Abstract
Choricotyle australiensis Roubal, Armitage & Rohde, 1983, a diclidophorid monogenean species, is redescribed and genetically characterised using the partial nuclear 28S ribosomal RNA gene (28S rRNA) and a fragment of mitochondrial cytochrome c oxidase subunit 1 (cox1) gene sequences for specimens collected from Chrysophrys auratus (Forster) off Australia and New Zealand. Previous studies have either provided morphological or genetic results, whereas this study combines morphological and advanced molecular methods. A total of 70 Ch. auratus were examined with 22 individuals of C. australiensis recovered from the gills (overall prevalence of 23%). This study has provided the first evidence for the exploration of mitochondrial cox1 region for C. australiensis. Comparison of the newly generated sequences with other available data supported the distinction of C. australiensis among diclidophorid Furhmann, 1928 species thus confirming its taxonomic status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chrysophrys auratus (Forster) is a large demersal predatory finfish of the family Sparidae Rafinesque (Order Perciformes). It is widely distributed in the warm to temperate Indo-Pacific waters which occur off New Zealand, Australia, Philippines, Indonesia, China (including Taiwan) and Japan (Paulin, 1990) at depths of 0–200 m. In the Southern Hemisphere, Ch. auratus is found in temperate to subtropical waters of the southern Great Barrier Reef, Lord Howe, and Norfolk Islands, whereas in the Northern Hemisphere it associated with temperate to tropical areas, penetrating nearly to the Equator in Indonesia (Paulin 1990). Chrysophrys auratus is also an iconic target species for recreational and commercial fisherman in each of the mainland states of Australia (Thurstan et al., 2016; Morgan et al., 2019) due to its quality of flesh (Gommon et al., 2008). This species has also been successfully cultured for many years in Japan (Foscarini, 1988). An assessment has been conducted in Australia to assess the suitability of Ch. auratus as an aquaculture species (Fielder et al., 2001) which Battaglene & Talbot (1992) have described as a prime candidate for successful aquaculture. However, there is limited knowledge on the key parasites hosted by Ch. auratus and which, if any, may pose potential risks to aquaculture (Hutson et al., 2007).
The genus Choricotyle van Beneden & Hesse, 1863 was first proposed by van Beneden & Hesse (1863) with the identification of C. chrysophryi van Beneden & Hesse, 1863 as the type-species which were obtained from the gills of Ch. auratus. There are approximately 25–30 valid species of Choricotyle identified to date with a broad geographical distribution globally (Fig. 1). The vast proportion of Choricotyle spp. parasitise sparid fish (see Supplementary Table S1) and appear to be region-specific.
The Monogenean Choricotyle australiensis Roubal, Armitage & Rohde, 1983 was first described by Roubal et al. (1983) from Ch. auratus from Coffs Harbour, New South Wales, Australia. It has subsequently been identified from five different geographical localities in Australia and New Zealand (Sharples & Evans, 1995b; Roubal et al., 1983; Roubal et al., 1996). There have been no studies which have used combined morphological and molecular methods to identify and describe C. australiensis and there is limited information on the genetics of species of Choricotyle with molecular data available for only three species: C. australiensis, C. cf. chrysophryii, and C. anisotremi Oliva, 1987, plus undescribed Choricotyle sp. 1, 2 and 3 of Mendoza-Franco, Tun, Anchevida & del Rio Rodríguez (2018) (see Supplementary Table S1). Morphological species identification presents many challenges as Choricotyle spp. are morphologically characterised by having eight fragile haptoral branches which are vulnerable to damage during preservation, processing and mounting. This may limit the quality and quantity of specimens for morphological examination (Sharples & Evans, 1995b) and provide insufficient evidence required for the redescription or revision of these species (Llewellyn, 1941b). In some cases, species remain undescribed such as Choricotyle sp. 1, 2 and 3 (Mendoza-Franco et al., 2018). Therefore, redescription for every species of Choricotyle using combined morphology and molecular tools is warranted.
Nowadays, molecular tools have been used extensively for the species identification of a number of parasites (McManus & Bowles, 1996). For example, PCR-based approaches have been used to differentiate among species of Monogenea using the partial 28S ribosomal RNA gene (28S rRNA), partial and complete 18S rRNA, as well as partial mitochondrial cytochrome c oxidase subunit 1 (cox1) gene (Catalano et al., 2010; Oliva et al., 2014; Jovelin & Justine, 2001). However, on some occasions, the characterisation of the Choricotyle spp. was based on a single or limited number of specimens, as well as the amplification of highly conserved region of the nuclear gene (28S rRNA for example) that shows a lower degree of genetic diversity. As a result, only a few species, including C. australiensis by Litvaitis & Rohde (1999) and Olson & Littlewood (2002), C. cf. chrysophryii by Jovelin & Justine (2001) and Mollaret et al. (2000) have been identified using the nuclear genes. Hence, the amplification of mitochondrial cox1 gene regarded as “the core of a global bioidentification system for animals” (Hebert et al., 2003; Ward et al., 2005). Mitochondrial cox1 gene shows high sequence variability at the isolate level compared to nuclear genes (such as 28S and 18S genes, those are much less variable). This inter-species variability in sequences is the major criterion for the molecular species characterisation and hence, cox1 is being used for characterisation of several Monogenean species (Bouguerche et al., 2019; Ayadi et al., 2017). Molecular characterisation may also lead to discovery of new or cryptic species as has been described in other parasites (Ayadi et al., 2017; Oliva et al., 2014; Bouguerche et al., 2019).
Therefore, the aim of the present study was to redescribe C. australiensis from Ch. auratus and to characterise the species genetically based on partial 28S ribosomal RNA (28S rDNA) and partial mitochondrial cox1 genes in order to validate its taxonomic status.
Materials and methods
Fish collection
A total of 70 fish were purchased from commercial catches at the Sydney fish market, sourced from three separate localities: off the coast of New South Wales (n = 30), the waters of New Zealand (n = 20) and an unknown location in Australia (n = 20). Fish were purchased on 11. x.2018, 29.viii.2018 and 28.vii.2018, respectively. Fish were transferred fresh to the Parasitology Laboratory of Charles Sturt University, Wagga Wagga Campus, in an insulated box filled with ice. All fish from each batch were examined on the day of arrival at the University.
Parasite collection
Fish were dissected and examined for the presence of monogeneans. In brief, the gills were removed and placed in an individual Petri dish containing seawater. The surfaces of all gills were thoroughly inspected under a dissecting microscope (Leica EZ4 Stereo Microscope, China) for the presence of parasites. Parasites were collected by using fine forceps (Jewellers forceps, Dumont no. 5). Collected monogeneans were counted and preserved in 70% ethanol for further morphological and genetic study.
Morphological examination
The processing and handling of specimens were carried out in accordance with the protocol provided by Barton et al. (2009), with the exception that a small piece of tissue was taken from the post-peduncle of each specimen prior to processing as per the methods of Bouguerche et al. (2019). The characteristics of systematic importance were measured directly with an eyepiece micrometre (BX‐43 Olympus Microscope, Olympus Corporation, Japan). All measurements are in micrometres and are given as the range, followed by the mean in parentheses unless otherwise stated. A dash (–) indicates that measurements could not be made or were not available. All drawings were made with the aid of a drawing tube on the same microscope.
Molecular sequencing
A tiny piece from the post peduncle of each parasite was transferred into 1.5 ml autoclaved Eppendorf tube for molecular study and the rest of the body (anterior end and peduncle containing haptoral branches) were processed for microscopy/morphological study. DNA was extracted using DNeasy Blood & Tissue Kits (Qiagen, Hilden, Germany), according to the manufacturerʼs instructions, and eluted in 40 µl of elution buffer. Polymerase Chain Reaction (PCR) amplification of the nuclear 28S rRNA (partial) gene and a fragment of the mitochondrial cox1 gene was carried out using the two primer sets: 28S-LSU5 (forward: 5′-TAG GTC GAC CCG CTG AAY TTA AGC A-3′) and EC-D2 (reverse: 5′-CCT TGG TCC GTG TTT CAA GAC GGG-3′) as well as COI-ASmit1 (forward: 5′-TTT TTT GGG CAT CCT GAG GTT TAT-3′) and COI-ASmit2 (reverse: 5′-TAA AGA AAG AAC ATA ATG AAA ATG-3′), respectively (Littlewood et al., 1997). The cycling conditions to amplify the nuclear gene was initial 95°C for 2 min, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 50°C for 1 min, extension at 72°C for 10 min and a final extension step at 72°C for 10 min. The mitochondrial gene was amplified using the same cycling conditions with the annealing temperature adjusted to 48°C for 45 s. An aliquot (2 µl) of each amplicon was examined on a 1.5% w/v agarose gel, stained with GelRedTM and photographed using a gel documentation system.
Representative samples were sent to the Australian Genome Research Facility (AGRF), Queensland, Australia, and were subjected to Sanger sequencing using the same primer sets as for PCR. Sequence data including chromatograms were observed initially through Sequence Scanner Software (Applied Biosystems® Genetic Analysers). Subsequently, sequences were aligned using ClustalX (Thompson et al., 1997) followed by manual adjustment.
Phylogenetic analysis
Phylogenetic trees for both gene regions were constructed from the sequences generated in this study along with representative (similar and closely related species) sequences from GenBank (Table 1). All sequences were then aligned with MUSCLE in MEGA v. 7 (Kumar et al., 2016) and adjusted manually where necessary. The phylogenetic relationships among species were determined using Bayesian method using MrBayes v 3.2 (Ronquist & Huelsenbeck, 2003). The GTR+G model was applied for both nuclear and mitochondrial genes as suggested by jModelTest 2 (Darriba et al., 2012). Kuhnia scombri (Kuhn, 1829) (GenBank: AF382044) and Microcotyle algeriensis Ayadi, Gey, Justine & Tazerouti, 2016 (GenBank: KX926444) were used as the outgroup for 28S and cox1 phylogenetic analyses, respectively. Sample frequency was set at 1,000, and calculated for 1,600,000 generations for both 28S and cox1 regions until the p-value < 0.01. After the mcmc run, the first 30% samples were discarded, and the sumt command was used to summarize the phylogenetic trees. Figtree v 1.4.3 was used to visualise the phylogenetic trees (Rambaut, 2014).
Data analyses
The prevalence, mean intensity and mean abundance of the monogeneans were calculated as follows: Prevalence (%) = Number of infected fish/Total number of examined fish ×100; Mean intensity = Number of parasites/Number of infected hosts; and Mean abundance = Number of parasites/Total number of examined hosts.
Results
A total of 22 C. australiensis were recovered from 16 of the 70 fish examined, with an overall prevalence of 23%. The intensity of infection of individual parasite was highest in Ch. auratus collected from off the coast of New South Wales (NSW) followed by the unknown location, Australia. The New Zealand Ch. auratus was the least infected with C. australiensis recorded on one out of twenty fish examined. The prevalence and abundance of C. australiensis from Ch. auratus in the present study are presented in Table 2.
Family Diclidophoridae Fuhrmann, 1928
Genus Choricotyle van Beneden & Hesse, 1863
Choricotyle australiensis Roubal, Armitage & Rohde, 1983
Host: Chrysophrys auratus (Forster).
Site in host: Gills.
Locality: Off the coast of NSW, Australia; an unknown location in Australia and waters off New Zealand.
Voucher material: Specimens (16 whole mount specimens: 8 mature and 8 immature/juvenile) were deposited in the South Australian Museum under the accession numbers (AHC 36789–36800).
Representative DNA sequences: 28S gene: GenBank: MT782270 (AHC 36789); MT782271 (AHC 36796). cox1 gene: GenBank: MT783685 (AHC 36789); MT783686 (AHC 36794); MT783687 (AHC 36798).
Redescription
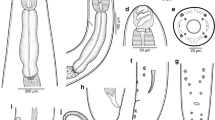
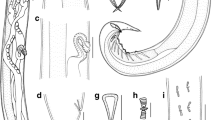
[Based on 16 specimens; Fig. 2 and Table 3.] Body proper divided into 2 regions, small region anterior to pharyngeal or postpharyngeal area, and main part of body proper (division not always clearly visible in immature specimens) (Fig. 2A). Maximum body width at level of reproductive system. Oesophagus with diverticula, bifurcation of intestine near genital atrium (Fig. 2A). Intestinal caeca in body proper not observed due to vitellaria.
Obvious peduncle present between body proper and haptor. Haptor with 8 branches (Fig. 2B), each with one clamp. Pair of anteriormost branches longest, and their origins contiguous, posterior branches progressively smaller. Clamps as described by Llewellyn (1941a) (Fig. 2C) but with 7–8 concentric arcs of small skeletal rods in dorsal walls of clamps, and one large and one slightly smaller sucker-like cup in basal quadrants of clamps.
Genital atrium mid-ventral, with well-developed radial muscles and 7–8 large spines (Fig. 2D). Uterus may be filled with numerous eggs. Vitellarium extend from level halfway between pharynx and genital atrium; vitelline follicles becoming much denser at 700–1,000 (950) from anterior extremity of body, extending into haptor and branches, with density decreasing nearer to the clamps. Eggs with a long abopercular filament, in clusters held together by long filaments (Fig. 2E); egg shape, size, number and maturity vary according to the stages of development of parasites. Yolk-sac visible in mature eggs.
Remarks
The morphometric and meristic data for the present material matched with the description provided by Roubal et al. (1983) (Table 3). The original description by Roubal et al. (1983) was based upon a small number of parasites (n = 7) from a single location (all from Coffs Harbour, NSW, Australia); in a subsequent collection from Port Hacking (NSW), Roubal et al. (1996) did not provide any morphological details. A subsequent observation of C. australiensis from New Zealand waters by Sharples & Evans (1995b) only provided data on the body length at 1,845–4,465 (3,519) µm for this parasite. The specimens of C. australiensis from Ch. auratus off the coast of NSW, Australia and New Zealand examined in this study did not significantly differ from the specimens described by Roubal et al. (1983) (Table 3). However, some minor differences were found as follows:
-
i.
The present study observed little variation in body shape, with all specimens elongated with a distinct peduncle. The original description of Roubal et al. (1983) showed most specimens to be more contracted and squat with a less obvious peduncle. This could be due to the collection method (their specimens were dropped into cold formalin). The present specimens were collected from fish that had been kept on ice and were relaxed at the time of collection. Thus, the elongate body and peduncle show a more “realistic” body shape.
-
ii.
Subsequently, the body length of the specimens examined here is greater than that described by Roubal et al. (1983) and Sharples & Evans (1995b).
-
iii.
Genital atrium spines were only between 7 and 8, whereas Roubal et al. (1983) reported 7 to 9.
-
iv.
This study provided some additional morphometric data, such as peduncle length and width, length and width of the individual branches of the haptor, egg diameter, location of pharynx, genital atrium and the distribution of the vitellarium.
Despite these differences in morphometric and meristic data, the morphology of the C. australiensis from Ch. auratus in Australian and New Zealand waters agrees overall with the previously descriptions of C. australiensis from Ch. auratus in Australian waters.
Molecular analyses
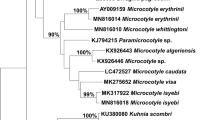
The alignments of 28S rRNA gene and cox1 gene sequences were 923 bp and 396 bp in length, respectively. All 28S rDNA sequences were identical; however, mutations in two loci were observed in cox1 sequences among our specimens. 28S sequences obtained from this study were clustered with the reference sequence of C. australiensis (GenBank: AF382046) in a well-supported clade comprising the two closely related Choricotyle spp. (Fig. 3A). The cox1 sequences obtained in the present study grouped within a single clade, with 95% posterior probability (Fig. 3B). The clade generated for the cox1 gene was distinct from the reference sequences with strong posterior probability values supporting the results of the morphological study.
Phylogenetic tree based on the Bayesian method for sequences of Choricotyle australiensis Roubal, Armitage & Rohde, 1983 generated in this study with the available sequences for diclidophorid species from the GenBank database (see Table 1 for details). A, 28S rRNA (partial) nucleotide sequences; B, mitochondrial cox1 (partial) nucleotide sequences. The sequences generated in this study are indicated with asterisks. Posterior probabilities branch support values higher than 90% are indicated
Discussion
This study confirmed the presence of C. australiensis on Ch. auratus in Australian and New Zealand waters through a combined morphological and molecular approach. The overall prevalence of C. australiensis in the study was 23%, the mean prevalence of parasite infection was highest in the fish sourced from the Australian waters and the lowest in specimens from New Zealand waters. Similar infection patterns were reported by Sharples & Evans (1995a) who found that C. australiensis was rare in Ch. auratus from New Zealand waters (prevalence of only 7.7%). The prevalence of C. australiensis from off the coast of NSW was higher (27%) than those reported from a similar geographical region off south-eastern Australia by Roubal et al. (1996) who reported a prevalence of 2.9%. This represents a marked increase in prevalence of infection and warrants further parasitological investigation with a greater sample size to reach in solid conclusion.
The body of the parasite was comparatively larger with a clear distinction of the peduncle in the specimens from this study (see Table 3 and Fig. 2B). The smaller body size and shape of specimens described by Roubal et al. (1983) and Sharples & Evans (1995b) may have been a result of the preservation methodology (for example, immersion in cold formalin) which can cause sample shrinkage (Baylis, 1922). This is supported by Cribb & Bray (2010) who suggested that the process of fixation and preservation of trematodes has impacts on both morphology and molecular study of trematodes: “We find that the quality of slide specimens produced by alcohol preservation does not quite match that of formalin preservation, but certainly the specimens are acceptable and the flexibility of this method has great advantages” (Cribb & Bray, 2010). Additionally, greater than double in number of specimens were examined in this study compared with the observations of Roubal et al. (1983). Differences in fixation and the use of more modern microscopy equipment and techniques may have influenced the quality of the morphometric observations in the present study.
Sequence data for C. australiensis are scarce, with three sequences available in GenBank for the nuclear genes only (see Supplementary Table S1). The present study incorporated the amplification of both nuclear and mitochondrial genes along with the morphological identification of C. australiensis. The mitochondrial cox1 (partial) gene sequences generated here are novel for C. australiensis. Since, almost all species of Choricotyle have been identified based solely on morphology, significant ambiguity still exists in Choricotyle spp. identification globally. For this reason, parasite taxonomists have suggested revision of the species within genus Choricotyle is required (Llewellyn, 1941b; Hargis, 1955).
Based on morphology records, the monogenean specimens from Ch. auratus were identified as C. australiensis. Phylogenetic trees generated from 28S rRNA and cox1 sequences supported the morphological conclusion, with C. australiensis grouping within the Choricotyle spp. clade (see Fig. 3A, B). Improved and comprehensive morphometric and meristic data along with nuclear and mitochondrial gene sequences of C. australiensis would provide an important foundation for the exploration of evolutionary history of other polyopisthocotyleans Monogenea including Choricotyle spp.
References
Ayadi, Z. E. M., Gey, D., Justine, J.-L., & Tazerouti, F. (2017). A new species of Microcotyle (monogenea: Microcotylidae) from Scorpaena notata (Teleostei: Scorpaenidae) in the Mediterranean Sea. Parasitology International, 66, 37–42.
Barton, D., Beaufrère, C., Justine, J.-L., & Whittington, I. (2009). Polyopisthocotylean monogeneans from carangid fishes off Queensland, Australia and New Caledonia, with a description of Heteromicrocotyloides megaspinosus sp. nov. Acta Parasitologica, 54, 205–217.
Battaglene, S. C., & Talbot, R. B. (1992). Induced spawning and larval rearing of snapper, Pagrus auratus (Pisces: Sparidae), from Australian waters. New Zealand Journal of Marine and Freshwater Research, 26, 179–185.
Baylis, H. A. (1922). Notes on the collection and preservation of parasitic worms. Parasitology, 14, 402–408.
Bouguerche, C., Gey, D., Justine, J.-L., & Tazerouti, F. (2019). Microcotyle visa n. sp. (Monogenea: Microcotylidae), a gill parasite of Pagrus caeruleostictus (Valenciennes) (Teleostei: Sparidae) off the Algerian coast, Western Mediterranean. Systematic Parasitology, 96, 131–147.
Catalano, S. R., Hutson, K. S., Ratcliff, R. M., & Whittington, I. D. (2010). Redescriptions of two species of microcotylid monogeneans from three arripid hosts in southern Australian waters. Systematic Parasitology, 76, 211–222.
Cribb, T. H., & Bray, R. A. (2010). Gut wash, body soak, blender and heat-fixation: approaches to the effective collection, fixation and preservation of trematodes of fishes. Systematic Parasitology, 76, 1–7.
Darriba, D., Taboada, G. L., Doallo, R., & Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9, 772.
Fielder, D. S., Bardsley, W. J., & Allan, G. L. (2001). Survival and growth of Australian snapper, Pagrus auratus, in saline groundwater from inland New South Wales, Australia. Aquaculture, 201, 73–90.
Foscarini, R. (1988). A review: intensive farming procedure for red sea bream (Pagrus major) in Japan. Aquaculture, 72, 191–246.
Gommon, M., Bray, D., & Kuiter, R. (Eds) (2008). Fishes of Australiaʼs Southern Coast. Reed New Holland Publishers.
Hargis, W. J. (1955). Monogenetic trematodes of Gulf of Mexico fishes. Part IX. The family Diclidophoridae Fuhrmann, 1928. Transactions of the American Microscopical Society, 74, 377–388.
Hebert, P. D. N., Cywinska, A., Ball, S. L., & Dewaard, J. R. (2003). Biological identifications through DNA barcodes. Proceedings of the Royal Society of London. Series B: Biological Sciences, 270, 313–321.
Hutson, K. S., Ernst, I., & Whittington, I. D. (2007). Risk assessment for metazoan parasites of yellowtail kingfish Seriola lalandi (Perciformes: Carangidae) in South Australian sea-cage aquaculture. Aquaculture, 271, 85–99.
Jovelin, R., & Justine, J.-L. (2001). Phylogenetic relationships within the polyopisthocotylean monogeneans (Platyhelminthes) inferred from partial 28S rDNA sequences. International Journal for Parasitology, 31, 393–401.
Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874.
Littlewood, D. T. J., Rohde, K., & Clough, K. A. (1997). Parasite speciation within or between host species? Phylogenetic evidence from site-specific polystome monogeneans. International Journal for Parasitology, 27, 1289–1297.
Litvaitis, M. K., & Rohde, K. (1999). A molecular test of platyhelminth phylogeny: inferences from partial 28S rDNA sequences. Invertebrate Biology, 118, 42–56.
Llewellyn, J. (1941a). A description of the anatomy of the monogenetic trematode Choricotyle chrysophryi van Beneden & Hesse. Parasitology, 33, 397–405.
Llewellyn, J. (1941b). A revision of the monogenean family Diclidophoridae Fuhrmann, 1928. Parasitology, 33, 416–430.
McManus, D. P., & Bowles, J. (1996). Molecular genetic approaches to parasite identification: their value in diagnostic parasitology and systematics. International Journal for Parasitology, 26, 687–704.
Mendoza-Franco, E. F., Tun, M. D. C. R., Anchevida, A. D. J. D., & del Rio Rodríguez, R. E. (2018). Morphological and molecular (28S rRNA) data of monogeneans (Platyhelminthes) infecting the gill lamellae of marine fishes in the Campeche Bank, southwest Gulf of Mexico. ZooKeys, 783, 125–161.
Mollaret, I., Jamieson, B. G. M., & Justine, J.-L. (2000). Phylogeny of the Monopisthocotylea and Polyopisthocotylea (Platyhelminthes) inferred from 28S rDNA sequences. International Journal for Parasitology, 30, 171–185.
Morgan, J. A. T., Sumpton, W. D., Jones, A. T., Campbell, A. B., Stewart, J., Hamer, P., et al. (2019). Assessment of genetic structure among Australian east coast populations of snapper Chrysophrys auratus (Sparidae). Marine and Freshwater Research, 70, 964–976.
Oliva, M. E., Sepulveda, F. A., & González, M. T. (2014). Parapedocotyle prolatili gen. n. et sp. n., a representative of a new subfamily of the Diclidophoridae (Monogenea), a gill parasite of Prolatilus jugularis (Teleostei: Pinguipedidae) from Chile. Folia Parasitologica, 61, 543.
Olson, P. D., & Littlewood, D. T. J. (2002). Phylogenetics of the Monogenea - evidence from a medley of molecules. International Journal for Parasitology, 32, 233–244.
Paulin, C. D. (1990). Pagrus auratus, a new combination for the species known as “snapper” in Australasian waters (Pisces: Sparidae). New Zealand Journal of Marine and Freshwater Research, 24, 259–265.
Rambaut, A. (2014). FigTree v1.4.2, a graphical viewer of phylogenetic trees. http://tree.bio.ed.ac.uk/software/figtree/. Accessed 31 January 2019.
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574.
Roubal, F. R., Armitage, J., & Rohde, K. (1983). Taxonomy of Metazoan ectoparasites of snapper, Chrysophrys autratus (Family Sparidae), from southern Australia, eastern Australia and New Zealand. Australian Journal of Zoology Supplementary Series, 31, 1–68.
Roubal, F. R., Quartararo, N., & West, A. (1996). Spatial and temporal variation in populations and community of ectoparasites on young snapper, Pagrus auratus (Bloch & Schneider) (Sparidae), from the wild and captivity at Port Hacking, Sydney, Australia. Marine and Freshwater Research, 47, 585–593.
Sharples, A. D., & Evans, C. W. (1995a). Metazoan parasites of the snapper, Pagrus auratus (Bloch & Schneider, 1801), in New Zealand: 1. Prevalence and abundance. New Zealand Journal of Marine and Freshwater Rsearch, 29, 195–201.
Sharples, A. D., & Evans, C. W. (1995b). Taxonomy of the metazoan parasites of the snapper Pagrus auratus in New Zealand: 1. Ectoparasites. New Zealand Journal of Zoology, 22, 143–161.
Thompson, J. D., Gibson, T. J., Plewniac, F., Jeanmougin, F., & Higgins, D. G. (1997). The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 24, 4876–4882.
Thurstan, R. H., Campbell, A. B., & Pandolfi, J. M. (2016). Nineteenth century narratives reveal historic catch rates for Australian snapper (Pagrus auratus). Fish and Fisheries, 17, 210–225.
Ward, R. D., Zemlak, T. S., Innes, B. H., Last, P. R., & Hebert, P. D. (2005). DNA barcoding Australiaʼs fish species. Philosophical Transactions of the Royal Society B: Biological Sciences, 360, 1847–1857.
Acknowledgements
Md. Shafaet Hossen is grateful to Australian Research Training Program Scholarship for providing PhD scholarship through Charles Sturt University. The authors would like to acknowledge the kind efforts of Craig Poynter from the Charles Sturt University Spatial Analysis Unit for the development of the sampling sites map included in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection Monogenea.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hossen, M.S., Barton, D.P., Zhu, X. et al. Re-description and molecular characterisation of Choricotyle australiensis Roubal, Armitage & Rohde, 1983 (Monogenea: Diclidophoridae) infecting Chrysophrys auratus (Forster) (Perciformes: Sparidae). Syst Parasitol 97, 815–825 (2020). https://doi.org/10.1007/s11230-020-09950-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-020-09950-4