Abstract
A new species of parasitic copepod, Caligus fajerae n. sp. (Caligidae), is described from Scomberomorus sierra Jordan & Starks (Scombridae) caught off the northwestern coast of Mexico. The new species morphologically resembles Caligus cybii Bassett-Smith, 1898, Caligus kanagurta Pillai, 1961, Caligus pelamydis Krøyer, 1863 and Caligus robustus Bassett-Smith, 1898, all of which have been reported from scombrid hosts. Caligus fajerae n. sp. differs from these species by having spinules on the abdomen and caudal ramus, two processes on the proximal antennulary segment, fine striations on the claw of the antenna and maxilliped, a stouter and more recurved maxillulary dentiform process, shorter tines on the sternal furca, two additional patches of spinules on the distal endopodal segment of leg 2, a sclerotised lobe on the anteromedian surface of the leg 3 protopod and serrations on both margins of the first exopodal spine of leg 3. Analysis of the DNA sequences of the mitochondrial cytochrome c oxidase subunit 1 gene for Caligus fajerae n. sp. and 28 congeners, including C. pelamydis and C. robustus, showed that the new species grouped with Caligus belones Krøyer, 1863 (with 20% divergence), a species known to occur predominantly on needlefishes. Caligus fajerae n. sp. is the fifth species of Caligus reported from S. sierra. An updated host-parasite list for Caligus spp. on scombrids is provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasitic copepods of the family Caligidae Burmeister, 1835 are frequently found on marine and brackish water fishes. Caligids have been intensively studied given the negative impact that some species have on finfish aquaculture, mostly salmoniculture, in different parts of the world (Johnson et al., 2004; Costello, 2009). There are about 490 described species of caligids classified in 30 genera (Dojiri & Ho, 2013; Özak et al., 2017), of which Caligus Müller, 1785 is the most speciose genus with approximately 250 species. In their comprehensive studies of parasitic copepods on fishes of the family Scombridae (tunas and mackerels), an important group in commercial and sports fisheries (Collette 2001), Cressey & Cressey (1980) and Cressey et al. (1983) reported a total of 17 Caligus species from 45 host taxa (Table 1). Nine additional species have been recorded subsequently from various scombrids, bringing the total number of Caligus spp. from tunas and mackerels to 26 (Table 1). During a recent parasitological survey of the Pacific sierra Scomberomorus sierra Jordan & Starks (Scombridae) occurring off the north-western Pacific coast of Mexico, specimens of an undescribed species of Caligus were collected. This paper provides a detailed description of the new species based on adult females, as well as comparisons of the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene sequence of the new species with that of selected congeners.
Materials and methods
A total of 109 Pacific sierra, caught and landed at Mazatlan Port on the north-western coast of Mexico, were purchased and then transferred in an icebox to the Aquatic Parasitology Laboratory at the Centro de Investigación en Alimentación y Desarrollo, Unidad Mazatlán (CIAD-Mazatlán), Sinaloa, Mexico, for parasitological examination. Copepod specimens were all collected from the hosts’ body surface and were fixed and preserved in 96% ethanol. Selected specimens were later cleared in lactophenol for about 1 h before dissection of the appendages on a slide under an Olympus SZ61 dissection microscope. The body parts and appendages were mounted on slides in lactophenol and examined under a Leica DMLB compound microscope with a series of magnifications up to 1000×. All drawings were made with the aid of a drawing tube attached to the compound microscope. Measurements were made using an ocular micrometer, and are given in millimeters as the range followed by the mean in parentheses. The type-material was deposited in the Colección de Parásitos de Peces del Noroeste del Pacífico (CPPNP) at CIAD- Mazatlán, Sinaloa, Mexico. Fish taxonomy and classification used herein follow FishBase (Froese & Pauly, 2017).
Two copepods were fixed in 96% ethanol and kept at 4°C until DNA extraction. Genomic DNA was extracted with the Animal and Fungi Preparation kit (Jena Bioscience, Jena, Germany). Primers used for the amplification reaction of the barcode region of the cox1 gene through the Polymerase Chain Reaction (PCR) were LCO1490 (5′-GGT CAA CAA ATC ATA AAG ATA TTG G-3′) and HCO2198 (5′-TAA ACT TCA GGG TGA CCA AAA AAT CA-3′) (Folmer et al., 1994). Amplification reactions contained 0.12 μl of Biolase Taq Polymerase (Bioline, London, UK), 3 μl of 5× My Taq reaction buffer, 0.25 μl of each primer, 2 μl of template DNA and 9.38 μl of water to reach 15 μl. Reactions were accomplished with an Arktik™ Thermal Cycler (Thermo Fisher Scientific, Waltham, USA) using an amplification protocol of 94°C for 4 min, followed by 35 cycles of 94°C for 40 s, 48°C for 45 s, 72°C for 45 s and a final extension at 72°C for 7 min. Amplification reactions were conducted at the Laboratorio Nacional de Biodiversidad, Instituto de Biología, Universidad Nacional Autónoma de México. DNA sequences of complementary strands were edited and assembled using the software Geneious 5.1.7 (Biomatters Ltd. Auckland, New Zealand). Newly generated sequences were aligned together with cox1 sequences for 28 species of the genus Caligus and Lepeophtheirus salmonis (Krøyer, 1837) (Caligidae) used as the outgroup (Table 2). Sequence alignment was performed in MUSCLE through the European Bioinformatics Institute webpage (http://www.ebi.ac.uk/Tools/msa/muscle/). The final data matrix included 36 terminals and 495 aligned nucleotides. A neighbor joining (NJ) analysis was performed in PAUP* 4.0 using the Kimura 2-parameter algorithm. Finally, cox1 sequences were used to calculate genetic distances between the sequences with the Kimura 2-parameter algorithm in PAUP* 4.0. GenBank accessions of cox1 sequences generated for the new species are given in Table 2.
Order Siphonostomatoida Thorell, 1859
Family Caligidae Burmeister, 1835
Genus Caligus Müller, 1785
Caligus fajerae n. sp.
Type-host: Scomberomorus sierra Jordan & Starks (Scombridae), Pacific sierra.
Type-locality: off Mazatlan Port (23°12′N, 106°26′W), Mexican Pacific, in the State of Sinaloa, Mexico.
Type-material: Holotype female (CPPNP 1369) and 6 paratype females (CPPNP 1370–1372).
Site on host: Upper body surface.
Prevalence: 43%.
Representative DNA sequences: MF069191 and MF069192
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Caligus fajerae n. sp. is urn:lsid:zoobank.org:act:C1D8F27D-9C11-43A5-9286-310F16515C21.
Etymology: The species is named in honor of Dr Emma Josefina Fajer Avila (CIAD, Mazatlán, Sinaloa, Mexico) for her work on fish parasitology.
Description (Figs. 1–2)
Adult female. [Based on 10 specimens.] Body (Fig. 1A) 4.6–5.2 (4.9) long, excluding setae on caudal ramus. Cephalothoracic shield longer than wide, 1.9–2.4 × 1.5–1.6 (2.1 × 1.5), excluding marginal hyaline membranes. Fourth pediger 2.4 times wider than long. Genital complex bell-shaped, 1.25 times longer than wide. Abdomen composed of 2 indistinctly separated somites, 1–1.3 (1.2) long in total, and 0.6 times as long as cephalothorax; proximal somite nearly 2 times wider and 4 times longer than distal (anal) somite; latter with patch of tiny spinules near posteroventral margin (Fig. 1B). Caudal ramus (Fig. 1B) 1.5 times longer than wide, with patch of tiny spinules on proximolateral corner, setules on median edge, and 3 short and 3 long, plumose setae.
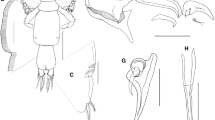
Caligus fajerae n. sp., adult female. A, Habitus, dorsal view; B, Right caudal ramus, ventral view; C, Left antennule, ventral view; D, Right antenna (A2) and postantennal process (PAP), ventral view; E, Mandible; F, Left maxillule, ventral view; G, Right maxilla, anterior view; H, Left maxilliped, posterior view; I, Sternal furca, ventral view. Scale-bars: A, 1 mm; B–I, 0.1 mm
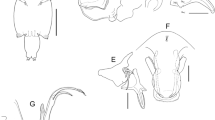
Caligus fajerae n. sp., adult female. A, Right leg 1 with detail of apical elements on second exopodal segment, anterior view; B, Right leg 2, anterior view; C, Left leg 3 with detail of first exopodal segment, ventral view; D, Right leg 4, ventral view; E, Left leg 5, ventral view. Scale-bars: A–D, 0.1 mm; E, 0.05 mm
Antennule (Fig. 1C) 2-segmented; proximal segment larger than distal segment, with proximal conical process, bifid process on posterodistal corner, and 23 plumose and 4 naked setae; distal segment with 1 subterminal seta on posterior margin and 11 setae plus 2 aesthetascs on distal margin. Antenna (Fig. 1D) 3-segmented, situated on pedestal; first segment (coxa) unarmed; second segment (basis) stout, subquadrate; terminal segment (endopod) a curved claw bearing 1 small, naked seta in proximal region and another one in middle region plus fine striations at tip. Postantennal process (Fig. 1D) small, subtriangular, with 2 papillae each bearing 3 sensilla; another similar sensilla-bearing papilla located posterior to tip of process. Mandible (Fig. 1E) composed of 4 sections, with 12 teeth on medial margin of distal blade and hyaline membrane on distal half of outer margin. Maxillule (Fig. 1F) comprising basal papilla with 3 unequal, naked setae and short, recurved dentiform process with tapered tip. Maxilla (Fig. 1G) 2-segmented, brachiform; proximal segment (lacertus) large, unarmed; distal segment (brachium) slender, carrying small subterminal hyaline membrane (flabellum) on inner edge, and short, finely serrated canna and long, finely serrated calamus distally. Maxilliped (Fig. 1H) subchelate, 3-segmented; proximal segment (corpus) robust, with tiny hyaline process on inner subdistal margin; middle segment (shaft) longer than distal segment (claw), with tiny hyaline process midway on posterior surface; claw with small, naked basal seta and fine striations at tip. Sternal furca (Fig. 1I) with subquadrate box and short, pointed tines.
Legs 1 to 3 (Fig. 2A–C) biramous; leg 4 (Fig. 2D) uniramous. Armature on rami of legs 1–4 as follows (Roman and Arabic numerals indicating spines and setae, respectively):
Exopod | Endopod | |
|---|---|---|
Leg 1 | I-0; III+1, 3 | vestigial |
Leg 2 | I-1; I-1; II, I, 5 | 0-1; 0-2; 6 |
Leg 3 | I-0; I-1; II, I, 4 | 0-0; 0-1; 6 |
Leg 4 | I-0; I-0; III | absent |
Leg 1 (Fig. 2A) intercoxal sclerite naked and elongate. Protopod with 1 long, outer plumose seta, 1 small, inner plumose seta and 1 outer papilla bearing 2 setules. Exopod 2-segmented; first segment elongate, with inner row of setules and small, outer distal spine furnished with tiny membrane at its base; second segment carrying 3 unequal apical spines each with inner row of teeth and pectinate membrane at base, 1 naked apical seta (as long as third spine) and 3 large, inner plumose setae. Endopod represented by small lobe tipped with 2 tiny processes.
Leg 2 (Fig. 2B) intercoxal sclerite subquadrate, with large hyaline membrane along distal margin. Coxa with large, inner plumose seta and 1 long setule on anterior surface. Basis with small, outer naked seta, 1 inner setule and hyaline membrane on outer posterior surface and along inner edge. Exopod 3-segmented; first segment with 1 large, outer serrated spine, 1 inner plumose seta, inner row of setules, pectinate membrane at base of outer spine and hyaline membrane on outer posterior surface; second segment with 1 large, serrated outer spine, 1 pore on anterior surface, inner row of setules and 1 inner plumose seta; third segment with 2 small outer spines (proximal spine with row of tiny teeth; distal spine with row of tiny teeth and flange along both margins), 1 long apical spine furnished with membrane along outer margin and setules along inner margin and 5 inner plumose setae. Endopod 3-segmented; first segment with long inner plumose seta; second segment with numerous fine spinules on outer surface, inner row of setules and 2 long, inner plumose setae; third segment with outer patch of fine spinules, 2 patches of short denticles on anterior surface, short inner row of setules and 6 plumose setae.
Leg 3 (Fig. 2C) protopod (apron) with 1 small, outer plumose seta, 1 long, inner plumose seta, 1 anteromedian sclerotised lobe, corrugated surface along outer proximal margin, 1 longitudinal and 1 patch of spinules near outer margin, 2 posteromedian setules and hyaline membranes on outer and posterior margins. Exopod 3-segmented; first segment with several sensilla and pectinate membrane at base of long (almost as long as second segment) serrated spine; second segment with outer row of setules, 1 short outer spine and 1 long, plumose inner seta; terminal segment with outer row of setules, 3 short outer spines and 4 plumose setae. Endopod 3-segmented; first segment forming broad, well developed velum fringed with setules along posterior margin; second segment with long, inner plumose seta; third segment with outer row of setules and 6 plumose setae.
Leg 4 (Fig. 2D) protopod large, with small plumose seta at outer distal corner. Exopod 3-segmented; first segment with sensillum on mid-lateral margin and conspicuous pectinate membrane at base of long, serrated outer spine; second segment ornamented with conspicuous pectinate membrane along posterior margin and armed with long, serrated outer spine; distal segment with 3 serrated spines (innermost spine longer than both middle and outermost spines) and pectinate membrane at base of each spine.
Leg 5 (Fig. 2E) represented by unisetose papilla and trisetose lobe on posterolateral margin of genital complex.
Molecular results
The cox1 sequences successfully obtained from the two specimens of Caligus fajerae n. sp. were identical and grouped with Caligus belones Krøyer, 1863 based on the NJ analysis (Fig. 3). Nonetheless, the genetic divergence between the new species and C. belones is 20.69%. Caligus robustus and C. pelamydis are morphologically similar to C. fajerae n. sp. (see below), but they did not group with the new taxon and genetically differ from it in 19.43 and 21.19%, respectively. In general, the genetic divergence between Caligus fajerae n. sp. and the 26 congeners included in the alignment ranged between 22–24%.
Neighbor-joining phylogram showing relatedness among cox1 gene sequences for species of Caligus and Lepeophtheirus (see Table 2 for details)
Discussion
Among the more than 250 species of Caligus considered valid, Caligus fajerae n. sp. resembles Caligus cybii Bassett-Smith, 1898, Caligus kanagurta Pillai, 1961, Caligus pelamydis Krøyer, 1863 and Caligus robustus Bassett-Smith, 1898 by having: (i) a long, indistinctly 2-segmented abdomen; (ii) no accessory process on the proximal segment of the antenna; (iii) a postantennal process composed of a broad base and short tip; (iv) no accessory process on the three apical spines of leg 1; (v) spinules on the last two endopodal segments of leg 2 and on the ventral surface of the leg 3 protopod; and (vi) a 3-segmented leg 4 exopod with an armature of I-0; I-0; III and furnished with a conspicuous pectinate membrane at the base of each exopodal spine. With regard to the aforementioned fourth character, we note here that Ho & Lin (2007) described two of the three apical spines of leg 1 as being bifid in their single adult female specimen of C. robustus collected from the bigeye trevally Caranx sexfasciatus Quoy & Gaimard captured off Taiwan. Whether this feature represents intra- or interspecific variation remains to be determined. Caligus fajerae n. sp. can be readily distinguished from C. cybii, C. kanagurta, C. pelamydis and C. robustus by the presence of a cluster of spinules on the posteroventral surface of the abdomen and on the anteroventral surface of the caudal ramus, a proximal conical process and posterodistal bifid process on the proximal antennulary segment, fine striations on the tip of the antennal and maxillipedal claw, a stouter and more recurved maxillulary dentiform process, shorter tines on the sternal furca, two additional patches of spinules on the distal endopodal segment of leg 2, a sclerotised lobe on the anteromedian surface of the leg 3 protopod and serrations on both margins of the first exopodal spine of leg 3.
Our molecular analysis revealed that C. fajerae n. sp. grouped with C. belones, and then with a group formed by C. gurnardi Krøyer, 1863, C. elongatus von Nordmann, 1832 and C. longirostris Heegaard, 1962. This grouping was unexpected because the latter four species were collected from non-scombrid hosts from either off Norway or Tasmania (Table 2), and possess a relatively short unsegmented abdomen and a 2-segmented exopod on leg 4 unlike C. fajerae n. sp. In contrast, C. pelamydis and C. robustus, two species morphologically similar to C. fajerae n. sp. and reported from scombrid hosts as noted above, grouped together separately from C. fajerae n. sp. based on the NJ analysis. Unfortunately, no cox1 sequences of C. cybii and C. kanagurta are yet available in public repositories, making the evaluation of their shared morphological characters with the new species (e.g. small postantennal process, indistinctly 2-segmented abdomen and 3-segmented exopod on leg 4) not possible at this time. As cox1 sequences have been obtained for a small fraction of Caligus spp., increased taxon sampling is needed to improve our understanding of the phylogenetic relationships within Caligus.
Four species of Caligus have been reported previously from Scomberomorus sierra: C. omissus Cressey & Cressey, 1980 from off Mexico, Panama, Colombia, Ecuador and Peru (Cressey & Cressey 1980; Morales-Serna et al., 2012, 2015) and C. mutabilis Wilson, 1905, C. productus Dana, 1852 and C. serratus Shiino, 1965 from off Mexico (Causey 1960; Morales-Serna et al., 2013). Pillai (1985) reported C. productus from S. sierra from off India, but the host identity is most likely erroneous as S. sierra is distributed from southern California to Chile (Froese & Pauly, 2017). In this study, C. omissus and C. fajerae n. sp. were found frequently on S. sierra, the former on the gills and the latter on the skin.
Thirty-two species of Caligus have been reported hitherto from Mexico, of which 23 are from the Mexican Pacific and three from the Mexican Caribbean (Morales-Serna et al., 2012, 2014; Suárez-Morales & Gasca, 2016). Therefore, the discovery of C. fajerae n. sp. represents the 33rd species of Caligus recorded from Mexico and the 24th species of Caligus for the Pacific Ocean off Mexico.
References
Andrews, M., Bott, N., Battaglene, S., & Nowak, B. (2009). A new species of copepod (Siphonostomatoida: Caligidae) parasitic on the striped trumpeter, Latris lineata (Forster), from Tasmania. Zootaxa, 1971, 59–68.
Causey, D. (1960). Parasitic Copepoda from Mexican coastal fishes. Bulletin of Marine Science, 10, 323–337.
Collette, B. B. (2001). Scombridae. Tunas (also, albacore, bonitos, mackerels, seerfishes, and wahoo). In: Karpenter, K. E. & Niem, V. H. (Eds), FAO species identification guide for fishery purposes. The living marine resources of the Western Central Pacific, vol. 6. Bony fishes, part 4 (Labridae to Latimeriidae). Rome: FAO, pp. 3721–3756.
Costello, M. J. (2009). The global economic cost of sea lice to the salmonid farming industry. Journal of Fish Diseases, 32, 115–118.
Cressey, R. (1991). Parasitic copepods from the Gulf of Mexico and Caribbean Sea, III: Caligus. Smithsonian Contributions to Zoology, 497, 1–53.
Cressey, R. F., & Collette, B. B. (1970). Copepods and needlefishes: a study in host-parasite relationships. Fishery Bulletin, 68, 347–432.
Cressey, R., & Cressey, H. B. (1980). Parasitic copepods of mackerel- and tuna-like fishes (Scombridae) of the world. Smithsonian Contributions to Zoology, 311, 1–186.
Cressey, R. F., Collette, B. B., & Russo, J. L. (1983). Copepods and scombrid fishes: A study in host-parasite relationships. Fishery Bulletin, 81, 227–265.
Cressey, R. F., & Nutter, P. (1987). Reidentification of David Causey’s Caligus collections (Crustacea: Copepoda). Proceedings of the Biological Society of Washington, 100, 600–602.
Dojiri, M., & Ho, J.-S. (2013). Systematics of the Caligidae, Copepods Parasitic on Marine Fishes. Crustaceana Monographs, 18. Leiden: Brill.
Folmer, O., Black, M., Hoeh, W., Lutz, R., & Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294–299.
Freeman, M. A., Anshary, H., & Ogawa, K. (2013). Multiple gene analyses of caligid copepods indicate that the reduction of thoracic appendage in Pseudocaligus represents convergent evolution. Parasites & Vectors, 6, 336.
Froese, R., & Pauly, D. (Eds) (2017). FishBase. World Wide Web electronic publication. Retrieved April 8, 2017, from http://www.fishbase.org, version (02/2017).
González, M. T., Castro, R., Muñoz, G., & López, Z. (2016). Sea lice (Siphonostomatoida: Caligidae) diversity on littoral fishes from the south-eastern Pacific coast determined from morphology and molecular analysis, with description of a new species (Lepeophtheirus confusum). Parasitology International, 65, 685–695.
Hayward, C. J., Aiken, H. M., & Nowak, B. F. (2008). An epizootic of Caligus chiastos on farmed southern bluefin tuna Thunnus maccoyii off South Australia. Diseases of Aquatic Organisms, 79, 57–63.
Hayward, C. J., Bott, N. J., & Nowak, B. F. (2009). Seasonal epizootics of sea lice, Caligus spp., on southern bluefin tuna, Thunnus maccoyii (Castelnau), in a long-term farming trial. Journal of Fish Diseases, 32, 101–106.
Ho, J.-S., & Lin, C.-L. (2004). Sea lice of Taiwan (Copepoda: Siphonostomatoida: Caligidae). Keelung: Sueichan Press.
Ho, J.-S., & Lin, C.-L. (2007). Three species of Caligus Müller, 1785 (Copepoda: Caligidae) parasitic on Caranx spp. (Teleostei: Carangidae) off Taiwan. Systematic Parasitology, 68, 33–43.
ICZN (2012). International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bulletin of Zoological Nomenclature, 69, 161–169.
Johnson, S. C., Treasurer, J. W., Bravo, S., Nagasawa, K., & Kabata, Z. (2004). A review of the impact of parasitic copepods on marine aquaculture. Zoological Studies, 43, 229–243.
Kim, I.-H. (1998). Illustrated encyclopedia of fauna & flora of Korea. Vol. 38. Cirripedia, symbiotic Copepoda, Pycnogonida. Seoul: Ministry of Education.
Lin, C.-L., & Ho, J.-S. (2007). Six species of sea lice (Copepoda, Caligidae) new to Taiwan. Journal of the Fisheries Society of Taiwan, 34, 41–67.
McBeath, A. J. A., Penston, M. J., Snow, M., Cook, P. F., Bricknell, I. R., & Cunningham, C. O. (2006). Development and application of real-time PCR for specific detection of Lepeophtheirus salmonis and Caligus elongatus larvae in Scottish plankton samples. Diseases of Aquatic Organisms, 73, 141–150.
Morales-Serna, F. N., Caña-Bozada, V., Mera-Loor, G., Loor-Andrade, P., Fajer-Ávila, E. J., & Ho, J.-S. (2015). New records of sea lice (Copepoda: Caligidae) from marine fishes in Jaramijó, an area with potential for sea-cage aquaculture in Ecuador. Zootaxa, 3920, 366–380.
Morales-Serna, F. N., Gómez, S., & Pérez-Ponce de León, G. (2012). Parasitic copepods reported from Mexico. Zootaxa, 3234, 43–68.
Morales-Serna, F. N., Hernández-Inda, Z. L., Gómez, S., & Pérez-Ponce de León, G. (2013). Redescription of Caligus serratus Shiino, 1965 (Copepoda: Caligidae) parasitic on eleven fish species from Chamela Bay in the Mexican Pacific. Acta Parasitologica, 58, 367–375.
Morales-Serna, F. N., Pinacho-Pinacho, C. D., Gómez, S., & Pérez-Ponce de León, G. (2014). Diversity of sea lice (Copepoda: Caligidae) parasitic on marine fishes with commercial and aquaculture importance in Chamela Bay, Pacific coast of Mexico by using morphology and DNA barcoding, with description of a new species of Caligus. Parasitology International, 63, 69–79.
Nagasawa, K. (2011). Caligus macarovi (Copepoda, Caligidae) from Pacific bluefin tuna, Thunnus orientalis, cultured in Japan. Crustaceana, 84, 1145–1147.
Nowak, B. F., Hayward, C. J., González, L., Bott, N. J., & Lester, R. J. G. (2011). Sea lice infections of salmonids farmed in Australia. Aquaculture, 320, 171–177.
Øines, Ø., & Heuch, P. A. (2005). Identification of sea louse species of the genus Caligus using mtDNA. Journal of the Marine Biological Association of the UK, 85, 73–79.
Øines, Ø., & Schram, T. (2008). Intra- or inter-specific differences in genotypes of Caligus elongatus Nordmann 1832. Acta Parasitologica, 53, 93–105.
Özak, A. A., Yanar, A., & Boxshall, G. A. (2017). The discovery of Caligus macrurus Heller, 1865 (Copepoda: Caligidae) in the Mediterranean Sea, and the recognition of Sciaenophilus van Beneden, 1852 as a junior synonym of Caligus Müller, 1785. Systematic Parasitology, 94, 97–109.
Parker, R. R. (1965). A review and redescription of Caligus gurnardi Krøyer, 1863 (Copepoda, Caligidae). Crustaceana, 9, 93–103.
Pillai, N. K. (1985). Fauna of India: Parasitic copepods of marine fishes. Calcutta: Technical and General Press.
Prabha, C., & Pillai, N. K. (1986). Additions to the copepods parasitic on the marine fishes of India. 4. On twenty six species of caligids. Records of the Zoological Survey of India Occasional Paper, 79, 1–139.
Suárez-Morales, E., & Gasca, R. (2016). A new species of Caligus Müller, 1785 (Copepoda: Siphonostomatoida: Caligidae) from coral reef plankton in the Mexican Caribbean. Zootaxa, 4174, 424–436.
van der Elst, R. P., & Collette, B. B. (1984). Game fishes of the east coast of southern Africa. 2. Biology and systematics of the queen mackerel Scomberomorus plurilineatus. Investigational Report. Oceanographic Research Institute, Durban, 55, 1–12.
Venmathi Maran, B. A., Cruz-Lacierda, E. R., Ohtsuka, S., & Nagasawa, K. (2016). New records of Caligidae (Copepoda, Siphonostomatoida) from the Philippines. Zootaxa, 4174, 237–248.
Williams, E. H., & Bunkley-Williams, L. (1996). Parasites of offshore big game fishes of Puerto Rico and the western Atlantic. Mayaguez: Antillean College Press.
Acknowledgements
We thank Rosa María Medina Guerrero for her help with fish examination.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Additional information
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as 98EA29B0-9D6F-4336-A3E6-9DC3841C5949. This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number. This is the Version of Record.
This article is part of the Topical Collection Arthropoda.
Rights and permissions
About this article
Cite this article
Morales-Serna, F.N., Oceguera-Figueroa, A. & Tang, D. Caligus fajerae n. sp. (Copepoda: Caligidae) parasitic on the Pacific sierra Scomberomurus sierra Jordan & Starks (Actinopterygii: Scombridae) in the Pacific Ocean off Mexico. Syst Parasitol 94, 927–939 (2017). https://doi.org/10.1007/s11230-017-9752-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-017-9752-2