Abstract
Microcotyle arripis Sandars, 1945 is redescribed from Arripis georgianus from four localities: Spencer Gulf, Gulf St. Vincent, off Kangaroo Island and Coffin Bay, South Australia, Australia. Kahawaia truttae (Dillon & Hargis, 1965) Lebedev, 1969 is reported from A. trutta off Bermagui, New South Wales and is redescribed from a new host, A. truttaceus, from four localities in South Australian waters: Spencer Gulf, Gulf St. Vincent, off Kangaroo Island and Coffin Bay. Phylogenetic analysis of the partial 28S ribosomal RNA gene (28S rRNA) nucleotide sequences for both microcotylid species and comparison with other available sequence data for microcotylid species across four genera contributes to our understanding of relationships in this monogenean family.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All four species of Arripis Jenyns (Pisces: Arripidae), A. georgianus (Valenciennes), A. trutta (Forster), A. truttaceus (Cuvier) and A. xylabion Paulin, are endemic to the waters of southern Australia and New Zealand (NZ), and are caught by commercial and recreational fishers (see Paulin, 1993, for a review). During a survey of the parasites of A. georgianus, A. trutta and A. truttaceus, two parasite species of the Microcotylidae Taschenberg, 1879 were recovered. Microcotyle arripis Sandars, 1945 is redescribed from A. georgianus, as the original description by Sandars (1945) and subsequent redescriptions by Dillon et al. (1984) and Williams (1991) are based upon limited material. Our study also provides new geographical localities for M. arripis in South Australian (SA) waters. Kahawaia truttae (Dillon & Hargis, 1965) Lebedev, 1969, originally described as Gonoplasius truttae Dillon & Hargis, 1965 from A. trutta at Timuru, South Island, NZ (Dillon & Hargis, 1965), is reported from A. trutta off the coast of New South Wales (NSW) and from A. truttaceus from SA waters, providing new geographical localities for this species in southern Australian waters. This microcotylid is redescribed, because the original description by Dillon & Hargis (1965) was based on limited material and the subsequent account by Lebedev (1969) was poorly illustrated. To improve our understanding of relationships within the Microcotylidae, newly sequenced partial 28S ribosomal RNA gene (28S rRNA) nucleotide sequence data for M. arripis from A. georgianus and K. truttae from A. trutta and A. truttaceus are compared with sequence data for other microcotylid species available in GenBank.
Materials and methods
Collection of hosts and parasites
Monogeneans were collected from the gills of Arripis georgianus and A. truttaceus at four localities in SA waters (Spencer Gulf, Gulf St. Vincent, off Kangaroo Island and Coffin Bay; see ‘Results’ for geographical coordinates), from A. trutta at one location off NSW (Bermagui; see ‘Results’ for geographical coordinates) and from Sillaginodes punctatus (Cuvier) in SA waters (specific locality unknown). Most fish were examined fresh; others were frozen immediately upon collection and processed later. Gills were removed, placed in individual Petri dishes of seawater and studied for parasites. Monogeneans were removed and preserved in either 10% formalin or 95% undenatured ethanol. Representative specimens were slivered along the body margin with the slivered section preserved in 95% ethanol and the remaining section preserved in formalin. Parasites preserved in formalin were washed twice in MiliQ water, stained in Mayer’s haematoxylin, destained in 3% HCl, dehydrated in an ethanol series (70%, 90%, 95% and 100%), cleared in cedar wood oil and mounted on a slide beneath a coverslip in Canada balsam.
Tissue samples were collected from each fish, stored in undenatured ethanol and lodged with the Australian Biological Tissue Collection (ABTC) at the South Australian Museum Australia (SAMA), North Terrace, Adelaide, South Australia 5000, Australia (ABTC 108509–777).
Morphological methods
Drawings of preserved, mounted parasites were made with the aid of a drawing tube and measurements were made using a computerised digitising system similar to that described by Roff & Hopcroft (1986). The terminology of Williams (1991) for microcotylid monogeneans is adopted, using buccal organs in preference to buccal suckers, and ovary in preference to germarium. Unless stated otherwise, all measurements are presented in micrometres as the mean with the range in parentheses, followed in brackets by the number of measurements made. A dash (-) indicates that measurements could not be made or were not available.
Measurements of lengths refer to the distance along the anteroposterior axis, except where otherwise noted. Length measurements of structures not orientated along the anteroposterior axis in some specimens, such as buccal organs, clamps, eggs and genital atrium armature, were measured along the longest axis of these structures. ‘Middle clamps’ are defined as being located halfway along the total haptor length and ‘posterior clamps’ are defined as being located at the posterior end of the haptor. Occasionally bodies and haptors were curved, so multiple linear measurements were made and summed to give the total length. Body width was measured across the ovary, and haptor width was measured across the anterior margin.
Molecular methods
Monogenean parasites for molecular analyses were preserved in 95% undenatured ethanol and collected from the gills of four fish species, A. georgianus, A. trutta, A. truttaceus and S. punctatus (Table 1). Additional monogenean sequences were obtained from GenBank (Table 1).
Total genomic DNA (gDNA) was extracted using the Gentra Kit (Gentra Systems) to digest tissue and release DNA, followed by the RNeasy Mini Kit (animal tissue protocol incorporating QIAshredder pretreatment, QIAGEN GmbH, Hilden, Germany) to extract the DNA. PCR amplification of partial 28S rRNA sequence was carried out using two universal primers (Jovelin & Justine, 2001), modified with a 5’ M13 tag, C1’M13 (5’GTAAAACGACGGCCAGACCCGCTGAATTTAAGCAT-3’) and reverse D2M13 (5’-CAGGAAACAGCTATGACTCCGTGTTTCAAGACGG-3’), corresponding to positions 25 and 1126 of the complete Mus musculus 28S rRNA nucleotide sequence (Hassouna et al., 1984). Two additional primers were designed for improved amplification of fish fin clips, SC-FM13 (5’-GTAAAACGACGGCCAGACCTCAGATCAGACGAGACAAC-3’) and reverse SC-RM13 (5’-CAGGAAACAGCTATGACCGTGCGTTAGACTCCTTGGTC-3’).
Amplification reactions were conducted in a final volume of 25 μl containing: 2.5 μl of GeneAmp 10x PCR Buffer II (Applied Biosystems, Inc. [ABI], Foster City, CA, USA), 1.5 mM MgCl2 (ABI), 200 μM of each dNTP, 10 pmol of each primer, 1.25 U of AmpliTaq Gold (ABI) and 1 μl gDNA extract. After enzyme activation at 94°C for 10 min, the reactions were subjected to 60 cycles of amplification (94°C for 30 s, 50°C for 1 min and 72°C for 2 min). Negative controls were included to determine any potential contamination of the PCR products. PCR products were visualised with UV following agarose gel (1.5%) electrophoresis and staining with EZ-Vision Three, 6X (Amresco, Solon, OH, USA). Products of the predicted size were cleaned (QIAquick PCR Purification Kit, QIAGEN) and sequenced in both directions using dye terminator chemistry (BigDye Terminator v3.1 cycle-sequencing kit, ABI) primed with M13 forward and reverse primers.
Sequence alignments, error correction and similarity analysis (neighbour-joining) were performed using Kodon (Applied Maths, St-Martens-Latem, Belgium) and PAUP* 4 (Swofford, 2003), including a bootstrap analysis (1,000 replicates). Percentages over 50% were included on the neighbour-joining (NJ) tree. Representative sequences were deposited in GenBank (Table 1).
Specimens requested and deposited
Five voucher specimens of Kahawaia truttae (as Gonoplasius truttae, W. 14952–56), lodged by Dillon & Hargis (1965) collected from A. trutta in NZ, were borrowed from the Australian Museum (6 College Street, Sydney, NSW, Australia) for comparison with newly collected material. Voucher specimens of taxa reported in this paper are deposited in the Australian Helminthological Collection (AHC) of SAMA, Parasitology Section, North Terrace, Adelaide, South Australia 5000, Australia, the Parasitic Worms Collection of the Natural History Museum (NHM), London, England and the United States National Parasitology Collection (USNPC).
-
Class Monogenea Carus, 1863
-
Family Microcotylidae Taschenberg, 1879
-
Subfamily Microcotylinae Taschenberg, 1879
-
Genus Microcotyle van Beneden & Hesse, 1863
Microcotyle arripis Sandars, 1945
Type-host: Arripis georgianus (Valenciennes), Arripidae, ‘Australian herring, tommy ruff’ (Sandars, 1945; Dillon et al., 1984; Williams, 1991; present study).
Site: Gills (Sandars, 1945; Dillon et al., 1984); gill filaments (Williams, 1991); primary gill filaments (present study).
Infection details: A total of 51 flukes collected from 50 fish; maximum intensity 4; mean intensity 2 (Sandars, 1945); a total of 67 flukes (Dillon et al., 1984); a total of 12 flukes collected from 8 fish; prevalence 63%; maximum intensity 4 (Williams, 1991); a total of 664 flukes collected from 183 fish; prevalence 72%; maximum intensity 21; mean intensity 5.1 (present study).
Geographical localities: North Beach, Swan River, Mandurah, Bunbury, Busselton, Albany, Woodman’s Point, Scarborough and Whitford’s Beach, Western Australia (WA), Australia (Sandars, 1945); Ardrossan and Adelaide, SA, Australia (Dillon et al., 1984); Swan River Estuary and Cockburn Sound, WA, Australia (Williams, 1991); Spencer Gulf (Pt. Pirie [33°10′37′′S, 138°0′36′′E], Pt. Broughton [33°36′0′S, 137°55′53′′E] and Tickera [33°47′8′′S, 137°42′23′E]), Gulf St. Vincent (Edithburgh [35°5′85′′S, 137°44′57′′E], Stansbury [34°54′48′′S, 137°48′29′′E], Port Parham [34°26′43′′S, 138°14′37′′E] and Rapid Bay [35°31′31′′S, 138°11′14′′E]), Kangaroo Island (Kingscote [35°39′27′′S, 137°38′38′′E] and Emu Bay [35°36′13′′S, 137°31′12′′E]) and Coffin Bay [34°37′20′′S, 135°27′46′′E], SA, Australia (present study).
Specimens studied: 3 whole-mounts (Dillon et al., 1984); 6 whole-mounts (Williams, 1991); 3 whole-mounts used for drawing (AHC 29751, AHC 29753, AHC 29884), 10 whole-mounts measured, 5 voucher specimens deposited in SAMA (AHC 29751–3, AHC 29883–4), 2 voucher specimens deposited in NHM (BMNH 2009.12.28.1–2) and 3 voucher specimens deposited in USNPC (USNPC 102673.00–102675.00) (present study).
Redescription (Fig. 1; Table 2)
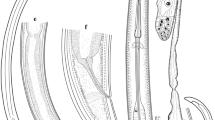
Body elongate; total length 1,887 (867–2,713) [10]; width 363 (155–564) [10]; body tapers anteriorly and posteriorly in most specimens (Fig. 1A). Haptor weakly delineated, appears continuous with body, 569 (148–939) [10] long; width across anterior margin 161 (94–233) [10]. Haptor armed with total of 76 (44–96) [10] clamps, in 2 nearly equal ventrolateral rows (Fig. 1E). Clamps of typical Microcotyle-type, similar in structure and size; middle clamps 22 (9–28) [10] long, 44 (26–54) wide (Fig. 1D); posterior clamps 21 (14–43) [10] long, 36 (24–42) [10] wide.
Microcotyle arripis Sandars, 1945. A. Whole-mount (ventral view), composite of two individuals (AHC 29751 and AHC 29753); B. Genital atrium drawn from AHC 29751; C. Egg, clearly distorted from normal shape, drawn in utero from AHC 29753; D. Clamp drawn from AHC 29751; E. Enlargement of unflattened, more typical symmetrical haptor showing opposing ventrolateral rows of equally-sized clamps drawn from AHC 29884. Abbreviations: BO, buccal organ; C, clamp; CVD, common vitelline duct; E, egg; GA, genital atrium; H, haptor; HR, haematin residues in gut branches (branches not drawn); IC, intestinal caeca; OES, oesophagus; O, ovary; PH, pharynx; T, testes; U, uterus; V, vitellarium; VIT D, vitelline duct. Scale-bars: A, 0.5 mm; B,C, 50 μm; D, 25 μm; E, 200 μm
Buccal organs 28 (20–35) [6] long, 28 (15–36) [6] wide; small, sclerotised, tooth-like papillae on rims. Pharynx 35 (29–40) [6] long, 38 (29–46) [6] wide, leading into oesophagus posteriorly. Oesophagus relatively long, 130 (115–144) [3], without diverticula. Genital atrium 38 (21–60) [9] long, 84 (39–113) [9] wide; located 208 (107–306) [9] from anterior end of body. Genital atrium arrangement and armature as in Fig. 1B; boundary lined with large spines except posterior edge with spine points facing inwards; inner portion armed with numerous smaller, conical spines with points directed posteriorly; inner spines randomly distributed in some specimens, spaced somewhat evenly in others. Gut bifurcates immediately posterior to genital atrium forming intestinal caeca with medial and lateral branching (branches not shown in Fig. 1A, but note haematin residues); unclear whether caeca unite posteriorly.
Testes post-ovarian, 19 (15–21) [7] in number; of irregular shape 34 (17–52) [7] long, 55 (41–104) [7] wide. Ovary tubular, tapers posteriorly, difficult to trace course in most specimens. Vitelline ducts anterior; common vitelline duct posterior; complete structure Y-shaped. Vaginal pore not observed. Vitellarium commences at central margin of genital atrium, occupies both lateral fields, extends into haptor. Uterus situated in central field, proceeds to genital atrium. Eggs in utero fusiform, 122 (99–145) [2] long, 25 (22–28) [2] wide, with filaments at both ends (Fig. 1C). Among live parasites collected, none was observed to lay eggs.
Remarks
Measurements for M. arripis collected in this study are presented in Table 2 for comparison with previously published measurements (Sandars, 1945; Dillon et al., 1984; Williams, 1991). This species is redescribed here as new specimens show some minor differences to previous descriptions, which were based upon limited material. The present specimens differ from those described by Sandars (1945), Dillon et al. (1984) and Williams (1991) as follows: (1) body length shorter than described by Williams (1991); (2) haptor width larger than described by Sandars (1945); (3) oesophagus shorter than described by Sandars (1945); and (4) middle clamp length, egg length and egg width smaller than all previous descriptions. However, despite these morphometric differences, the anatomy of the present material fits previous accounts of M. arripis.
Genus Kahawaia Lebedev, 1969
Kahawaia truttae(Dillon & Hargis, 1965) Lebedev, 1969 Syn.
Gonoplasius truttae Dillon & Hargis, 1965
Type-host: Arripis trutta (Forster), Arripidae, ‘eastern Australian salmon’ (Dillon & Hargis, 1965; Lebedev, 1969; present study).
Other hosts: A. truttaceus (Cuvier), Arripidae, ‘western Australian salmon’ (present study—new host record).
Site: A. trutta—gills (Dillon & Hargis, 1965; Lebedev, 1969); primary gill filaments (present study). A. truttaceus—primary gill filaments (present study).
Infection details: A. trutta—a total of 24 flukes collected from 23 fish; prevalence 48%; maximum intensity 5; mean intensity 2.2 (present study). A. truttaceus—a total of 83 flukes collected from 67 fish; prevalence 39%; maximum intensity 14; mean intensity 3.2 (present study).
Geographical localities: A. trutta—Timaru, Canterbury Province, South Island, NZ (Dillon & Hargis, 1965); Great Australian Bight, SA, Australia (Lebedev, 1969); Bermagui [36°25′12′′S, 150°4′55′′E], NSW, Australia (present study); A. truttaceus—Spencer Gulf (Whyalla [33°2′44′′S, 137°34′59′′E]), Gulf St. Vincent (Cape Jervis [35°36′29′′S, 138°5′45′′E]), Kangaroo Island (Kingscote [35°39′27′′S, 137°38′38′′E] and Emu Bay [35°36′13′′S, 137°3′12′′E]) and Coffin Bay [34°37′20′′S, 135°27′46′′E], SA, Australia (present study).
Specimens studied: A. trutta—2 (Dillon & Hargis, 1965); 5 (Lebedev, 1969); 10 whole-mounts measured, 5 voucher specimens deposited in SAMA (AHC 29781, 29782 [2 slides], 29887–8), 2 voucher specimens deposited in NHM (BMNH 2009.12.28.5–6) and 3 voucher specimens deposited in USNPC (USNPC 102678.00 [2 slides], 102679.00) (present study); A. truttaceus—2 whole-mounts used for drawings (AHC 29767, AHC 29882), 10 whole-mounts measured, 6 voucher specimens deposited in SAMA (AHC 29767–9, 29882, 29885–6), 2 voucher specimens deposited in NHM (BMNH 2009.12.28.3–4) and 2 voucher specimens deposited in USNPC (USNPC 102676.00–102677.00) (present study).
Redescription (Fig. 2; Table 3)
[Measurements of K. truttae from 2 localities (SA and NSW) on 2 host species (A. truttaceus and A. trutta) are presented in Table 3, with previously published measurements.] Body elongate, fusiform (Fig. 2A). Haptor clearly delineated from body, wedge-shaped, lined on both sides by single column of clamps. Clamps of typical Microcotyle-type, similar in structure (Fig. 2D), dissimilar in size; middle clamps longer and wider than posterior clamps (Table 3).
Kahawaia truttae (Dillon & Hargis, 1965) Lebedev, 1969 from Arripis truttaceus. A. Whole-mount (ventral view) drawn from AHC 29767; B. Genital atrium drawn from AHC 29767; C. Egg, clearly distorted from normal shape, drawn in utero from AHC 29882; D. Clamp drawn from AHC 29767. Abbreviations: As for Fig. 1, plus: CP, cuticularised pits; GIC, gastro-intestinal canal; VD, vas deferens. Scale-bars: A, 1.5 mm; B, 75 μm; C, 100 μm; D, 35 μm
Buccal organs of similar width and length; rim armed with small, sclerotised, tooth-like papillae. Pharynx similar in length and width, smaller than buccal organs. Oesophagus relatively long. Genital atrium (Fig. 2B) consists of 2 laterally placed pads (1 to right and 1 to left of mid-line); each pad armed with circle of spines with hooked tips; spines of similar structure, dissimilar in size. Gut bifurcate; intestinal caeca with medial and lateral branching (branches not shown in Fig. 2A, but note haematin residues); unclear whether caeca unite posteriorly.
Testes post-ovarian, abundant (number not determined), closely abutting but not touching, situated inter-caecally. Vas deferens wide, follows sinuous path anteriorly in mid-line to genital atrium. Vitellarium commences posterior to genital atrium, occupies both lateral fields, not extending into haptor. Ovary tubular, coiled irregularly in mid-line. Gastro-intestinal canal present, connects right intestinal caecum with common vitelline duct. Vitelline ducts anterior; common vitelline duct posterior; complete structure Y-shaped. Mehlis’ gland and vaginal pore not observed. Two dorsal cuticularised pits present, 1 on each side of mid-line (Fig. 2A). Uterus passes anteriorly in mid-line to genital atrium, obscured from view by vas deferens. Eggs in utero fusiform, with filaments at both ends (Fig. 2C). Among live parasites collected, none was observed to lay eggs.
Remarks
Measurements for K. truttae collected in this study are presented in Table 3 for comparison with previously published accounts by Dillon & Hargis (1965) and Lebedev (1969). The redescription given here is warranted because the original description by Dillon & Hargis (1965) was based upon limited material, and the subsequent redescription by Lebedev (1969) was poorly illustrated. This study also provides a new host record for K. truttae from A. truttaceus. Specimens of K. truttae from A. trutta off the coast of NSW did not differ from specimens collected from A. truttaceus in SA waters based on morphometrics (Table 3). However, there were minor differences between K. truttae from A. trutta and A. truttaceus in our study and specimens described by Dillon & Hargis (1965) and Lebedev (1969) from A. trutta as follows: (1) middle clamp width larger than described by Dillon & Hargis (1965), but smaller than described by Lebedev (1969); (2) posterior clamp length smaller than described by Dillon & Hargis (1965); (3) number of spines on left pad fewer than in all previous descriptions; and (4) egg length smaller than in all previous descriptions. Despite these differences in measurements and spine counts, the morphology of the new material from A. trutta and A. truttaceus agrees with K. truttae.
Molecular analyses
The 28S rRNA fragments for the majority of taxa sequenced were 850–950 bp, but shorter sequences were occasionally obtained due to poor primer binding. Analyses included sequences for Kahawaia truttae from A. truttaceus in SA waters and A. trutta off NSW, Microcotyle arripis from A. georgianus in SA waters, and five other microcotylid taxa and one outgroup taxon in the Discocotylidae (Table 1).
From the NJ analysis, the microcotylid taxa separated into four clades, Diplostamenides sciaenae, Polylabris sillaginae, Microcotyle spp. (M. arripis, M. sebastis, M. erythrinii and Microcotylidae sp. M10) and Kahawaia truttae (Fig. 3). Microcotyle arripis from A. georgianus grouped within the Microcotyle spp. clade, yet was distinct from other species within this clade. The 10 individuals within the P. sillaginae clade had identical sequences. Replicated samples of K. truttae from A. truttaceus and K. truttae from A. trutta grouped together within a single clade, with strong support (Bootstrap value 100% after 1,000 replicates; Fig. 3). Within the K. truttae clade, 52 individuals of K. truttae from A. truttaceus and 9 individuals of K. truttae from A. trutta were identical (Fig. 3), providing confidence in the morphological study that the microcotylid species from A. truttaceus and A. trutta is a single taxon, K. truttae.
Neighbour-joining tree from analyses of the 28S rRNA nucleotide sequence data for the Polyopisthocotylea, including seven microcotylid taxa and one outgroup taxon (Table 1). Note that the top branch represents a total of 61 identical Kahawaia truttae sequences from Arripis trutta and A. truttaceus. Bootstrap values (≥50) are shown above the branches
Discussion
Based on morphology and host, the monogenean species from Arripis georgianus was identified as Microcotyle arripis, originally described by Sandars (1945). Phylogenetic analysis of partial 28S rRNA nucleotide sequence data using NJ supported the morphological conclusion, with M. arripis grouping within the Microcotyle spp. clade, but observed to be distinct from all other species (Fig. 3). There is no published record of this parasite species from any other fish species, and therefore M. arripis likely exhibits host-specificity to A. georgianus. This is not unexpected, as monogeneans are among the most host-specific of parasites in general (Rohde, 1979; Whittington et al., 2000).
Kahawaia truttae was first recorded as Gonoplasius truttae Dillon & Hargis, 1965 from A. trutta at Timura, South Island, NZ (Dillon & Hargis, 1965), but, after analysis of its morphological structures, was reassigned by Lebedev (1969) to a new genus, Kahawaia. Morphological study suggested the monogenean species from A. truttaceus in SA waters was identical to the monogenean species from A. trutta in waters off the coast of NSW, namely K. truttae, which therefore represents a new host record for K. truttae on A. truttaceus. Molecular analyses supported this hypothesis, with representatives of K. truttae from both arripid host species grouping together within the same clade with strong support (Fig. 3).
Prior to 1993, there was considerable confusion surrounding the identification and discrimination of A. truttaceus and A. trutta (see Paulin, 1993, for a review). Lebedev (1969) identified K. truttae from A. trutta in SA waters (Great Australian Bight); however, this record may have been a misidentification of A. truttaceus, because in 1969 there were no reports of A. trutta from SA waters (Stanley & Malcolm, 1977). As Lebedev (1969) did not accession corresponding host specimens, this identification cannot be verified.
To enable further insight into the relationships within the Microcotylidae established here, it would be preferable to analyse more sequence data from a number of nuclear genes, including both coding and non-coding regions (McManus & Bowles, 1996), and run analyses to incorporate these multi-gene data. Furthermore, incorporation of some sequence data from mitochondrial genes may shed further light on the phylogenetic relationships between microcotylids. An improved appreciation and understanding of informative morphological characters that separate microcotylid genera and species would contribute significantly to unravelling the evolutionary history of these higher polyopisthocotyleans.
References
Aiken, H. M., Bott, N. J., Mladineo, I., Montero, F. E., Nowak, B. F., & Hayward, C. J. (2007). Molecular evidence for cosmopolitan distribution of platyhelminth parasites of tunas (Thunnus spp.). Fish and Fisheries, 8, 167–180.
Dillon, W. A., & Hargis, W. J. (1965). Monogenean trematodes from the southern Pacific Ocean. 2. Polyopisthocotyleids from New Zealand fishes: the families Discocotylidae, Microcotylidae, Axinidae, and Gastrocotylidae. Biology of the Antarctic Seas II, Antarctic Research Series, 5, 251–280.
Dillon, W. A., Hargis, W. J., & Harrises, A. E. (1984). Monogeneans from the southern Pacific Ocean. Polypisthocotyleids from Australian fishes. The subfamily Microcotylinae (English version). Zoologicheskii Zhurnal, 63, 348–359.
Hassouna, N., Michot, B., & Bachellerie, J. (1984). The complete nucleotide sequence of mouse 28S rRNA gene. Implications for the process of size increase of the large subunit rRNA in higher eukaryotes. Nucleic Acids Research, 12, 3563–3583.
Hayward, C. J. (1996). Revision of the monogenean genus Polylabris (Microcotylidae). Invertebrate Taxonomy, 10, 995–1039.
Jovelin, R., & Justine, J. (2001). Phylogenetic relationships within the polyopisthocotylean monogeneans (Platyhelminthes) inferred from partial 28S rDNA sequences. International Journal for Parasitology, 31, 393–401.
Lebedev, B. I. (1969). Substantiation of the new genus Kahawaia gen. n., for Gonoplasius truttae Dillon et Hargis, 1965 (Microcotylidae: Monogenoidea). Parazitologiya, 3, 69–73. (In Russian).
McManus, D. P., & Bowles, J. (1996). Molecular genetic approaches to parasite identification: their value in diagnostic parasitology and systematics. International Journal for Parasitology, 26, 687–704.
Olson, P. D., & Littlewood, D. T. J. (2002). Phylogenetics of the Monogenea–evidence from a medley of molecules. International Journal for Parasitology, 32, 233–244.
Paulin, C. (1993). Review of the Australasian fish family Arripididae (Percomorpha), with the description of a new species. Australian Journal of Marine and Freshwater Research, 44, 459–471.
Roff, J. C., & Hopcroft, R. R. (1986). High precision microcomputer based measuring system for ecological research. Canadian Journal of Fisheries and Aquatic Sciences, 43, 2044–2048.
Rohde, K. (1979). A critical evaluation of intrinsic and extrinsic factors responsible for niche restriction in parasites. The American Naturalist, 114, 648–671.
Sandars, D. F. (1945). Five new microcotylids from fish from Western Australian waters. Journal of the Royal Society of Western Australia, 29, 107–135.
Stanley, C. A., & Malcolm, W. B. (1977). Reproductive cycles in the eastern subspecies of the Australian salmon, Arripis trutta marginata (Cuvier & Valenciennes). Australian Journal of Marine and Freshwater Research, 28, 287–301.
Swofford, D. L. (2003). PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer Associates, Sunderland, Massachusetts.
Whittington, I. D., Cribb, B. W., Hamwood, T. E., & Halliday, J. A. (2000). Host-specificity of monogenean (platyhelminth) parasites: a role for anterior adhesive areas? International Journal for Parasitology, 30, 305–320.
Williams, A. (1991). Monogeneans of the families Microcotylidae Taschenberg, 1879 and Heteraxinidae Price, 1962 from Western Australia, including the description of Polylabris sandarsae n. sp. (Microcotylidae). Systematic Parasitology, 18, 17–43.
Acknowledgements
We wish to thank Leonie Barnett, Kieran Brazell, Mieke Burger, Angelo, Domenico, Margaret and Pepperoni Catalano, Bruce Jackson, Matt Padula, Pep Severino, Richard and Brian Saunders, and Mike Steer for assistance in the field, as well as Stephen Keable from the Australian Museum, Sydney, for providing the Kahawaia truttae specimens. We are also grateful to the Institute of Medical and Veterinary Science for access to resources and to the Infectious Diseases Laboratories group who provided constructive comments throughout the study period. Scholarship support for SRC was provided from Unibooks (University of Adelaide) and Playford Memorial Trust. This research was funded by an Australian Biological Resources Study grant (207-44) and a Fisheries Research and Development Corporation grant (207/225) awarded to KSH and IDW.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Catalano, S.R., Hutson, K.S., Ratcliff, R.M. et al. Redescriptions of two species of microcotylid monogeneans from three arripid hosts in southern Australian waters. Syst Parasitol 76, 211–222 (2010). https://doi.org/10.1007/s11230-010-9247-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-010-9247-x