Abstract
A systematic study on the effect of extra hydrogen bonding (H-bonding) and water chain on ground-state multiple proton transfer (GSMPT) in 2-aminopyridine (2AP) complexes bonded with 1–3 water molecules was explored at the M06-2X/6-311+G(d, p) level. In 2AP-(H2O)n+m (n = 1–3, m = 1) complexes, n and m represented the number of water molecules which involved in PT process and formed an extra H-bonding with 2AP-(H2O)1–3, respectively. The analyses of structural parameter and correlation plot indicated that the H-bonded chain did not influence the GSPT mechanism, but affected the barrier height of GSPT. The H-bonded chain composed of water dimer in 2AP complex is more favorable to PT process than that of one water or water trimer. However, the extra H-bonding formed at different region had different effects on GSPT process. Evidently, when the extra H-bonding is formed in the proton donating and proton accepting regions, the concerted but asynchronous solvolysis pathway is not changed, but the asynchronicity of proton transfer is enlarged. When the extra H-bonding is formed at the bridging water region, 2AP-(H2O)3 maintains the concerted but asynchronous solvolysis pathway to occur GSPT, while 2AP-H2O undergo GSPT process in a concerted but asynchronous protolysis pathway. In addition, the extra H-bond formed in the proton accepting region is favorable to GSPT process. The GSPT mechanisms in 2AP-(H2O)1+1(B) and 2AP-(H2O)2+1(B1) in water change from protolysis pattern to solvolysis pattern. The solvation effect augments the asynchronicity of the concerted pathway and decreases the barrier height of GSPT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proton transfer (PT) occurring in the bifunctional organic compounds with proton donating group and accepting group is broadly existed in life and chemical processes [1,2,3,4,5]. The structure of bifunctional compound itself determines the type of PT process (intramolecular or intermolecular PT). The intramolecular PT (IntraPT) can take place along the strong intramolecular hydrogen bonding (H-bonding) formed between proton donor and proton acceptor within one compound. The intermolecular PT (InterPT) usually occurs with the assistance of bridging molecule such as protic solvent (e.g., H2O, CH3OH, NH3, etc.) which can connect the proton donor and proton acceptor by intermolecular H-bonding [6,7,8,9]. The solvent-mediated InterPT process has aroused great interest and been widely investigated on the reaction mechanism which may give more useful information to interpret and control the chemical and biological processes [10,11,12,13,14,15,16].
2-Aminopyridine (2AP) is a suitable example to study proton transfer reaction, which has a proton donor (N-H) and a proton acceptor (aromatic N) and can be used as structural minic of pyrimidine bases. And its dimer has been used as model for investigating the hydrogen-bonded (H-bonded) DNA base pairs [17,18,19,20,21,22,23,24]. Hager and Wallace [25, 26] investigated the complexes of 2AP with H2O and NH3 by using multiphoton ionization and photoionization spectroscopy. Wu et al. have studied the clusters of 2AP with one and two water molecules by R2PI and IR/R2PI spectroscopy, as well as ab initio calculation [27]. They reported that one water molecule forms a very strong H-bond with the aromatic nitrogen and a second H-bond with the amino group. While two water molecules form a H-bonded water dimer bridge between amino N and the aromatic N. Both the complexes 2AP-(H2O)1-2 displayed cyclic H-bonded structures at very low temperatures. And the H-bond to the aromatic N atom is stronger in the 2AP-(H2O)2 than in the 2AP-H2O complex. Wu et al. also investigated the electronic and vibrational spectra of 2AP-NH3 complex experimentally and theoretically [28].
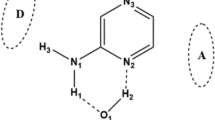
However, solvent-mediated ground state multiple proton transfer (GSMPT) process and the effect of the extra hydrogen bonding (H-bonding) which is not directly involved in PT process in 2AP molecule have not been investigated. Based on this, we studied the GSMPT process in the 2AP complexes with 1–3 water molecule(s) by using density functional theory (DFT) method. Since the proton donating (N-H) and accepting (aromatic N) groups in the 2AP molecule are too far, protic water molecule is chosen as the bridging molecule to form an H-bonded chain between proton donor and proton acceptor, and to participate in proton transfer process. The structures of 2AP with 1–3 bridging water molecules are stable. If the H-bonded chain is formed by more than three bridging molecules, the H-bonded chain will be too flexible to maintain the chain-like structure which would be favorable to proton transfer process. Furthermore, in order to study the effect of the extra H-bonding on GSPT process, the fourth water molecule is introduced by the extra H-bonding (see Fig. 1). The main goal in this work is to demonstrate the effect of water chain and extra H-bonding on the structure, GSMPT mechanism and activation energy in water-mediated 2AP complexes. We hope our theoretical studies may provide much useful information for the development and utilization of compounds with PT features in the future.
Computational details
All the studies were carried out by using M06-2X [29] functional and 6-311+G (d,p) basis set in Gaussian 09 program [30]. The ground-state structures of the stationary points (reactant (R), transition state (TS), and product (P)) along the proton transfer potential curve in the 2AP-(H2O)n+m (n = 1–3, m = 1) clusters were completed optimized. The optimized structures of all the stationary points were verified by frequency calculations at the same computational level. Both reactant and product have no imaginary frequency, and the TS structure has only one imaginary frequency. All the TS structures were confirmed by intrinsic reaction coordinate (IRC) calculations. We also performed polarizable continuum model calculations by using the integral equation formalism (IEFPCM) [31,32,33] to study the solvent effect on GSPT process in water.
The TS property during proton transfer process can be well presented by the correlation plot between the proton transfer coordinate and the H-bond distance. In the X–H. . .Y complex, under the assumption that the sum of bond orders is conserved (nXH + nYH = 1), the distances of rXH and rYH correlate with each other, and are in accordance with Pauling equations [34]:
where rXH0/rYH0 is the bond distance in the free XH/YH, and bXH/bYH is the parameter denoting to the decreasing bond valence. The relationship between rXH and rYH in X–H. . .Y complex can be described by the H-bond coordinates q1 = 1/2(rXH-rYH) and q2 = rXH + rYH [35, 36]. For a linear H-bond, q1 is the distance from H to the H-bonding center, and q2 is the distance from X to Y.
Results and discussion
In this work, the complex between 2AP and water molecule is denoted as 2AP-(H2O)n+m (n = 1–3, m = 1), where n represents the number of bridging water molecule involving in PT process, and m represents the number of water molecule forming the extra H-bonding between H2O and 2AP-(H2O)n. As shown in Fig. 1, the extra H-bonding can be formed in three regions (D/A/B), where D, A, and B denote to the proton donating region, proton accepting region and bridged water region, respectively. In order to clearly mark the position where the additional H-bond is formed, D/A/B is labeled in parenthesis (e.g., 2AP-(H2O)n+m(D), 2AP-(H2O)n+m(A) and 2AP-(H2O)n+m(B)).
GSDPT mechanism in 2AP-H2O and 2AP-(H2O)1+1
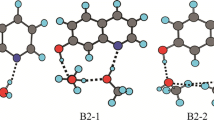
At first, we investigated the ground state double proton transfer process (GSDPT) in the 2AP-H2O and 2AP-(H2O)1+1 complexes. For the 2AP-H2O and 2AP-(H2O)1+1 complexes, the bridging water molecule connects the proton donor (amino N-H) and proton acceptor (aromatic N atom) of 2AP via intermolecular H-bond, and forms a cyclic six-membered H-bonded ring structure. Except the bridging water molecule, 2AP-(H2O)1+1 complex has an extra H-bonding forming between the second water and 2AP-H2O. Two stable 2AP-(H2O)1+1 complexes are obtained and denoted as 2AP-(H2O)1+1(D) and 2AP-(H2O)1+1(B), respectively. In the 2AP-(H2O)1+1(D) and 2AP-(H2O)1+1(B) complexes, the extra H-bonding is formed between water and another N1-H group, and bridging water in 2AP-H2O, respectively. The extra H-bond distances of the reactants and products in the optimized 2AP-(H2O)1+1(D) and 2AP-(H2O)1+1(B) complexes are 2.047 Å and 1.940 Å, which are displayed in Supporting Information (Figs. S1 and S2). The fully optimized TS structures of 2AP-H2O and 2AP-(H2O)1+1 were shown in Fig. 2, and some structural parameters involving in proton transfer process were listed in Table 1. For the 2AP-H2O, 2AP-(H2O)1+1(D) and 2AP-(H2O)1+1(B) complexes, there is only one TS but no intermediate obtained during the GSDPT process.
For the 2AP-H2O cluster, N1-H1, H1-O1, O1-H2 and H2-N2 distances in TS are 1.234 Å, 1.262 Å, 1.357 Å, and 1.167 Å, respectively. N1-H1 distance is 0.123 Å shorter than the O1-H2 distance, which indicates that H2 proton starts the GSDPT process via transferring more than halfway from O1 to N2. At the same time, N1-H1 distance is 0.028 Å shorter than H1-O1 distance, and O1-H2 distance is 0.190 Å longer than H2-N2 distance, which means that H1 moves less than halfway from N1 to O1 and generates an OH–-like portion at O1. A concerted but asynchronous GSDPT process in 2AP-H2O occurs in a solvolysis [37, 38] pattern, in which proton moves firstly from the bridging water molecule to the aromatic N, afterwards the bridging water molecule obtains the proton by deprotonation of N-H group. The Mulliken charge of the OH–-like moiety of the TS in 2AP-H2O (see Table 1) is − 0.526, which proves the asynchronous solvolysis pathway in 2AP-H2O.
For the 2AP-(H2O)1+1(D), the N1-H1, H1-O1, O1-H2, and H2-N2 distances in the TS are 1.217 Å, 1.285 Å, 1.410 Å, and 1.138 Å, respectively. N1-H1 distance is 0.193 Å shorter than the O1-H2 distance, which indicates that H2 proton moves first via transferring more than halfway from O1 to N2, and H1 moves less than halfway from N1 to O1, and generates an OH–-like portion at O1. GSDPT process in 2AP-(H2O)1+1(D) also takes place in a concerted but asynchronous solvolysis [37, 38] pattern. However, GSDPT process in 2AP-(H2O)1+1(B) prefers to occur in a concerted but asynchronous protolysis [37, 38] pattern, in which the proton shifts from N-H group to the bridging water molecule firstly, following the aromatic N atom obtains a proton from the bridging water. As shown in the TS of 2AP-(H2O)1+1(B), N1-H1, H1-O1, O1-H2 and H2-N2 distances in the TS are 1.345 Å, 1.166 Å, 1.230 Å, and 1.268 Å, respectively. N1-H1 distance is 0.179 Å longer than H1-O1 distance, and O1-H2 distance is 0.038 Å shorter than H2-N2 distance, which indicates that H1 proton moves first via transferring more than halfway from N1 to O1, H2 moves less than halfway from O1 to N2, and produces a H3O+-like portion at O1. The Mulliken charges of the OH–-like and H3O+-like moiety of the TS (see Table 1) in the 2AP-(H2O)1+1(D) and 2AP-(H2O)1+1(B) are − 0.575 and 0.619, respectively, which proves the asynchronous solvolysis and protolysis pathway in 2AP-(H2O)1+1(D) and 2AP-(H2O)1+1(B), respectively. It is obvious that the extra H-bonding formed in the different position can adjust the GDSPT process.
We can figure out the property of TS, such as earliness or lateness, synchronicity and bond order during PT process, in the correlation plot between the proton transfer coordinate and the H-bond distance. When H transfers from X to Y in the X-H. . .Y complex, q1 changes from negative to positive and q2 positions at q1=0 after going through a minimum. A positive or negative q1 value means a late or an early TS, respectively. For the multiple proton transfer process, the multiple similar or different q1 values of TS mean the synchronous or asynchronous mechanism. For the 2AP-H2O, 2AP-(H2O)1+1(D) and 2AP-(H2O)1+1(B) complexes, the correlations between N1-H1 and H1-O1 distances (H1 transfer), and O1-H2 and H2-N2 distances (H2 transfer) are showed in Fig. 3. The reactant (R), transition state (TS) and product (P) of 2AP-H2O, 2AP-(H2O)1+1(D) and 2AP-(H2O)1+1(B) are all at or near to the Pauling line, which demonstrates that the total bond orders at all stationary points are conserved. As shown in Fig. 3, the q1 values of H1 and H2 transfer at the TS in the 2AP-H2O, 2AP-(H2O)1+1(D) are very close to zero and a little positive, respectively, which indicates that H1 is almost in the center between N1 and O1, and H2 is close to N2. For 2AP-(H2O)1+1(B), the q1 values of H1 and H2 transfer at the TS are a little positive and very close to zero, respectively, which indicates that H1 is close to O1, and H2 is almost in the center between O1 and N2. These results prove that GSDPT in the 2AP-H2O, 2AP-(H2O)1+1(D) and 2AP-(H2O)1+1(B) occur in a highly asynchronously concerted pattern.
Correlation of the H-bond distances, q2 = r1 + r2, with the proton transfer coordinate, q1= 1/2(r1-r2), for the 2AP-H2O and 2AP-(H2O)1+1complexes in the gas phase (a) and in water (b). Top: H1 transfer; bottom: H2 transfer. All points are for the reactant (R), transition state (TS) and product (P) at the M06-2X/6-311+G(d, p) level. The solid lines designate the correlation that satisfies conservation of the bond order. The parameters for Pauling equations were from the literature [35]
The barrier heights (ΔV) and ground state tautomerization energies (ΔE) of the GSDPT in the 2AP-H2O, 2AP-(H2O)1+1(D) and 2AP-(H2O)1+1(B) complexes were listed in Table 2. The tautomerization energies of the 2AP-H2O complexe are in the range of 10.8~12.6 and 11.2~12.8 kcal/mol without and with zero-point energy (ZPE) correction, respectively. The GSDPT reaction for the 2AP-H2O complex is endothermic. The barrier heights of GSDPT in the 2AP-H2O complex are in the range of 21.5~22.3 and 17.9~18.6 kcal/mol without and with zero-point energy (ZPE) correction, respectively.
GSTPT mechanism in 2AP-(H2O)2 and 2AP-(H2O)2+1
The structures of 2AP-(H2O)2 and 2AP-(H2O)2+1 are fully optimized at the M06-2X/6-311+G(d,p) level. In the 2AP-(H2O)2 complex, an eight-memberred cyclic H-bonded structure was formed, and triple proton can transfer along the H-bonded water dimer chain. In addition, two extra H-bondings can be formed between 2AP-(H2O)2 and the third water molecule, and four stable 2AP-(H2O)2+1 complexes are obtained and denoted to 2AP-(H2O)2+1(D), 2AP-(H2O)2+1(A), 2AP-(H2O)2+1(B1) and 2AP-(H2O)2+1(B2). Their fully optimized TS structures were shown in Fig. 4, and some primary structural parameters were listed in Tables 3 and 4. The extra H-bond distances of the reactants and products in the optimized 2AP-(H2O)2+1(D), 2AP-(H2O)2+1(A), 2AP-(H2O)2+1(B1) and 2AP-(H2O)2+1(B2) are displayed in Supporting Information (Figs. S1 and S2). For the 2AP-(H2O)2+1(D) complex, one extra H-bonding between the third water and another N1-H group is 2.408 Å, the other extra H-bonding between the third water and O1 atom is 1.807 Å. For the 2AP-(H2O)2+1(A) complex, the two extra H-bonds are 2.198 Å and 1.761 Å, respectively, which forms between the third water molecule and the C-H group next to aromatic N, and between the third water molecule and the O2 atom, respectively. For the 2AP-(H2O)2+1(B1) complex, one extra H-bonding between the third water and N1 atom is 2.018 Å, the other extra H-bonding between the third water and the O2-H group of bridging water is 1.974 Å. For the 2AP-(H2O)2+1(B2) complex, the two extra H-bonds formed between the third water and the two bridging water molecules are 2.006 Å and 1.872 Å, respectively. For the 2AP-(H2O)2 and 2AP-(H2O)2+1 complexes, there is only one TS structure obtained and confirmed by IRC calculation. The ground state triple proton transfer (GSTPT) mechanism of 2AP-(H2O)2 and 2AP-(H2O)2+1 can be explored by analyzing the structural parameters of TS.
For the 2AP-(H2O)2 complex, N1-H1, H1-O1, O1-H2, H2-O2, O2-H3, and H3-N2 distances in TS are 1.186 Å, 1.310 Å, 1.244 Å, 1.159 Å, 1.380 Å, and 1.141 Å, respectively. N1-H1 distance is 0.194 Å shorter than the O2-H3 distance, which indicates that H3 proton starts the GSTPT process via transferring more than halfway from O2 to N2. At the same time, N1-H1 distance is 0.124 Å shorter than H1-O1 distance, O1-H2 distance is 0.085 Å longer than H2-O2 distance, O2-H3 distance is 0.239 Å longer than H3-N2 distance. These results indicate that H1 moves less than halfway from N1 to O1, H2 moves more than halfway from O1 to O2, and H3 moves much more than halfway from O2 to N2, which generates an OH–-like portion at O1 atom. GSTPT process in 2AP-(H2O)2 occurs in a concerted but asynchronous solvolysis [37, 38] pathway.
For the 2AP-(H2O)2+1(D) cluster, N1-H1, H1-O1, O1-H2, H2-O2, O2-H3, and H3-N2 distances in the TS are 1.123 Å, 1.418 Å, 1.395 Å, 1.068 Å, 1.537 Å, and 1.077 Å, respectively. N1-H1 distance is 0.295 Å shorter than H1-O1 distance, O1-H2 distance is 0.327 Å longer than H2-O2 distance, O2-H3 distance is 0.460 Å longer than H3-N2 distance. It is obvious that H3 transfers firstly and moves much more than halfway from N1 to O1, and H2 and H1 moves subsequently, which generates an OH–-like portion at O1 atom. A concerted but highly asynchronous GSTPT process in 2AP-(H2O)2+1(D) occurs in a solvolysis manner. The GSTPT process occurring in the 2AP-(H2O)2+1(A) complex also prefers in a concertedly asynchronous solvolysis [37, 38] pathway. As shown in Table 3, N1-H1 distance in the TS is 0.167 Å shorter than H1-O1 distance, O1-H2 distance is 0.051 Å longer than H2-O2 distance, O2-H3 distance is 0.538 Å longer than H3-N2 distance. This result indicates that H3 starts the PT process and a OH–-like moiety at O1 atom as part of TS appears, which proves the concertedly asynchronous solvolysis mechanism exists in the 2AP-(H2O)2+1(A) complex. The Mulliken charges of the OH–-like moiety at O1 atom in 2AP-(H2O)2, 2AP-(H2O)2+1(D), 2AP-(H2O)2+1(A) (see Table 3) is − 0.544, − 0.695, and − 0.555, respectively, which proves the asynchronous solvolysis pathway in the above complexes.
When the third water molecule is connected to the bridging water, the GSTPT process in the two complexes 2AP-(H2O)2+1(B2) and 2AP-(H2O)2+1(B1) take place in a highly concertedly asynchronous solvolysis and provolysis [37, 38] pathway, respectively. For the 2AP-(H2O)2+1(B2) complex, N1-H1, H1-O1, O1-H2, H2-O2, O2-H3, and H3-N2 distances in the TS are 1.282 Å, 1.198 Å, 1.150 Å, 1.261 Å, 1.508 Å, and 1.087 Å, respectively. N1-H1 distance is 0.084 Å longer than H1-O1 distance, O1-H2 distance is 0.111 Å shorter than H2-O2 distance, O2-H3 distance is 0.421 Å longer than H3-N2 distance. H3 starts the GSTPT process and a OH–-like moiety at O2 appears, which means a concerted but highly asynchronous solvolysis pathway exists in 2AP-(H2O)2+1(B2). However, for the 2AP-(H2O)2+1(B1) complex, N1-H1, H1-O1, O1-H2, H2-O2, O2-H3 and H3-N2 distances in the TS are 1.336 Å, 1.159 Å, 1.304 Å, 1.120 Å, 1.111 Å and 1.410 Å, respectively. N1-H1 distance is 0.225 Å longer than O2-H3 distance, which indicates that H1 triggers the PT process and moves more than halfway from N1 to O1 and generates a H5O2+-like moiety as part of TS. GSTPT process in 2AP-(H2O)2+1(B1) obviously occurs in a concerted but highly asynchronous protolysis manner. The Mulliken charges of the OH–-like moiety at O2 atom in 2AP-(H2O)2+1(B2) and H2O5+-like moiety as the part of TS in 2AP-(H2O)2+1(B1) are –0.647 and 0.724, respectively, which provides an evidence that the GSTPT processes in 2AP-(H2O)2+1(B2) and 2AP-(H2O)2+1(B1) undergo in an asynchronous solvolysis and protolysis pathway, respectively.
The correlation between q1 and q2 for the GSTPT in 2AP-(H2O)2, 2AP-(H2O)2+1(D), 2AP-(H2O)2+1(A), 2AP-(H2O)2+1(B1), and 2AP-(H2O)2+1(B2) complexes are presented in Fig. 5. The reactant, TS and product of 2AP-(H2O)2, 2AP-(H2O)2+1(D), 2AP-(H2O)2+1(A), 2AP-(H2O)2+1(B1) and 2AP-(H2O)2+1(B2) are all at or near the Pauling line, which demonstrates that the total bond orders at all stationary points are conserved. As shown in Fig. 5, the q1 values of H1, H2 and H3 transfer at the TS in the 2AP-(H2O)2, 2AP-(H2O)2+1(D), and 2AP-(H2O)2+1(A) are a little negative, near zero/a little positive and much positive, respectively, which indicates that H1 is close to N1, H2 is almost in the center between N1 and O1 or near O2, and H3 is close to N2. For 2AP-(H2O)2+1(B2), the q1 values of H1, H2 and H3 at the TS are near zero, a little negative and much positive, respectively, which means that H1 and H2 are close to O1, and H3 is close to N2. For 2AP-(H2O)2+1(B1), the q1 values of both H1 and H2 at the TS are a little positive and much positive, respectively, and the q1 values of H3 transfer are much negative, which indicates that H1 is close to O1, H2 is close to O2, and H3 is also close to O2. These results prove that GSTPT in the 2AP-(H2O)2, 2AP-(H2O)2+1(D), 2AP-(H2O)2+1(A), 2AP-(H2O)2+1(B1), and 2AP-(H2O)2+1(B2) occur in a highly asynchronously concerted manner.
Correlation of the H-bond distances, q2 = r1 + r2, with the proton transfer coordinate, q1= 1/2(r1-r2), for the 2AP-(H2O)2 and 2AP-(H2O)2+1complexes in the gas phase (a) and in water (b). Top: H1 transfer; bottom: H2 transfer. All points are for the reactant (R), transition state (TS) and product (P) at the M06-2X/6-311+G(d, p) level. The solid lines designate the correlation that satisfies conservation of the bond order. The parameters for Pauling equations were from the literature [35]
The barrier heights (ΔV) and ground state tautomerization energies (ΔE) of the GSTPT in the 2AP-(H2O)2, 2AP-(H2O)2+1(D), 2AP-(H2O)2+1(A), 2AP-(H2O)2+1(B1), and 2AP-(H2O)2+1(B2) complexes were listed in Table 2. The tautomerization energies of the 2AP-(H2O)2 and 2AP-(H2O)2+1 complexes are in the range of 6.49~12.3 and 7.02~12.4 kcal/mol without and with zero-point energy (ZPE) correction, respectively. The GSTPT reaction for the 2AP-(H2O)2 and 2AP-(H2O)2+1 complexes are endothermic. The barrier heights of GSTPT in these complexes are in the range of 14.9~18.8 and 11.3~14.8 kcal/mol without and with zero-point energy (ZPE) correction, respectively.
GSQPT mechanism in 2AP-(H2O)3 and 2AP-(H2O)3+1
A H-bonded bridge consisting of three water molecules was formed to connect proton donor and proton acceptor of 2AP, which produced a 10-membered cyclic 2AP-(H2O)3 complex. The fully optimized TS structures were shown in Fig. 6, and some primary structural parameters were listed in Table 5. In the 2AP-(H2O)3 complex, with the help of bridging water trimer chain, quadruple proton transfer may occur. In the TS, N1-H1, H1-O1, O1-H2, H2-O2, O2-H3, H3-O3, O3-H4 and H4-N2 distances are 1.130 Å, 1.396 Å, 1.169 Å, 1.227 Å, 1.317 Å, 1.102 Å, 1.460 Å, and 1.104 Å, respectively. N1-H1 distance is 0.266 Å shorter than H1-O1 distance, O1-H2 distance is 0.058 Å shorter than H2-O2 distance, O2-H3 distance is 0.215 Å longer than H3-O3 distance, O3-H4 distance is 0.356 Å longer than H4-N2 distance. At the same time, N1-H1 distance is 0.330 Å shorter than O3-H4 distance. All these results indicate that H4 moves firstly from O3 to N2 and triggers the whole proton transfer process, subsequently H3, H2, and H1 transfer, and generates a OH–-like moiety at O2 atom. GSQPT process in the 2AP-(H2O)3 complex takes place in a concerted but asynchronous solvolysis [37, 38] pattern. The Mulliken charges of the OH–-like moiety of the TS (see Table 5) in 2AP-(H2O)3 is − 0.616. This result verifies that the GSQPT process in 2AP-(H2O)3 occur in a asynchronous solvolysis pathway.
2AP-(H2O)3 can form an extra H-bonding with the fouth water molecule via another N1-H group, C-H group next to aromatic N and bridging water molecules, then 2AP-(H2O)3+1(D), 2AP-(H2O)3+1(A) and 2AP-(H2O)3+1(B) complexes are obtained. The extra H-bond distance of the reactant in the optimized 2AP-(H2O)3+1(D), 2AP-(H2O)3+1(A) and 2AP-(H2O)3+1(B) are displayed in Supporting Information (Fig. S1). In the TS of 2AP-(H2O)3+1(D) and 2AP-(H2O)3+1(A) and 2AP-(H2O)3+1(B) complexes, N1-H1 distance is average 0.491 Å shorter than O3-H4 distance, which means that GSQPT process in the above complexes occur in a concerted but highly asynchronous solvolysis [37, 38] pathway. H4 Proton triggers the GSQPT process, the other protons shift successively. In the TS of 2AP-(H2O)3+1(B) complex, N1-H1 distance is 0.456 Å and 0.444 Å shorter than O2-H3 and O3-H4 distances, respectively. This result means that GSQPT process in 2AP-(H2O)3+1(B) occur in a concerted but highly asynchronous solvolysis pathway, which is triggered by H3 proton, and H4, H2 and H1 protons moves subsequently. For 2AP-(H2O)3+1(D), 2AP-(H2O)3+1(A) and 2AP-(H2O)3+1(B), OH–-like moieties at O2 atom as part of TS appear. The Mulliken charges of OH–-like moiety of 2AP-(H2O)3+1(D), 2AP-(H2O)3+1(A) and 2AP-(H2O)3+1(B) have proved their GSPT mechanism.
For the GSQPT processes of 2AP-(H2O)3, 2AP-(H2O)3+1(D), 2AP-(H2O)3+1(A), and 2AP-(H2O)3+1(B) complexes, the correlation between q1 and q2 are showed in Fig. 7. The reactants, TSs and products of 2AP-(H2O)3, 2AP-(H2O)3+1(D), 2AP-(H2O)3+1(A) and 2AP-(H2O)3+1(B) are all at or near to the Pauling line, which demonstrates that the total bond orders at all stationary points are conserved. As shown in Fig. 7, the q1 values of H1, H2, H3 and H4 transfer at the TS in the above complexes are a little negative, very close to zero, a little positive and much positive, respectively, which indicates that H1 is near to N1, H2 is close to O1, H3 is close to O3 and H4 is N2. These results prove that GSQPT in the 2AP-(H2O)3, 2AP-(H2O)3+1(D), 2AP-(H2O)3+1(A), and 2AP-(H2O)3+1(B) complexes occur in a highly asynchronously concerted pattern.
Correlation of the H-bond distances, q2 = r1 + r2, with the proton transfer coordinate, q1= 1/2(r1-r2), for the 2AP-(H2O)3 and 2AP-(H2O)3+1complexes in the gas phase (a) and in water (b). Top: H1 transfer; bottom: H2 transfer. All points are for the reactant (R), transition state (TS), and product (P) at the M06-2X/6-311+G(d, p) level. The solid lines designate the correlation that satisfies conservation of the bond order. The parameters for Pauling equations were from the literature [35]
The barrier heights (ΔV) and ground state tautomerization energies (ΔE) of the GSQPT in the 2AP-(H2O)3, 2AP-(H2O)3+1(D), 2AP-(H2O)3+1(A), and 2AP-(H2O)3+1(B) complexes were listed in Table 2. The tautomerization energies of these complexes are in the range of 7.90~9.88 and 8.19~9.71 kcal/mol without and with zero-point energy (ZPE) correction, respectively. The GSQPT reactions for the 2AP-(H2O)3 and 2AP-(H2O)3+1 complexes are endothermic. The barrier heights of GSQPT in these complexes are in the range of 17.8~21.0 and 13.6~16.2 kcal/mol without and with zero-point energy (ZPE) correction, respectively.
The effect of H-bonded water chain on GSPT
Since the proton donor (N-H) and proton acceptor (aromatic N) of 2AP is not close enough, a H-bonded bridge composed of 1–3 water molecules is necessary to 2AP to occur GSPT process. Hence, 2AP-(H2O)1–3 complexes with cyclic H-bonded structure were obtained. For these complexes, the length of H-bonded chain would affect the GSPT process. After comparing the results of GSPT in the 2AP-(H2O)1–3 complexes, it can be found that the GSPT processes in the 2AP-(H2O)1–3 complexes all prefer to occur in a concerted but asynchronous solvolysis pathway. In addition, some differences of GSPT processes in the 2AP-(H2O)1–3 complexes are discovered. Obviously, the H-bond parameters in the 2AP-(H2O)1–3 complexes are influenced by the H-bonded water chain. For the 2AP-H2O complex, the H-bond distances are 2.038 Å and 1.924 Å for H1-O1 and H2-N2 in the reactant, respectively. The H-bond N1-H1...O1 and O1-H2...N2 bond angles are 144.6° and 151.5°, respectively. When the H-bonded chain is composed of water dimer, the H-bond H1-O1, H2-O2 and H3-N2 distances of 2AP-(H2O)2 in the reactant are 1.924 Å, 1.792 Å and 1.800 Å, respectively. The corresponding H-bond N1-H1...O1, O1-H2...O2, and O2-H3...N2 bond angles are 178.8°, 157.4°, and 177.3°, respectively. For the 2AP-(H2O)3 complex with the H-bonded chain consisted of water trimer, the H-bond H1-O1, H2-O2, H3-O3, and H4-N2 distances in the reactant are 1.918 Å, 1.779 Å, 1.744 Å, and 1.781 Å, respectively. The corresponding H-bond N1-H1...O1, O1-H2...O2, O2-H3...O3, and O3-H4...N2 bond angles are 163.2°, 166.2°, 167.0°, and 169.0°, respectively. It is interesting to note that the H-bond angles in the 2AP-(H2O)2 are almost linear, those in the 2AP-H2O complex are much more bent, and those in 2AP-(H2O)3 are in the middle. Linear H-bonds are generally stronger than bent H-bonds, which would be more prone to GSPT process. Hence, the GSPT process would be easier for 2AP-(H2O)2 than 2AP-H2O and 2AP-(H2O)3, which have been proved by their barrier heights. As shown in Table 2, the GSPT barrier height with ZPE correction in 2AP-(H2O)2 is 4.00 kcal/mol and 2.30 kcal/mol lower than those in 2AP-H2O and 2AP-(H2O)3, which indicates that the H-bonded chain formed with water dimer in 2AP complex is more favorable to PT process than that with one water or water trimer.
The effect of extra H-bonding on GSPT
The dynamics of GSPT process are influenced by the H-bonding strength. Adding an extra H-bonding in the cyclic 2AP-(H2O)1–3 complex can adjust the H-bonding strength, and then have an effect on the GSPT process. The effect of an extra H-bonding at different position in the 2AP-(H2O)1–3 complexes on GSPT process can be obtained by comparing the results in the 2AP-(H2O)1–3 complexes to those complexes without extra H-bonding.
For the 2AP-H2O complex, the extra H-bonding in 2AP-(H2O)1+1(B) can change the GSPT mechanism from solvolysis pattern to protolysis pattern, while the extra H-bonding in 2AP-(H2O)1+1(D) maintains the GSPT process to occur in a solvolysis pathway, but a little enlarges the asynchronicity of proton transfer. As shown in the correlation plot (see Fig. 3), the q1 values of H1 transfer at the TS in the 2AP-H2O and 2AP-(H2O)1+1(D) are very close to zero, but the corresponding q1 values of H2 transfer in 2AP-(H2O)1+1(D) is more positive than that in 2AP-H2O. These differences existing in the correlation plot come from the changes on the TS structures due to the extra H-bonding, and expand the asynchronicity of GSDPT process. Furthermore, the barrier height of GSDPT process was also affected by the extra H-bonding. The ZPE-corrected barrier heights of 2AP-(H2O)1+1(D) and 2AP-(H2O)1+1(B) are 0.70 kcal/mol and 0.30 kcal/mol higher than that of 2AP-H2O, respectively. Both the extra H-bondings with another N1-H group and bridging water are not favorable to GSPT process.
For the 2AP-(H2O)2 complex, the extra H-bonding in 2AP-(H2O)2+1(D) and 2AP-(H2O)2+1(A) can not affect the GSPT mechanism, but enlarge the asynchronicity of proton transfer. As shown in Fig. 5, the q1 values of H1 and H3 transfer at the TS in the 2AP-(H2O)2+1(D) and 2AP-(H2O)2+1(A) are more negative and positive, respectively, with the comparison to those corresponding values in 2AP-(H2O)2, which means that the asynchronicity of GSTPT is expanded. When the extra H-bonding formed with the two bridging water molecules, the 2AP-(H2O) 2+1(B2) complex is obtained, and its GSTPT process occurs in a solvolysis pattern. When the extra H-bonding formed with the bridging water and the N1-H group, 2AP-(H2O) 2+1(B1) complex is generated, and the GSTPT process in this complex takes place in a protolysis manner. Furthermore, the extra H-bonding also adjusts the barrier height of GSTPT process. The ZPE-corrected barrier height of 2AP-(H2O)2+1(A) is 2.60 kcal/mol lower than that of 2AP-(H2O)2, and those values of 2AP-(H2O)2+1(D), 2AP-(H2O)2+1(B2) and 2AP-(H2O)2+1(B1) are 0.90 kcal/mol, 0.80 kcal/mol and 0.40 kcal/mol higher than that of 2AP-(H2O)2. These results indicate that the extra H-bond in the proton accepting region would promote GSTPT process in 2AP-(H2O)2.
For the 2AP-(H2O)3 complex, by forming an extra H-bonding at the proton donating region, proton accepting region and bridging water region, respectively, three new complexes 2AP-(H2O)3+1(D), 2AP-(H2O)3+1(A), and 2AP-(H2O)3+1(B) are obtained. The extra H-bonding can not affect the GSQPT mechanism of 2AP-(H2O)3 complex. The 2AP-(H2O)3+1(D), 2AP-(H2O)3+1(A), and 2AP-(H2O)3+1(B) all take place proton transfer in a solvolysis pattern. But the asynchronicity of GSQPT in 2AP-(H2O)3 is also enlarged by the extra H-bonding. As shown in Fig. 7, the H1 and H4 correlation points for TS in the 2AP-(H2O)3+1 are close to each other and move more to the upper-right side, respectively, with the comparison to those corresponding values in 2AP-(H2O)3. This result indicates that the asynchronicity of GSQPT process in 2AP-(H2O)3+1 is enlarged due to the changes on TS structures. In addition, the extra H-bonding also can alter the barrier height of GSQPT in 2AP-(H2O)3. For the 2AP-(H2O)3+1(D), 2AP-(H2O)3+1(A) and 2AP-(H2O)3+1(B) complexes, the GSQPT barrier height is 0.5 kcal/mol, 2.6 kcal/mol, and 0.3 kcal/mol lower than that in 2AP-(H2O)3, respectively. It is obvious that all the extra H-bondings formed in the 2AP-(H2O)3 complex help to accelerate the proton transfer process.
The effect of solvation
In order to consider solvent effect on the mechanism of PT process, we carried out IEFPCM calculations for the reactant, TS and product in water. The optimized structures of all the stationary points were also confirmed by the frequency calculations. Some optimized structural parameters for 2AP-(H2O)n+m (n = 1–3, m = 1) complexes in water are listed in Tables S2, S3 and S4, respectively, and the corresponding structures of reactant and product are shown in Figs. S3 and S4 in the Supporting Information. The optimized TS structures are displayed in Fig. 8. For the 2AP-(H2O)n+m (n = 1–3, m = 1) complexes, their structures of reactant and product in water are similar to those in the gas phase. Only one TS but no intermediate in 2AP-(H2O)n+m (n = 1–3, m = 1) are obtained in water during GSPT process. All the GSPT processes in 2AP-(H2O)n+m (n = 1–3, m = 1) occur in a concerted but asynchronous solvolysis manner, which are the same to those in gas except the GSPT mechanisms in 2AP-(H2O)1+1(B) and 2AP-(H2O)2+1(B1). The solvent effect changes the GSPT mechanisms in 2AP-(H2O)1+1(B) and 2AP-(H2O)2+1(B1) from protolysis pattern to solvolysis pattern. The correlations between q1 and q2 for proton transfer in the 2AP-(H2O)n+m (n = 1–3, m = 1) complexes are displayed in Figs. 3b, 5b, and 7b, respectively. It is useful to deeply understand the solvation effect on the structures after comparing Figs. 3b, 5b, and 7b with Figs. 3a, 5a, and 7a, respectively. It is evident that the correlation points at the TS rely greatly on the solvent effect. For 2AP-H2O and 2AP-(H2O)1+1 complexes, the correlation points at the TS of H1 and H2 move a little to the right and left side along the Pauling equation line in water, respectively, as shown in Fig. 3b. This result means that the solvent effect increases the asynchronicity of the concerted GSPT mechanism in 2AP-H2O and 2AP-(H2O)1+1 complexes. The enlarged asynchronicity of the concerted GSPT mechanism are also found in 2AP-(H2O)2,3, 2AP-(H2O)2+1 and 2AP-(H2O)3+1 complexes. Furthermore, the barrier heights depend on the solvent effect (Table 6). As shown in Table 6, the barrier heights in water were a little smaller than those in the gas phase. With ZPE-correction, the barrier heights in 2AP-(H2O)1–3, 2AP-(H2O)1+1(D), 2AP-(H2O)3+1(D), and 2AP-(H2O)3+1(A) are lower than the corresponding values in the gas phase. For 2AP-(H2O)1+1(B), 2AP-(H2O)2+1(B1), and 2AP-(H2O)2+1(A), their barrier heights are 0.2~0.4 kcal/mol higher than those in the gas phase. The effect of extra H-bonding on the GSPT barrier in water is the same to that in the gas phase. The extra H-bonding formed in the proton accepting region and in the bridged water region will promote and hinder the GSPT process in water, respectively.
Conclusions
In the present study, the effect of extra H-bonding and water chain on ground-state multiple proton transfer in 2AP complex have been investigated in detail at the M06-2X/6-311+G(d, p) level. The results from the structural parameters and correlation plots showed that the H-bonded water chain did not influence the GSPT mechanism, but affected the barrier height of GSPT. For the 2AP-(H2O)1–3 complexes, proton transfer occurred in a concerted but asynchronous solvolysis pathway. However, the H-bonded chain composed of water dimer in 2AP complex is more favorable to PT process than that of one water or water trimer. When the extra H-bonding is formed in different regions in the 2AP-(H2O)1–3 complexes, the GSPT process would be totally similar or different with comparison to 2AP-(H2O)1–3. Apparently, when the extra H-bonding is formed in the proton donating and proton accepting regions, the concerted but asynchronous solvolysis pathway in 2AP-(H2O)1–3 is not changed, but the asynchronicity of proton transfer is enlarged. When the extra H-bonding is formed in the bridging water region, 2AP-(H2O)3 still undergos GSPT process in a concerted but asynchronous solvolysis pathway, whereas 2AP-H2O takes place GSPT process in a concertedly asynchronous protolysis pathway. In addition, the barrier height of GSPT process is altered by the extra H-bond. For 2AP-H2O, 2AP-(H2O)2, and 2AP-(H2O)3, the extra H-bonding in the proton donating region and bridged water region made the corresponding barrier height of GSPT increase or decrease a little. While the extra H-bond formed in the proton accepting region is benefical for the GSPT process in 2AP-(H2O)1–3. The GSPT mechanisms in 2AP-(H2O)1+1(B) and 2AP-(H2O)2+1(B1) in water change from protolysis pattern to solvolysis pattern. The solvation effect augments the asynchronicity of the concerted pathway and decreases the barrier height of GSPT. The effect of extra H-bonding on the GSPT barrier in water is the same to that in the gas phase.
Data availability
Data can be obtained from the corresponding authors through email.
References
Zhao JF, Dong H, Zheng YJ (2018) Theoretical insights into the excited state double proton transfer mechanism of deep red pigment Alkannin. J Phys Chem A 122(5):1200–1208
Denis LG, Vladimir AK, Kharlanov Robert GB, Wolfgang R (2000) Excited-state intramolecular proton transfer (ESIPT) in 2-(20-hydroxyphenyl)-oxazole and-thiazole. J Photo Photobio A 130:101–111
Goodman J, Brus LE (1978) Proton transfer and tautomerism in an excited state of methyl salicylate. J Am Chem Soc 100:24
Stasyuk AJ, Cyrański MK, Gryko DT, Solà M (2015) Acidic C-H bond as a proton donor in excited state intramolecular proton transfer reactions. J Chem Theory Comput 11(3):1046–1054
Luo X, Yang YF, Li YQ (2020) Theoretical insights into ESIPT mechanism of the two protons system BH-BA in dichloromethane solution. J Mol Liq 319:114154
Zhao GJ, Han KL (2012) Hydrogen bonding in the electronic excited state. Acc Chem Res 45:404–413
Yi J, Fang H (2020) Effect of water on excited-state double proton transfer in 7-azaindole-H2O complex: a theoretical study. J Phys Org Chem 33:e4060
Fang H (2020) A theoretical study on water-assisted excited state double proton transfer process in substituted 2,7-diazaindole-H2O complex. Theor Chem Acc 139:139
Fang H (2015) Theoretical study on excited-state proton transfer via hydrogen-bonded ethanol (EtOH) wire for 7AI in the gas phase. Theor Chem Acc 134:142
Yamabe S, Tsuchida N, Hayashida Y (2005) Reaction paths of the water-assisted neutral hydrolysis of ethyl acetate. J Phys Chem A 109(32):7216–7224
Tortonda FR, Pascul-Ahuir JL, Silica E, Tunon I (1996) Why is glycine a zwitterion in aqueous solution? A theoretical study of solvent stabilising factors. Chem Phys Lett 260(1):21–26
Markova N, Enchev V, Timtchera I (2005) Oxo−hydroxy tautomerism of 5-Fluorouracil: water-assisted proton transfer. J Phys Chem A 109(9):1981–1988
Dkhissi A, Adamowick L, Maes G (2000) Hybrid density functionals and ab initio studies of 2-pyridone-H2O and 2-pyridone-(H2O)2. Chem Phys Lett 324(1):127–136
Kim Y, Lim S, Kim HJ, Kim Y (1999) Theoretical study of the double proton transfer in hydrogen-bonded complexes in the gas phase and in solution: Prototropic tautomerization of formamide. J Phys Chem 103(5):617–624
Tsuchida N, Yambe S (2005) Reaction paths of tautomerization between hydroxypyridines and pyridones. J Phys Chem 109(9):1974–1980
Li QS, Fang WH (2003) Theoretical studies on structures and reactivity of 8-hydroxyquinoline and its one-water complex in the ground and excited states. Chem Phys Lett 367(5):637–644
Müller A, Talbot F, Leutwyler S (2002) Hydrogen bond vibrations of 2-aminopyridine-2-pyridone, a Watson-Crick analogue of Adenine-Uracil. J Am Chem Soc 124(48):14486
Roscioli JR, Pratt DW (2003) Base pair analogs in the gas phase. Proc Natl Acad Sci USA 100(24):13752
Sobolewski AL, Domcke W (2003) Ab initio study of the excited-state coupled electron–proton-transfer process in the 2-aminopyridine dimer. Chem Phys 294(1):73–83
Frey JA, Müller A, Frey HM, Leutwyler S (2004) Infrared depletion spectra of 2-aminopyridine-2-pyridone, a Watson-Crick mimic of adenine-uracil. J Chem Phys 121:8237
Wu R, Brutschy B (2004) Infrared depletion spectroscopy and structure of the 2-aminopyridine dimer. J Phys Chem A 108(45):9715
Schultz T, Samoylova E, Radloff W, I. Hertel V, Sobolewski AL, Domcke W (2004) Efficient deactivation of a model base pair via excited-state hydrogen transfer. Science 306(5702):1765
Samoylova E, Smith VR, Ritze HH, Radloff W, Kabelac M, Schultz T (2006) Ultrafast deactivation processes in Aminopyridine clusters: Excitation energy dependence and isotope effects. J Am Chem Soc 128(49):15652
Alkorta I, Elguero J (2002) Influence of intermolecular hydrogen bonds on the tautomerism of pyridine derivatives. J Org Chem 67:1515–1519
Hager J, Wallace SC (1985) Solvation effects in jet-cooled 2-aminopyridine clusters: excited-state dynamics and two-color threshold photoionization spectroscopy. J Phys Chem 89(18):3833–3841
Hager JW, Leach GW, Demmer DR, Wallace SC (1987) Structure and excited-state dynamics of 2-aminopyridine van der Waals molecules and hydrogen-bonded complexes. J Phys Chem 91(14):3750
Wu RH, Nachtigal P, Brutschy B (2004) Structure and hydrogen bonding of 2-aminopyridine·(H2O)n (n = 1, 2) studied by infrared ion depletion spectroscopy. Phys Chem Chem Phys 6:515–521
Wu RH, Vaupel S, Nachtigall P, Brutschy B (2004) Structure and hydrogen bonding of different isomers of 2-Aminopyridine·NH3 studied by IR/R2PI spectroscopy. J Phys Chem A 108(16):3338–3343
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited-states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Frisch MJ, Truck GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision D.01, Gaussian, Inc, Wallingford CT
Cances E, Mennucci B, Tomasi J (1997) A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys 107:3032–3041
Cossi M, Barone V, Mennucci B, Tomasi J (1998) Ab initio study of ionic solutions by a polarizable continuum dielectric model. Chem Phys Lett 286(3–4):253–260
Mennucci B, Tomasi J (1997) Adsorption isotherm for flexible molecules in random porous media. Can we regard the system as a binary mixture? J Chem Phys 106:5151–5158
Brown ID (1992) Chemical and steric constraints in inorganic solids. Acta Cryst B 48:553–572
Limbach HH, Pietrzak M, Benedict H, Tolstoy PM, Golubev NS, Denisov GS (2004) Empirical corrections for anharmonic zero-point vibrations of hydrogen and deuterium in geometric hydrogen bond correlations. J Mol Struct 706(1):115–119
Limbach HH, Lopez JM, Kohen A (2006) Arrhenius curves of hydrogen transfers: tunnel effects, isotope effects and effects of pre-equilibria. Philos Trans R Soc B 361(1472):1399–1415
Mohammed OF, Pines D, Nibbering ETJ, Pines E (2007) Base-induced solvent switches in acid–base reactions. Agnew Chem Int Ed 46(9):1458
Park SY, Jang DJ (2010) Accumulated proton-donating ability of solvent molecules in proton transfer. J Am Chem Soc 132(1):297–302
Author information
Authors and Affiliations
Contributions
All authors (Guotao Sun and Hua Fang) made substantial contribution.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, G., Fang, H. Extra hydrogen-bonding and water chain altering water-mediated ground-state multiple proton transfer in 2-aminopyridine: a theoretical study. Struct Chem 33, 335–349 (2022). https://doi.org/10.1007/s11224-021-01848-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-021-01848-1