Abstract
The siphonostomatoid parasitic copepod Caligus macrurus Heller, 1865 is redescribed based on new material collected from the gill filaments and pharynx of tripletail Lobotes surinamensis Bloch (Lobotidae) caught in Iskenderun Bay, Turkey. Key diagnostic characters and newly observed taxonomic features are reported, supported by light and scanning electron microscopy observations. This is the first report of C. macrurus from Mediterranean waters. Caligus macrurus is also recognised as conspecific with the better known Caligus bennetti Causey, 1953, found on the same host, which becomes a junior subjective synonym of C. macrurus. Caligus O.F. Müller, 1785 and Sciaenophilus van Beneden, 1852 have both been treated as valid genera within the family Caligidae although numerous doubts have been expressed over the validity of the latter. The morphological evidence does not support generic level distinction and we recommend the transfer of all species currently placed in Sciaenophilus into Caligus as C. tenuis (van Beneden, 1852), C. pharaonis von Nordmann, 1832, C. nibeae Shen, 1957 and C. macrurus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The copepod family Caligidae Burmeister, 1835, known as sea lice, currently contains about 490 valid species and it is the most species rich group in the entire order Siphonostomatoida Thorell, 1859 (see Boxshall & Halsey 2004). Members of this family are typically ectoparasitic on their fish hosts and can infest the outer body surface, the gills and the internal walls of the branchial and oral cavities. Caligid sea lice are a major health problem for finfish in marine aquaculture (Johnson et al., 2004) with heavy infestations often resulting in high mortalities due to surface lesions being susceptible to secondary bacterial infections.
The family currently consists of 31 valid genera according to the most recent overview by Dojiri & Ho (2013). Of these, the genus Caligus O. F. Müller, 1785 is the most species-rich taxon within the family and currently contains over 250 valid species (Hayes et al., 2012). In the Mediterranean, the number of known species of Caligus reached 28 with the addition of the most recently described new species Caligus solea Demirkale, Özak, Yanar & Boxshall, 2014 (see Demirkale et al., 2014). To the best of our knowledge, these 28 species of Caligus have thus far been reported from hosts representing 16 different families of teleost fishes (Raibaut et al., 1998; Özak et al., 2012, 2013). In this study, Caligus macrurus is reported for the first time in Mediterranean waters and redescribed based on new material collected from tripletail Lobotes surinamensis Bloch. In addition, the taxonomic position of C. macrurus is discussed in detail based on the re-examination of key diagnostic characters and newly observed taxonomic features.
Materials and methods
Three Atlantic tripletail Lobotes surinamensis (Bloch) (Lobotidae) caught by otter trawl in İskenderun Bay near Yumurtalık, Turkey (36°45′30.11″N, 35°43′08.75″E) were examined for the presence of parasitic copepods. The body surfaces, gill cavities, gill filaments and buccal cavity of the fish were examined. The fish ranged in total length from 55 to 91 cm. Parasitic copepods were collected from the gill cavities, gill filaments and pharynx of the infested fish and were immediately preserved in 70% ethanol. Specimens were cleared in lactic acid for 2 h prior to examination using an Olympus SZX16 dissecting microscope and Olympus BX51 compound microscope. Intact specimens and individual appendages were photographed with a digital camera on both microscopes. The scientific and common names of fishes follow Froese & Pauly (2016) and the morphological terminology for the copepods follows Boxshall (1990). All measurements are in millimetres unless otherwise stated and are reported as the range followed by the mean in parentheses. The protocols for preparing crustaceans for scanning electron microscopy (SEM) outlined by Felgenhauer (1987) were followed. Ethanol-fixed specimens were hydrated to distilled water and post-fixed in 1–2% osmium tetroxide (OsO4) in buffer for 2 h, washed in distilled water, dehydrated through graded acetone series, critical point dried using liquid carbon dioxide as the exchange medium, mounted on aluminium stubs and sputter coated with platinum. Coated specimens were examined on a Zeiss Supra 55 (FE-SEM, Germany) field emission scanning electron microscope at 1–3 kV.
Family Caligidae Burmeister, 1835
Genus Caligus O.F. Müller, 1785
Caligus macrurus Heller, 1865
Syns Sciaenophilus macrurus (Heller, 1865); Caligus bennetti Causey, 1953; Sciaenophilus bennetti (Causey, 1953)
Host: Lobotes surinamensis (Bloch) (Lobotidae).
Type-locality: Java, Indonesia.
New locality: Northeastern Mediterranean waters off Yumurtalık in Iskenderun Bay, Turkey collected by Alper Yanar (24.x.2015), depth range 50–70 m; mean surface water temperature 13°C; salinity 36 ppt.
Site in host: Females collected from the gill filaments and branchial cavity, single male found in pharynx.
Prevalence: 33% (1 fish infected out of 3 examined).
Material examined: Twelve ovigerous females collected from gill filaments and one male specimen from pharynx. Newly collected specimens of C. macrurus were fixed in ethanol containing vials and deposited in the Parasitology Museum of Çukurova University in Adana, Turkey (CUPM-COP/2015-1).
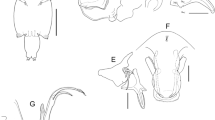
Adult female. Body (Fig. 1A) comprising caligiform cephalothorax, incorporating first to third pedigerous somites, free fourth pedigerous somite, genital complex and unusually long indistinctly 2-segmented abdomen. Total body length 7.19–8.43 (7.79) (n = 10). Cephalothorax subrectangular, longer than wide, 1.53–1.66 × 1.34–1.6 (1.58 × 1.42). Frontal plates bearing paired lunules. Thoracic zone of dorsal cephalothoracic shield distinctly wider than long 0.61–0.68 × 0.93–0.98 (0.64 × 0.95), posterior margin extending slightly beyond posterior ends of lateral zones. Fourth pedigerous somite wider than long 0.10–0.18 × 0.61–0.69 (0.12 × 0.64), posterior margin not clearly demarcated from genital complex and forming a neck-like transition region. Genital complex (Fig. 1A) narrower anteriorly, with weakly convex lateral margins and rounded posterolateral corners, just longer than wide, 1.58–1.64 × 1.50–1.58 (1.61 × 1.53). Abdomen (Fig. 1A, C) unusually long 4.64–4.90 × 0.50–0.55 (4.70 × 0.52), indistinctly 2-segmented, divided into narrow anterior part and broader posterior part comprising two-thirds of entire abdomen, 1.28 times wider and 2.51 times longer than anterior third of abdomen; entire abdomen approximately 1.46 times longer than combined length of cephalothorax, fourth pedigerous somite, and genital complex. Caudal rami (Fig. 1D) about 3.4 times longer than wide, 0.60–0.63 × 0.17–0.19 (0.62 × 0.18), each armed with 6 pinnate setae; inner and outermost setae (Fig. 1D, arrows) smallest; rami ornamented with fringe of pinnules along inner margin.
Antennule (Fig. 2A) 2-segmented, with elongate distal segment about 1.31 times longer than proximal; proximal segment with 25 setae on anteroventral surface, and 2 setae anterodorsally, distal segment with 12 setae plus 2 aesthetascs around apex. Antenna (Fig. 2B) 3-segmented; proximal and middle segments unarmed; distal segment produced into strongly curved claw armed with spine-like seta proximally plus another seta in mid section. Postantennal process (Fig. 2C) small, triangular, with 2 papillae each with 2 sensillae; similar papilla with 2 sensillae located on body surface adjacent to process. Sternal furca (Fig. 2D arrowed) small, with diverging tines rounded at tips (Fig. 2D inset). Maxilliped (Fig. 2E) comprising robust protopod (corpus) with smooth medial margin, and distal subchela terminating in strongly curved, tapering claw; inner surface of claw ornamented with fine longitudinal ridges (Fig. 2E arrowed, inset).
Caligus macrurus Heller, 1865. Female. A, Antennule and lunule; B, Terminal segment of antenna; C, Postantennal process; D, Light microscopy image of sternal furca, ventral view (arrow), inset: scanning electron microscopy image of sternal furca; E, Maxilliped, inner surface of claw (arrow) ornamented with fine longitidunal ridges (inset). Scale-bars: A, 100 µm; B, 10 µm; C, 20 µm; D inset, 10 µm; E, 50 µm; E inset, 1 µm
Swimming leg 1 biramous; with 2-segmented exopod and vestigial, lobate endopod. Protopod armed with plumose seta at anterodistal corner and plumose seta on posteromedial margin; ornamented with patch of spinules (Fig. 3A) on ventral surface. Spinules about 1 µm long, with rounded tips (Fig. 3B, C). Vestigial endopod (Fig. 3D) tapering distally bearing 1 minute, acutely-pointed setal vestige on apex (Fig. 3D arrowed, inset). Distal exopodal segment with 3 plumose setae on posterior margin plus 4 spiniform elements along distal margin. Outermost element (spine 1) (Fig. 5A, arrowed) small, finely serrated on both sides (Fig. 5A inset); originating proximal to distal corner of segment. Middle spines (spines 2 and 3) each with accessory process (Fig. 4A arrowed) and ornamented with fine serrations along inner and outer margins (Fig. 4A–C). Innermost element (spine 4) (Fig. 5B arrowed) at inner distal angle finely serrated along distal part of inner and outer margins (Fig. 5B inset).
Leg 2 biramous, with 3-segmented rami. First and second exopodal segments (Fig. 6A) each with pinnate seta on inner margin and long spine at outer distal corner: spines not reflexed but directed distally. Third segment (Fig. 6B) with 3 outer spines and 5 pinnate setae; first 2 outer spines unequal and serrated along inner and outer margins (Fig. 6C), third spine longest, fringed with hyaline membrane.
Caligus macrurus Heller, 1865. Female. A, Scanning electron microscopy image of exopod of leg 2; B, Light microscopy image of exopod of leg 2 showing spines and setae on each segment; C, Ornamentation of first spine on third exopodal segment of leg 2; D, Spines (black arrows) and setae (white arrows) on third exopodal segment of leg 3; E, Setae (*) on second endopodal segment of leg 3; F, Spines on 3-segmented exopod of leg 4, distal spine on first exopodal (arrow) segment well extending beyond second exopodal segment, inset: groove (arrow) at tip of distal spine. Scale-bars: A, 20 µm; B, 10 µm; C, 5 µm; D–F, 50 µm; F inset, 5 µm
Leg 3 (Fig. 6D) with 3-segmented exopod, armed with slightly curved outer spine on first segment, extending just beyond distal margin of second segment and lying more-or-less parallel with long axis of ramus. Second exopodal segment with outer spine and inner plumose seta. Third exopodal segment with 3 unequal outer spines (Fig. 6D black arrows) and 4 pinnate setae (Fig. 6D white arrows). Endopod (Fig. 6E) 2-segmented; first segment with long inner pinnate seta, second with 5 pinnate setae (Fig. 6E asterisks).
Leg 4 uniramous, comprising long protopodal segment and 3-segmented exopod (Fig. 6F); first and second exopodal segments each with single distal spine, third segment with 3 equal apical spines, each spine with pecten at base; distal spine on first segment (Fig. 6F arrowed) extending well beyond second exopodal segment; spines on all exopodal segments ornamented with fine serrations and grooved at tip (Fig. 6F inset).
Spine (Roman numerals) and seta (Arabic numerals) formula of legs 1–4 as follows:
Leg | Exopod | Endopod |
|---|---|---|
Leg 1 | I-0; I, III, 3 | vestigial |
Leg 2 | I-1; I-1; II, 1, 5 | 0–1; 0–2; 6 |
Leg 3 | I-0; I-1; III, 4 | 0–1; 5 |
Leg 4 | I; I; III | absent |
Female leg 5 (Fig. 7E) represented by elongate process with single lateral seta plus 3 apical plumose setae.
Caligus macrurus Heller, 1865. Male. A, Distal segment of antenna; B, Postantennal process; C, Light microscopy image of sternal furca (arrow), inset: diverging tines of sternal furca; D, Maxilliped; E, Female leg 5; F, Male leg 5 with four setae; leg 6 with three setae, outer seta smallest (arrow). Scale-bars: A, 40 µm; B, 20 µm; C inset, 10 µm; D, 100 µm; E–F, 50 µm
Adult male. Body length 3.91 (n = 1). Cephalothorax suborbicular (Fig. 1B), slightly wider than long, 1.56 × 1.6. Thoracic zone of shield distinctly wider than long 0.76 × 1.02, posterior margin extending beyond posterior ends of lateral zones. Fourth pedigerous somite wider than long 0.26 × 0.40. Genital complex (Fig. 1B) subrectangular, longer than wide, 0.62 × 0.56. Abdomen (Fig. 1B) distinctly 2-segmented; first free abdominal somite slightly wider than long 0.21 × 0.29; anal somite rectangular, longer than wide 0.61 × 0.32; entire abdomen about 1.32 times longer than genital complex. Caudal rami as in female; caudal ramus about 1.9 times longer than wide and about equal in length to anal somite. Antennule as in female. Antenna 3-segmented; proximal segment bearing corrugated adhesion pad on mid-outer surface; middle segment longest, with corrugated pads on medial and distal surfaces; distal segment (Fig. 7A) forming recurved, tapering claw armed with smaller accessory process and 2 slender setae. Postantennal process (Fig. 7B) small, with elongate process directed laterally; carrying 2 multi-sensillate papillae; similar multi-sensillate papilla located on body surface adjacent to process. Sternal furca (Fig. 7C arrowed) with more strongly divergent tines than female (Fig. 7C inset). Maxilliped (Fig. 7D) with massive corpus carrying conspicuous process on myxal margin, opposing tip of claw; process conical with minute pore at apex; distal subchela with short, curved claw plus long seta at base of claw. Leg 5 (Fig. 7F) represented by single process located on mid-lateral margin of genital complex, armed with 4 setae. Leg 6 (Fig. 7F) represented by single papilla bearing 3 setae on posteroventral side of genital complex; outer seta smallest (Fig. 7F arrowed).
Remarks
Caligus macrurus was first described by Heller (1865) based on females collected from the gills of the lobotid teleost Lobotes surinamensis (as Labotes erato) taken off Java, Indonesia. However, the type-material of C. macrurus is unavailable. Heller (1865) made explicit comparisons with the genus Sciaenophilus van Beneden, 1852, established some years earlier by van Beneden (1852), but concluded that his species was simply a Caligus with an elongate abdomen and did not merit separate generic status. Despite Heller’s conclusion, C. macrurus was subsequently transferred to Sciaenophilus by Yamaguti (1963), who gave no justification in support of this change, although Ho & Bashirullah (1977) returned it to its original combination due to its possession of a sternal furca (absent in the type-species of Sciaenophilus). No subsequent discoveries of C. macrurus have been reported in the literature.
Caligus bennetti Causey, 1953 was established by Causey (1953) based on material from Lobotes surinamensis from Grand Isle, Louisiana, United States. This widespread copepod has since been reported from the same host from Port Aransas (Texas, USA), Gairia (Venezuela), Trivandrum (India), and Dong-shi (Taiwan) (Causey, 1955; Ho & Bashirullah, 1977; Prabha & Pillai, 1983; Ho & Lin, 2004). Detailed redescriptions of C. bennetti are available in Ho & Bashirullah (1977), based on females from Venezuela, and in Ho & Lin (2004), based on material of both sexes from Taiwan.
Ho & Bashirullah (1977) suspected that C. bennetti and C. macrurus were conspecific but could not confirm this from Heller’s (1865) description. Prabha & Pillai (1983) stated that their Indian Ocean material of C. bennetti supported the suggestion of synonymy between C. macrurus and C. bennetti made by Ho & Bashirullah (1977). We also consider that there is sufficient evidence to support their suspicion. The unique body form, characterised by the hyper-development of the free abdomen, is shared by both species and both occur on the same host, Lobotes surinamensis. Both have elongate fourth legs and these carry the armature of five spines towards the end of the limb. We consider the segmentation pattern shown by Heller (1865: table XV, figure 2a) to be an observational error because the combination of a 2-segmented exopod on leg 4 with 5 outer spines on the distal exopodal segment does not exist anywhere in the family Caligidae (or in the order Siphonostomatoida). The exopod of leg 1 has three long distal elements (spines 2 to 4) and spine 1 is small and offset, inserting proximal to the outer distal corner of the segment in both species. These are the only limbs figured by Heller (1865) and his text description does not provide much detail, although it does confirm the presence of a sternal furca. The body length of Heller’s material is 8 mm, very close to the 8.61 mm given by Ho & Lin (2004) for Taiwanese material and the 8.8 mm for the Indian material (Prabha & Pillai 1983), although smaller than the 11.37 mm given for the Venezuelan material by Ho & Bashirullah (1977). Our Turkish material (7.79 mm) is most similar to Heller’s in body length. The length of the abdomen alone also matches most closely between the Turkish (4.70 mm) and Taiwanese females (4.87 mm), compared with the Venezuelan females (7.44 mm). The male from Turkey has a longer body than males reported by Prabha & Pillai (1983) from India and by Lin & Ho (2004) from Taiwan (3.91 mm vs 3.26 mm and 3.13 mm, respectively).
In addition to these morphometric differences, we observed some minor morphological differences, for example the middle two elements (spines 2 and 3) on the distal exopodal segment of leg 1 have accessory processes which have not been reported in previous descriptions. However, the accessory processes are best visualised using SEM (e.g. Fig. 4A) while light microscopy (cf. Fig. 4C) gives an image that closely resembles the line drawing provided by Ho & Bashirullah (1977: figure 2B). The inner and outermost elements (spines 1 and 4) on the distal exopodal segment of leg 1 (Fig. 5A, B) are ornamented with marginal serrations but are depicted as smooth in Ho & Lin (2004: figure 65A). However, both are shown as serrated in Ho & Bashirullah (1977) and this may reflect only the style of the drawings. Similar differences in fine scale ornamentation are apparent in other limbs, for example, the presence or absence of serrations on the two proximal spines on the third exopodal segment of leg 2. There are some additional differences apparent between other published descriptions: for example, Prabha & Pillai (1983) show only four setae on the distal endopod segment of leg 3 whereas all other descriptions show five, and Ho & Lin (2004) show III,3 elements on the distal exopod segment of leg 3 whereas other descriptions show III,4. We interpret these atypical states as either observational errors or damaged specimens. We also note some variation in the degree of divergence of the tines of the sternal furca in both sexes between our Turkish material and published descriptions. Despite these apparent differences between previous reports and the Turkish specimens, the overwhelming number of shared similarities supports the identification of this material as conspecific with C. bennetti as described by Ho & Bashirullah (1977), Prabha & Pillai (1983) and Ho & Lin (2004). We also accept that this species is identical with C. macrurus of Heller (1865) and this name has priority, therefore C. bennetti becomes a junior subjective synonym of C. macrurus.
Caligus macrurus is primarily a parasite of the buccal and branchial cavities and gills of the lobotid fish, Lobotes surinamensis. This is the type-host and C. macrurus has been reported on this host from Indonesia (Java), the Gulf of Mexico (Louisiana and Texas), Venezuela, India and Taiwan (Heller, 1865; Causey, 1953, 1955; Ho & Bashirullah, 1977; Prabha & Pillai, 1983; Ho & Lin, 2004). We now extend the known distribution range into the Mediterranean, at Iskenderun Bay, Turkey. The species reported here, Caligus macrurus Heller, 1865 brings the total number for the Mediterranean to 29, and the number of fish families parasitized by these Caligus species in the Mediterranean is now 17. This parasite has also been reported from two other fish hosts: Paralabrax maculatofasciatus (Steindachner) (Serranidae) and Kyphosus sectatrix (Linnaeus) (Kyphosidae) caught from Veracruz on the Mexican Gulf coast and Sinaloa respectively, on the Pacific coast of Mexico (Causey, 1960).
Discussion
Our examination of C. macrurus prompted us to re-consider the validity of the genus Sciaenophilus, established by van Beneden (1852) to accommodate his new species Sciaenophilus tenuis van Beneden, 1852, a widely distributed parasite of sciaenids (Dojiri & Ho, 2013: table XVIII). Heller (1865) did not consider that a genus distinguished from Caligus on the basis of an elongate abdomen was justified. Capart (1941, 1959) considered Sciaenophilus as a junior synonym of Caligus, and Kabata (1979) referred to it as having questionable validity, although he retained it. In contrast, Heegaard (1966) actually proposed a new family, Sciaenophilidae, based around Sciaenophilus although he neither gave a definition of the family nor discussed his reasons. This proposal has not been followed. Ho & Bashirullah (1977) and Ho & Lin (2004) treated Sciaenophilus as valid but specifically excluded Caligus macrurus (as C. bennetti) which they retained in Caligus.
Dojiri & Ho’s (2013) monograph on the systematics of the Caligidae did not test the validity of Sciaenophilus, but they revisited the evidence supporting its generic level status. They discussed a set of character states that were shared by species of Sciaenophilus: firstly the female abdomen is as long as or longer than the rest of the body combined; secondly, the sternal furca was typically absent; thirdly, the distal armature of the exopod of leg 1 (offset spine 1, lack of accessory processes on spines 2 and 3, large size of spine 3, and spiniform appearance of spine 4), and lastly, the lack of a posteriorly-directed process on the second segment of the female antenna. Dojiri & Ho (2013) noted that most of these character states, for example, the shape of the distal armature on the exopod of leg 1, were shared with other clusters of Caligus species. However, they retained Sciaenophilus based on this set of characters and stated that it contained five species: S. tenuis, S. pharaonis (von Nordmann, 1832), S. nibeae (Shen, 1957), S. macrurus and S. bennetti. Prior to this study, both Ho & Bashirullah (1977) and Ho & Lin (2004) placed the latter two species in Caligus, based largely on their possession of a sternal furca. The possession of an elongate abdomen is the only character that unites these five species, and there seems no justification for recognising this as a generic level character given that abdomen length is variable within other caligid genera, including Caligus, and the removal of these five species would simply leave Caligus as a paraphyletic taxon. We consider that there is no justification for maintaining Sciaenophilus as a distinct genus and that its species should all be classified within Caligus. These are not new combinations since all have at some time previously been considered as species of Caligus. We reach this conclusion after consideration of the morphological evidence, but we note that Hayes et al. (submitted) have reached the same conclusion after analysis of new molecular sequence data.
References
Boxshall, G. A. (1990). The skeletomusculature of siphonostomatoid copepods, with an analysis of adaptive radiation in structure of the oral cone. Philosophical Transactions of the Royal Society, London B, 328, 167–212.
Boxshall, G. A., & Halsey, S. (2004). An Introduction to Copepod Diversity. London, Ray Society, 966 pp.
Capart, A. (1941). Copepoda parasitica. V. Résultats scientifiques des croisières du Navire-École belge “Mercator”. Mémoires du Musée Royal d’Historie Naturelle de Belgique, 2, 171–197.
Capart, A. (1959). Copépodes parasites. Résultats Scientifiques de l’Expédition Océanographique Belge dans les Eaux Côtieres Africaines de l’Atlantique Sud (1948-1949). Institut Royale des Sciences Naturelles de Belgique, 3, 55–126.
Causey, D. L. (1953). Parasitic Copepoda from Grand Isle, Louisiana. Occasional Papers of the Marine Laboratory, Louisiana State University, 7, 1–18.
Causey, D. L. (1955). Parasitic Copepoda from Gulf of Mexico fish. Occasional Papers of the Marine Laboratory, Louisiana State University, 9, 1–19.
Causey, D. L. (1960). Parasitic Copepoda from Mexican coastal fishes. Bulletin of Marine Science of the Gulf and Caribbean, 10(3), 323–337.
Demirkale, I., Özak, A., Yanar, A., & Boxshall, G. A. (2014). Caligus solea n. sp. (Copepoda: Caligidae) parasitic on the common sole Solea solea (Linnaeus) from the north-eastern Mediterranean off the Turkish coast. Systematic Parasitology, 89, 23–32.
Dojiri, M., & Ho, J.-S. (2013). Systematics of the Caligidae, Copepods Parasitic on Marine Fishes. Crustaceana Monographs, 18. Leiden & Boston: Brill, 448 pp.
Felgenhauer, B. (1987). Techniques for preparing crustaceans for scanning electron microscopy. Journal of Crustacean Biology, 7, 71–76.
Froese, R., & Pauly, D. (Eds) (2016). FishBase. World Wide Web electronic publication. Available at: www.fishbase.org (accessed April 2016).
Hayes, P., Justine, J.-L., & Boxshall, G. A. (2012). The genus Caligus Müller, 1785 (Copepoda: Siphonostomatoida): two new species from reef associated fishes in New Caledonia, and some nomenclatural problems resolved. Zootaxa, 3534, 21–39.
Heegaard, P. (1966). Parasitic copepods from Texas. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening i Kjøbenhavn, 129, 187–197.
Heller, C. (1865). Crustaceen. Reise der osterreichischen Fregatte Novara um die Erde in den Jahren 1857, -58, 59 unter den Befehlen des Commodore B. Von Wüllerstorf-Urbair. Zoolgischer Theil, 2(3), 1–280.
Ho, J.-S., & Bashirullah, A. (1977). Two species of caligid copepods (Crustacea) parasitic on marine fishes of Venezuela, with discussion of Metacaligus Thomsen, 1949. Journal of Natural History, 11, 703–714.
Ho, J.-S., & Lin, C. (2004). Sea Lice of Taiwan: Copepoda, Siphonostomatoida, Caligidae. Keelung, Taiwan: The Sueichan Press, 388 pp.
Johnson, S. C., Treasurer, J. W., Bravo, S., Nagasawa, K., & Kabata, Z. (2004). A review of the impact of parasitic copepods on marine aquaculture. Zoological Studies, 43, 229–243.
Kabata, Z. (1979). Parasitic Copepoda of British Fishes. London: Ray Society, 468 pp.
Müller, O. F. (1785). Entomostraca, seu Insecta testacea quae in aquis Daniae et Novegiae resperit. XI. Caligus. Leipzig and Copenhagen, pp. 128–134.
Özak, A., Demirkale, İ., Boxshall, G. A., & Etyemez, M. (2013). Parasitic copepods of the common sole, Solea solea (L.), from the Eastern Mediterranean coast of Turkey. Systematic Parasitology, 86, 173–185.
Özak, A. A., Demirkale, İ., & Yanar, A. (2012). First record of two species of parasitic copepods on immigrant Pufferfishes (Tetraodontiformes: Tetraodontidae) caught in the eastern Mediterranean sea. Turkish Journal of Fisheries and Aquatic Sciences, 12, 751–760.
Prabha, C., & Pillai, N. K. (1983). Additions to the copepods parasitic on the marine fishes of India. 1. On twelve species of caligids. Records of the Zoological Survey of India Occasional Paper, 46, 1–46.
Raibaut, A., Combes, C., & Benoit, F. (1998). Analysis of the parasitic copepod species richness among Mediterranean fish. Journal of Marine Systems, 15(1–4), 185–206.
van Beneden, P. J. (1852). Note sur quelques parasites d’un poisson rare sur nos cotes (le Maigre d’Europe, Sciaena aquila Cuv). Bulletin de l’Académie Royale des Sciences, des Lettres et des Beaux-Artes de Belgique, 19, 98–109.
Yamaguti, S. (1963). Parasitic Copepoda and Branchiura of Fishes. New York: Wiley Interscience, 1104 pp.
Acknowledgements
We would like to thank Assoc. Prof. Dr. Kasım Ocakoglu, Head of Advanced Technologies Research & Application Center (MEITAM) of the University of Mersin, Turkey, for his administrative support. We also would like to thank Prof. Suphan Karaytug and Ms. Seher Kuru from Mersin University, Turkey, for their technical help and comments during the SEM and LM studies.
Funding
This study was funded by the Cukurova University Academic Research Projects Unit (SUF2013BAP2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Özak, A.A., Yanar, A. & Boxshall, G.A. The discovery of Caligus macrurus Heller, 1865 (Copepoda: Caligidae) in the Mediterranean Sea, and the recognition of Sciaenophilus van Beneden, 1852 as a junior synonym of Caligus Müller, 1785. Syst Parasitol 94, 97–109 (2017). https://doi.org/10.1007/s11230-016-9682-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-016-9682-4