poor outcome in patients treating tirofiban combined with EVT. The following reasons may have attributed to this discrepancy. On the one hand, this study [12] was published in 2013, while the other studies included in our meta-analysis were published after 2015. Therefore, it should be noted that the clinical guidelines regarding the indication for EVT for AIS patients were updated in 2015 [24]. On the other hand, this study was the only non-Chinese study. It is known that the etiology and pathology of AIS in Chinese population is different from that in Western population. Furthermore, a study by Wu et al. [14] showed that tirofiban was associated with an increased risk of bleeding during EVT in AIS patients. However, after removing this study [14] from our sensitivity analysis, it showed that patients treated with tirofiban and EVT had lower mortality rates than those treated with EVT alone. This may be due to the fact that, unlike the other included studies, this study [14] focused on the relationship between different doses of tirofiban and the risk of bleeding during EVT and mortality. Different doses of tirofiban may result in different outcomes. Fortunately, the sensitivity analysis for our other outcomes showed that these two studies did not change the final result, thereby indicating the stability of our results.
Furthermore, the rate of recanalization after EVT is one of the main predictors for functional outcomes in AIS patients. Microvascular thrombosis may remain in situ after blood vessel occlusions are recanalized via EVT [25]. Several studies have reported that tirofiban can prevent platelet aggregation, thereby inhibiting microthrombus formation and improving the level of tissue reperfusion [25, 26]. After removing Zhao et al.’s [13] study from our sensitivity analysis, it showed that patients treated with tirofiban and EVT had higher recanalization rates and lower 3-month mRS scores than those treated with EVT alone. Zhao et al.’s [13] study demonstrated that interventionists were prone to use tirofiban in patients with a high risk of reocclusion after arterial occlusions were recanalized. This selection bias may have undervalued the rate of recanalization in patients treated with tirofiban and EVT. Therefore, we speculate that tirofiban is effective in treating AIS patients who undergo EVT. More randomized controlled trials are needed to further evaluate whether tirofiban can improve post-EVT recanalization rates.
Additionally, sICH is a major complication of EVT for AIS patients. The main reason that sICH occurs after EVT may be due to the combination of antiplatelet therapy [27]. However, our meta-analysis showed that tirofiban combined with EVT did not increase the risk of sICH and mortality. This may be attributed to the possible advantages that tirofiban has over other antiplatelet drugs. Tirofiban is a fast-acting and fast-deactivated GP IIb/IIIa antagonist that is rapidly eliminated after infusion cessation due to its short half-life (about 2 h) [4]. Moreover, tirofiban can selectively inhibit fibrinogen from binding to platelets and prevent subsequent platelet aggregation, which makes platelet function reversible after infusion cessation [28]. Therefore, the incidence of bleeding caused by tirofiban is lower than other antiplatelet drugs, meaning that tirofiban therapy may be safe when combined with EVT.
This study has several limitations. Firstly, our meta-analysis included only one RCT, while the other studies were cohort studies. This may increase the risk of bias because of insufficient random sequence generation and blinding. Secondly, the included studies reported several different EVT strategies, including mechanical thrombectomy, stenting, and balloon angioplasty. Furthermore, these studies had different occlusion locations, rates of bridging therapy, and the information on the use of tirofiban. These differences may have influenced our final outcomes. Lastly, our sensitivity analysis showed that the incidence rates of favorable functional outcomes in our meta-analysis were not stable. Thus, more RCTs are needed to fully elucidate the efficacy of tirofiban combined with EVT in treating AIS patients.
In conclusion, we found that tirofiban therapy significantly increased the incidence of favorable functional outcomes and did not increase the risk of sICH and mortality in the Chinese population. Considering that there was only one RCT and one non-Chinese study among the 11 included studies, more RCTs and non-Chinese studies are needed to evaluate the efficacy and safety of tirofiban combined with EVT in the future.
Data availability
Not applicable.
References
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, AlMazroa MA, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Abdulhak AB, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng ATA, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, de Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FGR, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Memish ZA, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KMV, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA III, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, de Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Silberberg D, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJC, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SRM, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AKM, Zheng ZJ, Zonies D, Lopez AD, Murray CJL (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2163–2196. https://doi.org/10.1016/S0140-6736(12)61729-2
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL (2019) Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50(12):e344–e418. https://doi.org/10.1161/STR.0000000000000211
Teng D, Pannell JS, Rennert RC, Li J, Li YS, Wong VW, Chien S, Khalessi AA (2015) Endothelial trauma from mechanical thrombectomy in acute stroke: in vitro live-cell platform with animal validation. Stroke 46(4):1099–1106. https://doi.org/10.1161/STROKEAHA.114.007494
Schwarz M, Meade G, Stoll P, Ylanne J, Bassler N, Chen YC, Hagemeyer CE, Ahrens I, Moran N, Kenny D, Fitzgerald D, Bode C, Peter K (2006) Conformation-specific blockade of the integrin GPIIb/IIIa: a novel antiplatelet strategy that selectively targets activated platelets. Circ Res 99(1):25–33. https://doi.org/10.1161/01.RES.0000232317.84122.0c
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. https://doi.org/10.1136/bmj.b2700
Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, Investigators E (2008) Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359(13):1317–1329. https://doi.org/10.1056/NEJMoa0804656
Adams HP Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, Woolson RF, Hansen MD (1999) Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 53(1):126–131. https://doi.org/10.1212/wnl.53.1.126
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629
Zhang Y, Zhang QQ, Fu C, Wang L, Zhang GQ, Cao PW, Chen GF, Fu XM (2019) Clinical efficacy of tirofiban combined with a Solitaire stent in treating acute ischemic stroke. Braz J Med Biol Res 52(10):e8396. https://doi.org/10.1590/1414-431x20198396
Kellert L, Hametner C, Rohde S, Bendszus M, Hacke W, Ringleb P, Stampfl S (2013) Endovascular stroke therapy: tirofiban is associated with risk of fatal intracerebral hemorrhage and poor outcome. Stroke 44(5):1453–1455. https://doi.org/10.1161/strokeaha.111.000502
Zhao W, Che R, Shang S, Wu C, Li C, Wu L, Chen J, Duan J, Song H, Zhang H, Ling F, Wang Y, Liebeskind D, Feng W, Ji X (2017) Low-dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke 48(12):3289–3294. https://doi.org/10.1161/strokeaha.117.019193
Wu Y, Yin C, Yang J, Jiang L, Parsons MW, Lin L (2018) Endovascular thrombectomy: tirofiban increases bleeding risk in acute stroke patients. Stroke 49(11):2783–2785. https://doi.org/10.1161/strokeaha.118.022919
Pan X, Zheng D, Zheng Y, Chan PWL, Lin Y, Zou J, Zhou J, Yang J (2019) Safety and efficacy of tirofiban combined with endovascular treatment in acute ischaemic stroke. Eur J Neurol 26(8):1105–1110. https://doi.org/10.1111/ene.13946
Sun C, Li X, Zhao Z, Chen X, Huang C, Li X, Shan Y, Zou Y, Liu Y, Ibrahim M, Nyame L, Song B, Wang F, Zheng X, Hu J, Zhao Z, Zhou J, Zou J (2019) Safety and efficacy of tirofiban combined with mechanical thrombectomy depend on ischemic stroke etiology. Front Neurol 10:1100. https://doi.org/10.3389/fneur.2019.01100
Yang M, Huo X, Gao F, Wang A, Ma N, Shi H, Chen W, Wang S, Wang Y, Miao Z (2020) Low-dose rescue tirofiban in mechanical thrombectomy for acute cerebral large artery occlusion. Eur J Neurol 27(6):1056–1061. https://doi.org/10.1111/ene.14170
Yu T, Lin Y, Jin A, Zhang P, Zhou X, Fang M, Liu X (2018) Safety and efficiency of low dose intra-arterial tirofiban in mechanical thrombectomy during acute ischemic stroke. Curr Neurovasc Res 15(2):145–150. https://doi.org/10.2174/1567202615666180605104931
Quan T, Hou H, Xue W, Yu G, Ma H, Sun J, Guan S, Xu Y, Xu H (2019) Endovascular treatment of acute intracranial vertebrobasilar artery occlusion: a multicenter retrospective observational study. Neuroradiology 61(12):1477–1484. https://doi.org/10.1007/s00234-019-02282-1
Yi HJ, Sung JH, Lee DH (2019) Safety and efficacy of intra-arterial tirofiban injection during mechanical thrombectomy for large artery occlusion. Curr Neurovasc Res 16(5):416–424. https://doi.org/10.2174/1567202616666191023154956
Luo Y, Yang Y, Xie Y, Yuan Z, Li X, Li J (2019) Therapeutic effect of pre-operative tirofiban on patients with acute ischemic stroke with mechanical thrombectomy within 6-24 hours. Interv Neuroradiol 25(6):705–709. https://doi.org/10.1177/1591019919851167
Gong J, Shang J, Yu H, Wan Q, Su D, Sun Z, Liu G (2020) Tirofiban for acute ischemic stroke: systematic review and meta-analysis. Eur J Clin Pharmacol 76:475–481. https://doi.org/10.1007/s00228-019-02817-8
Zhou J, Gao Y, Ma QL (2020) Safety and efficacy of tirofiban in acute ischemic stroke patients not receiving endovascular treatment: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci 24(3):1492–1503. https://doi.org/10.26355/eurrev_202002_20208
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM, Investigators E-I (2015) Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 372(11):1009–1018. https://doi.org/10.1056/NEJMoa1414792
Kang DH, Kim YW, Hwang YH, Park SP, Kim YS, Baik SK (2014) Instant reocclusion following mechanical thrombectomy of in situ thromboocclusion and the role of low-dose intra-arterial tirofiban. Cerebrovasc Dis 37(5):350–355. https://doi.org/10.1159/000362435
Choudhri TF, Hoh BL, Zerwes HG, Prestigiacomo CJ, Kim SC, Connolly ES Jr, Kottirsch G, Pinsky DJ (1998) Reduced microvascular thrombosis and improved outcome in acute murine stroke by inhibiting GP IIb/IIIa receptor-mediated platelet aggregation. J Clin Invest 102(7):1301–1310. https://doi.org/10.1172/JCI3338
Demchuk AM, Goyal M, Menon BK, Eesa M, Ryckborst KJ, Kamal N, Patil S, Mishra S, Almekhlafi M, Randhawa PA, Roy D, Willinsky R, Montanera W, Silver FL, Shuaib A, Rempel J, Jovin T, Frei D, Sapkota B, Thornton JM, Poppe A, Tampieri D, Lum C, Weill A, Sajobi TT, Hill MD, Investigators ET (2015) Endovascular treatment for small core and anterior circulation proximal occlusion with emphasis on minimizing CT to recanalization times (ESCAPE) trial: methodology. Int J Stroke 10(3):429–438. https://doi.org/10.1111/ijs.12424
Yang M, Huo X, Miao Z, Wang Y (2019) Platelet glycoprotein IIb/IIIa receptor inhibitor tirofiban in acute ischemic stroke. Drugs 79(5):515–529. https://doi.org/10.1007/s40265-019-01078-0
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This study was funded by the National Key R&D Program of China (2016YFC1301600), JLUSTIRT (2017TD-12), and Science and Technology Department of Jilin Province (20180623052TC) to Yi Yang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval for this study was not required because this manuscript did not contain patient data and we only performed data analysis based on published studies.
Informed consent
Informed consent was not required because this manuscript did not contain patient data and it is a meta-analysis based on published studies.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supply 1
The results of a sensitivity analysis for the incidence of symptomatic intracranial hemorrhage in acute ischemic stroke patients who underwent endovascular therapy (PNG 2063 kb)
Supply 2
The results of a sensitivity analysis for 3-month mortality in acute ischemic stroke patients who underwent endovascular therapy (PNG 2063 kb)
Supply 3
The results of a sensitivity analysis for the incidence of favorable functional outcomes in acute ischemic stroke patients who underwent endovascular therapy (PNG 2063 kb)
Supply 4
The results of a sensitivity analysis for recanalization rates in acute ischemic stroke patients who underwent endovascular therapy (PNG 2063 kb)
Supply 5
A funnel plot for assessing publication bias in regards to studies reporting the incidence of symptomatic intracranial hemorrhage in acute ischemic stroke patients who underwent endovascular therapy (PNG 2018 kb)
Supply 6
A funnel plot for assessing publication bias in regards to studies reporting 3-month mortality in acute ischemic stroke patients who underwent endovascular therapy (PNG 2060 kb)
Supply 7
A funnel plot for assessing publication bias in regards to studies reporting the incidence of favorable functional outcomes in acute ischemic stroke patients who underwent endovascular therapy (PNG 49 kb)
Supply 8
A funnel plot for assessing publication bias in regards to studies reporting recanalization rates in acute ischemic stroke patients who underwent endovascular therapy (PNG 2057 kb)
Rights and permissions
About this article
Cite this article
Sun, Y., Guo, ZN., Yan, X. et al. Safety and efficacy of tirofiban combined with endovascular therapy compared with endovascular therapy alone in acute ischemic stroke: a meta-analysis. Neuroradiology 63, 17–25 (2021). https://doi.org/10.1007/s00234-020-02530-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02530-9