Abstract
The [4+2] cycloaddition reaction of 1,3-dimethylindole and ortho-quinone methide (obtained from tautomerization of 2,6-dimethylquinone under basic conditions), experimentally studied by Wen and co-workers, was theoretically studied at the B3LYP/6-311G** and M062X/6-311G** computational levels in both gas and ethanol solution phases. Two possible reactive channels were considered between the reactants and their theoretical parameters were calculated. The results indicated that the formation of the experimentally reported product is clearly confirmed by the analysis of the calculated Fukui and Parr functions reactivity indices. Transition states analysis showed that the experimentally reported product is both kinetically and thermodynamically preferred than the other regioisomeric adduct. The intrinsic reaction coordinates analysis indicated that in contrast to the proposed stepwise mechanism by Wen and co-workers, the reaction proceeds in one step without formation of any stable intermediate. Analysis of the global electron density transfer (GEDT) showed that the corresponding transition state is relatively polar and the electron density is fluxed from 1,3-dimethylindole toward ortho-quinone methide. Because of the polar character of the transition state, the reaction accelerates in ethanol, as a polar solvent, in comparison to the gas phase. Analysis of the frontier molecular orbitals showed that the HOMO orbital of 1,3-dimethylindole as a donor is also the frontier effective-for-reaction molecular orbital (FERMO). According to the Wiberg bond indexes and atoms in molecules analysis, it was found that both reactive channels take place via an asynchronous concerted mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A reaction in which two molecules combine to produce a cyclic adduct is known as cycloaddition. The [4+2] cycloaddition, known also as Diels-Alder reaction, is one of the most well-known and useful reactions, and is extensively used in chemistry. This reaction takes place when a four-atom component, namely diene, is attacked by an unsaturated bond, namely dienophile, to generate a six-membered homocyclic and heterocyclic compounds usually in a highly regio- and stereoselective fashion [1]. In the transition state relevant to the Diels-Alder reaction, the diene and dienophile approach together in approximately parallel planes. For most systems, the experimental and theoretical studies are in agreement with a concerted mechanism [2]. It has been found that in a reaction that involves unsymmetrical diene and dienophiles, the degree of advancement of bond formation might be different at one pair of termini than that at the other. This situation is indicative of a stepwise asynchronous mechanism along with formation of a zwitterionic intermediate [3]. Usually, the reaction between dienes and dienophiles with a very different electronic character takes place via a stepwise asynchronous mechanism. Alternatively, in some cases which the first single bond is formed between the diene and the dienophile in the rate-determining step, the second bond is barely beginning to form in the transition state. This two-stage mechanism is known as an asynchronous concerted one according to Houk’s definition, in which the formation of two bonds takes place in separate, but overlapping, processes [4].
The solvent plays an important role on the rate of Diels-Alder reactions. In many cases, the rate and mechanism of reaction are sensitive to solvent polarity. For instance, water and other highly polar solvents enhance the rate of some Diels-Alder reactions [5,6,7]. The “enforced hydrophobic interaction” effect is responsible of the accelerating effect of water, in which the strong hydrogen bonding network in water causes to exclude non-polar reactants and closes them together, resulting in the increase of effective concentrations [8]. In some cases, more solvation and consequently more stabilization of transition states than the reactants can contribute to the rate acceleration [9].
Polycyclic indolines, which are one class of nitrogen-containing heterocycles, have received considerable attention because of their ubiquitous presence in natural products and biologically active compounds [10,11,12]. It has proven that certain compounds containing a quinoline-fused indoline core exhibit anticancer and insecticidal activities [13, 14]. Due to various pharmacological activities, the development of efficient synthetic protocols to build such polyheterocyclic skeletons is of interest and many organic researchers have focused their attention on the synthesis of indole alkaloids [15, 16].
In 2017, Wen et al. reported an intermolecular [4+2] cycloaddition reaction between 2,6-dimethylquinone 1a and 1,3-disubstituted indoles 2 in basic conditions for the synthesis of polycyclic indolines 3 (Scheme 1) [17].
The proposed mechanism includes the initial tautomerization of 2,6-dimethylquinone 1a under basic conditions which leads to the formation of ortho-quinone methide QM. Nucleophilic attack of 1,3-disubstituted indoles on ortho-quinone methide QM generates a zwitterionic intermediate which undergoes a ring closure to afford corresponding polycyclic indoline 3 (Scheme 2).
Since reliable theoretical studies can support the experimental outcomes, we have investigated theoretically the [4+2] cycloaddition reactions of 1,3-dimethylindoles and 2,6-dimethylquinone by computational methods in continuous of our previous theoretical studies on various organic compounds [18,19,20,21]. The important aims of this work are as follows:
-
Analysis of global and local reactivity indices at the ground state of the reagents involved in the Diels-Alder reaction of 1,3-dimethylindole toward ortho-quinone methide QM.
-
To elucidate the kinetic and thermodynamic aspects of the reaction.
-
To study the regioselectivity of the reaction.
-
Determination of solvation effects on the reaction mechanism.
-
To study the charge transfer at transition states.
-
To study the bond formation and structural changes during the reaction using atoms in molecules (AIM) analysis.
-
Investigation of the reaction synchronicity.
Results and discussion
Analysis of the global reactivity indices of the reactants
Global reactivity indices, namely chemical potential (μ), chemical hardness (η), global electrophilicity (ω), and global nucleophilicity (N), are one of the important tools to elucidate the reactivity of the reactants and regioselectivity in the cycloaddition reactions [22, 23]. Also, based on the model established by Domingo and co-workers, the polar or non-polar character of a reaction can be evaluated using analysis of the reactivity indices [24]. Thus, to study the reactivity of the reactants and polar character of the reaction, the global reactivity indices were calculated for ortho-quinone methide QM and 1,3-dimethylindole (2a, R1 = H, R2 = R3 = Me) in both gas and ethanol phases which are given in Table 1.
A comparison of the results presented in Table 1 reveals that the electronic chemical potential of 2a is higher than that of QM, which is indicative of fluxing the electron density from 2a toward QM along the corresponding cycloaddition reaction. Also, the calculated values of the nucleophilicity N and electrophilicity ω indices identify that QM and 2a being classified as a strong electrophile and strong nucleophile, respectively. According to Domingo’s model, the polar character of the reactions can be evaluated using calculation of Δω between the reagents [24]. High values of Δω predict fast reactions between the reactants due to the low barriers. In the gas phase, the value of Δω, 4.18 (for B3LYP/6-311G** method) and 2.33 (for M062X/6-311G** method), predicts a high polar reaction between QM and 2a. The same trend is also observed in the solution phase in which a large value of Δω is obtained by using two computational methods (4.24 for B3LYP/6-311G** and 2.32 for M062X/6-311G** method).
Study of the regioselectivity of the reaction
To study the regioselectivity in the title reaction, two possible reactive channels were considered between the reactants, QM and 2a, in which they are added together to generate two regioisomeric adducts 3a and 3a′ (Scheme 3). In the first reactive channels, the Cθ and Cϕ atoms of 2a interact respectively with Cδ and Oα atoms of QM to afford the corresponding cycloadduct 3a. Alternatively, another regioisomer, 3a′, is formed when Cθ and Cϕ atoms of 2a interact respectively with Oα and Cδ atoms of QM during the second reactive channel. As discussed in the “Introduction” section, Wen et al. reported experimentally the regioselective formation of the 3a adduct from cycloaddition reaction between QM and 2a [17].
When a non-symmetric nucleophile/electrophile pair approaches together, the most probable event is that the most nucleophilic center of nucleophile interacts with the most electrophilic center of electrophile. The Fukui functions reactivity indices are one of the powerful tools to study the reactivity of the reactants and regioselectivity. In 1984, Yang and Mortier introduced an approach to calculate the Fukui functions using the variation of the Mulliken population of an atom, qk, which undergoes electrophilic or nucleophilic attack [25]:
the local electrophilicity ωk and local nucleophilicity Nk are calculated from the following equations where ω and N refer to the global electrophilicity and nucleophilicity indices, respectively [26, 27]:
The local reactivity indices, ωk and Nk, can be used satisfactorily to explain the reactivity and regioselectivity in various polar cycloadditions.
To investigate the observed regioselectivity in the reaction between 2a and QM which is responsible for the formation of 3a adduct [17], the local electrophilicity and nucleophilicity indices were calculated using NBO and Hirshfeld and Mulliken population analyses. Since, in this reaction 2a acts as the nucleophile and QM acts as the electrophile, the local nucleophilicity (Nk) of 2a and local electrophilicity (ωk) of QM were calculated. The results are given in Table 2.
Analysis of the local Fukui functions presented in Table 2 indicates that QM is more electrophilically activated at the δ-carbon atom. On the other hand, in contrast to the Mulliken analysis, the NBO and Hirshfeld ones indicate that 2a is more nucleophilically activated at the θ-carbon atom than the ϕ-carbon atom. Accordingly, it is proposed that the most favorable nucleophile-electrophile interaction along the nucleophilic attack of 2a on QM will take place between the δ-carbon atom as the most electrophilic center of QM and the θ-carbon atom as the most nucleophilic center of 2a. This interaction leads to the formation of 3a cycloadduct which is excellently consistent with the experimental reports, because this cycloadduct is the regiospecific product for the title reaction [17]. It is noteworthy that the Mulliken population analysis fails to explain regioselective formation of 3a adduct, because the corresponding results indicate that 2a is more nucleophilically activated at the ϕ-carbon atom. Thus, the corresponding interaction leads to the formation of 3a′ adduct.
An alternative way to study the local reactivity and regioselectivity in polar reactions is based on nucleophilic Pk− and electrophilic Pk+ Parr functions which was recently introduced by Domingo and co-workers [28, 29]. Parr functions are obtained in terms of the variations of spin electron density that originate from electron transfer processes in polar reactions. As mentioned above, the compound 2a acts as the nucleophile (toward QM); therefore, the Pk− Parr functions of 2a and Pk+of QM were calculated. Table 3 summarizes the calculated Parr functions of 2a and QM molecules.
Analysis of the calculated nucleophilic and electrophilic Parr functions presented in Table 3 designates that both Mulliken and Hirshfeld analyses can satisfactorily explain the formation of 3a adduct. It is noteworthy that the most nucleophilic/electrophilic interaction takes place between the δ-carbon as the most electrophilic site of QM and the θ-carbon atom as the most nucleophilic center of 2a which leads to the formation of 3a adduct. So again, the electrophilic and nucleophilic Parr functions give a satisfactory description about the σ-bond formation between QM and 2a fragments.
As an attempt to test the aforementioned local reactivity indices and also to study the regioselectivity, the transition states associated with the two reactive channels between QM and 2a were calculated and detected by using two computational methods. Analysis of the intrinsic reaction coordinates (IRC) profiles associated with the two transition states involved in two reactive channels between QM and 2a evidently revealed that formation of the corresponding cycloadducts 3a as well as 3a′ takes place via a one-step mechanism without generation of any stable intermediate. While Wen et al. believe that the reaction takes place via a stepwise mechanism along with generation of a zwitterionic intermediate (Scheme 2) [17], the IRC analysis ruled out the formation of any stable intermediate in both gas and solution phases.
In order to compare two reactive channels from an energy viewpoint, the thermodynamic and kinetic parameters were calculated from optimized geometries of the reactants, transition states, and products. Table 4 gives the calculated results in both gas and ethanol phases obtained from M062X/6-311G** method. Also, the results obtained from B3LYP/6-311G** method are given in Table S1 of the supporting information.
In order to portray the energetic aspects of the studied reactive channels in a more evident and comparable manner, the corresponding free energies diagrams were plotted for both gas and solution phases which are depicted in Fig. 1.
Analysis of the results presented in Table 4 and Fig. 1 indicates that:
(a) Both reactive channels are possible thermodynamically because the corresponding products lie lower than the reactants. According to kinetic principles, the rate of 3a and 3a′ formation can be considered as k[2a][QM] and k′[2a][QM], respectively, where k and k′ are the second-order rate constants. Consequently, the concentration ratio of 3a to 3a′ in the reaction mixture becomes:
according to the Eyring equation, the rate constant is defined as:
where KB is the Boltzmann constant, h is Plank’s constant, T is the Kelvin temperature, ΔG‡ is the Gibbs free energy of activation, and R is the universal gas constant [30].
Thus, it can be concluded that:
The calculated values of ΔG‡ and ΔG‡′ at the solution phase are 81.13 and 95.31 kJ mol−1, respectively, and the \( \frac{\left[\mathbf{3}\mathbf{a}\ \right]}{\left[\mathbf{3}{\mathbf{a}}^{\prime }\ \right]} \) ratio turn into 305.0. Accordingly, the percentage of 3a becomes 99.66% which indicates that is approximately the only product present in the reaction mixture and the title cycloaddition reaction takes place via a stereospecific fashion to generate 3a.
(b) The reactive channel 1 is more kinetically and themodynamically favored than the reactive channel 2 which is consistent with the experimental outcomes [17]. The reactive channel 1 produces 3a adduct with a rate constant of 3.31 × 10−3 s−1 in the gas phase which is approximately 5400 times faster than the reactive channel 2 under the same conditions. Also, the first reactive channel proceeds with a rate constant of 3.77 × 10−2 s−1 in ethanol solution which is 300-fold higher than the second reactive channel. Thus, it can be concluded that ethanol as a polar solvent affects the reactive channel 2 more than the reactive channel 1.
(c) Generally, the polarity of the solvent, reactants, intermediates, and transition states plays an important role on the reactions rate. Since in the title reaction the transition states have more polar character compared to the reactants, the rate of the reactions is increased when they are carried out in ethanol with dielectric constant of 24.5. Ethanol as a polar solvent lowers the polar transition state energy level more than the reactants and decreases the activation energies.
(d) Because of the decreasing of the freedom degrees of the reactants along the reaction coordinate, the entropy of the reactions (ΔS) is decreased.
Study of charge transfer in transition states
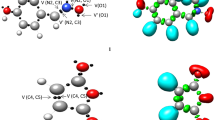
When an electrophilic/nucleophilic pair interacts together, the charge transfer can occur along the reaction and consequently, we deal with a polar reaction. Global electron density transfer (GEDT) can be considered as a relative criterion of charge transfer in transition states which is sometimes responsible for the barrier energies [31]. Therefore, to investigate the charge transfer associated with the reaction studied, the GEDT values were calculated for all transition states in both gas and ethanol phases. The molecular electrostatic potential (MESP) map of transition states was also obtained in order to portray the charge transfer in a better comparable manner. Figure 2 depicts the calculated values of GEDTs along with the MESP maps, which therein the red and blue colors represent respectively more negative (higher electron density) and more positive (lower electron density) regions.
Analysis of the GEDT values and MESP maps reveals that two reactive channels are polar and the electron density is fluxed from 2a as nucleophile toward QM as electrophile. The red color in the molecular electrostatic map depicts the regions with high electron density around the α-oxygen atom of QM as electron acceptor. As said in the previous section, ethanol as a polar solvent affects the second reactive channel more than the first one, which can be attributed to this fact that the GEDT value of the reactive channel 2 increases more than the other, when going from the gas phase to the solution.
The changes in NBO atomic charges during the reaction were also calculated for both reactive channels. The results obtained from M062X/6-311G** method are given in Fig. 3 and those obtained from B3LYP/6-311G** method are given in Fig. S1 of supporting information. Because of electron transfer from 2a toward QM, the charge of the ε, ϕ, and θ atoms (corresponding to 2a) is increased in the transition states and that for the α, β, γ, and δ atoms (corresponding to QM) is decreased.
Study of the interaction between frontier molecular orbitals of the reactants
Since, in the title reaction QM and 2a respectively act as electron acceptor and donor, the interaction between the LUMO of the former and the HOMO of the later is concluded. Recently, the frontier effective-for-reaction molecular orbital (FERMO) concept has been introduced as a powerful approach to study the interaction between the frontier orbitals of the reactants. This theory considers the HOMO or an occupied molecular orbital close to the HOMO with large contribution of active sites. For many reactions, the FERMOs can work better than HOMO [32], because to determinate the interacting frontier orbitals, it considers both energy difference and contribution of active sites. We used satisfactorily the FERMO concept to describe the reactivity of the active sites of the pyrene [18]. Therefore, for determination of the FERMO of 2a as the donor, the relative contribution of the active sites (θ, ϕ, and ε atoms) was calculated in the three highest occupied MOs. The results are given in Fig. 4. It is clear that in terms of the composition and location of the molecular orbitals, the FERMO is also the HOMO for the reaction, because the active sites of 2a have a larger contribution in the HOMO in comparison to the other occupied MOs (13.99%, 12.82%, and 20.28%, respectively, for the ε, ϕ, and θ atoms). The larger contribution of the θ-carbon atom in the FERMO compared to the other active sites (ε, ϕ atoms) indicates that 2a is activated nucleophilically at the θ-carbon atom, which is consistent with the local reactivity indices obtained from Fukui and Parr function analysis. On the other hand, analysis of the carbon atoms corresponding to the six-membered ring of 2a indicates a lower contribution in FERMO and consequently lower reactivity compared to the five-membered ring atoms.
Study of the synchronicity of the reactions
Cycloaddition reactions usually proceed via a synchronous mechanism in which the structural variations take place in one step. Therefore, in order to understand the level of the synchronicity, the structural features of the transition states were studied by using Wiberg bond index analysis. Table 5 summarizes the calculated values of the synchronicity of both reactive channels under gas and solution conditions using M062X/6-311G** method. The results obtained from B3LYP/6-311G** are given in Table S2 of supporting information. Also, Fig. 5 depicts the optimized structure of the transition states along with their imaginary frequencies and the distances between interacting atoms of two fragments.
Analysis of the results presented in Table 5 reveals that both reactive channels are not completely synchronous. According to the bond distances presented in Fig. 5, the degree of advancement of the two new bonds between two fragments is not equivalent in the corresponding transition states. For instance, the bond lengths of Cθ–Cδ and Cϕ–Cδ respectively in TSa and TSa′ are less than those for Cϕ–Oα and Cθ–Oα ones, respectively. Therefore, it can be said that the Cθ–Cδ and Cφ–Cδ bonds in TSa and TSa′, respectively, are formed faster than the other bonds. This trend is completely consistent with the results of the local Fukui functions obtained from NBO and Hirshfeld analyses, because the most favorable interaction was found to be between the Cθ and Cδ atoms which results in 3a adduct. A similar agreement is also observed for the reactive channel 2 between the Fukui function analysis and Wiberg bond indexes. Thus, the synchronicity and bond length analysis reveal that both cycloaddition channels take place via an asynchronous concerted mechanism.
AIM study of the cycloaddition reaction of QM and 2a
AIM analysis is one of the suitable methods to study the structural changes during the cycloaddition reactions. Thus, the AIM analysis was performed along the IRC profile associated with the most preferred transition state, TSa. The molecular topological graphs and the electron densities (ρ) of the bond critical points (BCP) corresponding to the two forming bonds, Cθ–Cδ and Cϕ–Oα, were obtained for certain points on the IRC profile. The variations of the BCP electron densities during the reaction coordinate were also obtained. The results are given in Figs. 6 and 7. At the first point associated with the IRC profile, P1, where the Cθ–Cδ and Cϕ–Oα distances are 2.9081 and 2.9659 Å, respectively, two interacting moieties, QM and 2a, are sufficiently far away from each other so that the corresponding BCP electron densities for Cθ–Cδ and Cϕ–Oα bonds are relatively low (ρ1 = 0.0136 and ρ2 = 0.0104). When two reactants gradually approach each other, the BCP electron density for Cθ–Cδ and Cϕ–Oα bonds is increased. For instance, the values of ρ1 and ρ2 in the transition state are 0.0828 and 0.0236, respectively. The increasing trend in the BCP electron densities continues to produce 3a adduct at the P7 point, in which the ρ1 and ρ2 values reach to 0.2318 and 0.2397, respectively. A comparison of the variation of the BCP electron densities corresponding to the Cθ–Cδ and Cϕ–Oα bonds presented in Fig. 6 indicates that the increasing trend of the electron density for the former bond is more than the later. Thus, it is concluded that the Cθ–Cδ bond is formed faster than the Cϕ–Oα one, and consequently we deal with an asynchronous concerted cycloaddition reaction between QM and 2a. These results indicate that the AIM analysis has satisfactory agreement with the Fukui function as well as synchronicity analysis.
Conclusion
Computational methods at B3LYP/6-311G** and M062X/6-311G** levels of theory were applied to study [4+2] cycloaddition of 1,3-dimethylindole and ortho-quinone methide (obtained from tautomerization of 2,6-dimethylquinone) which was experimentally investigated by Wen et al. For this purpose, two possible reactive channels were considered between 1,3-dimethyindole and 2,6-dimethylquinone in both gas and ethanol solution which leads to generation of two possible regioisomeric adducts. Regioselective formation of the experimentally reported adduct was confirmed with Fukui function reactivity indices obtained from NBO and Hirshfeld analysis and also with the Parr functions reactivity indices obtained from Mulliken and Hirshfeld analyses. Transition state calculations confirmed that the reactive channel which generates the experimentally reported adduct is kinetically and thermodynamically the preferred path. Although Wen et al. believe that the reaction proceeds via a stepwise mechanism with formation of a zwitterionic intermediate, the IRC analysis ruled out the formation of any stable intermediate. For investigation of the charge transfer during the reaction, the global electron density transfer (GEDT) values were calculated for the transition states and the results indicated that all transition states are relatively polar in both gas and solution phases. Also, it was found that the electron density is fluxed from 2a toward QM. AIM analysis and calculation of synchronicity revealed that all reactive channels proceed via an asynchronous concerted mechanism.
Computational details
To study the cycloaddition reactions between 1,3-dimethylindole and ortho-quinone methide, the optimization of the reactants and products was carried out using B3LYP/6-311G** and M062X/6-311G methods [33, 34]. To calculate the solvation effects on the studied molecules, the conductor-like polarizable continuum model (CPCM) was used [35]. The structures of transition states were determined by using the Berny algorithm or the synchronous transit-guided quasi-Newton (STQN) procedure [36, 37]. The stationary points were characterized by frequency calculations to verify that all transition states have one and only one imaginary frequency. The accuracy of transition states was also verified by using IRC calculations [38, 39].
The atomic charges of the reactants, TS structures, and products were calculated using NBO analysis [40, 41].
The nucleophilicity index N is defined as the following equation in which EHOMO(Nu), the HOMO energy of tetracyanoethylene (TCE), is taken as the reference and EHOMO(Nu) refers to the HOMO energy of nucleophile [42]:
The electrophilicity index ω is given by the following equation based on the chemical hardness η and electronic chemical potential μ [43]. Both η and μ quantities were calculated from the frontier molecular orbitals energies as μ ≈ (EHOMO + ELUMO)/2 and η ≈ (ELUMO − EHOMO)/2 [44, 45].
The synchronicity (Sy) in transition states was calculated with the following equation [46]:
where n is the number of directly participating bonds in the reaction, δBi is a relative variation of bond index Bi at the transition state, and δBav is a measure of the degree of advancement of the transition state along the reaction coordinate. The values of synchronicity vary from 0 for stepwise to 1 for concerted mechanisms.
All calculations were performed with the Gaussian 09 program package [47].
References
Fringuelli F, Taticchi A (2002) The Diels-Alder reaction: selected practical methods. Wiley, Chichester
Gajewski JJ, Peterson KB, Kagel JR (1987). J Am Chem Soc 109:5545–5546
Linder M, Brinck T (2012). J Org Chem 77:6563–6573
Houk KN, Gonzalez J, Li Y (1995). Acc Chem Res 28:81–90
Rideout D, Breslow R (1980). J Am Chem Soc 102:7816–7817
Breslow R, Guo T (1988). J Am Chem Soc 110:5613–5617
Dunams T, Hoekstra W, Pentaleri M, Liotta D (1988). Tetrahedron Lett 29:3745–3748
Otto S, Engberts JBFN (2000). Pure Appl Chem 72:1365–1372
Breslow R, Rizzo CJ (1991). J Am Chem Soc 113:4340–4341
Dewick PM (2002) Medicinal natural products: a biosynthetic approach2nd edn. Wiley, New York
Fattorusso E, Scafati OT (2008) Modern alkaloids. Wiley-VCH, Weinheim
Sanchis PR, Savina SA, Albericio F, Alvarez MA (2011). Chem Eur J 17:1388–1408
Trost BM, Osipov M (2015). Chem Eur J 21:16318–16343
Popov K, Hoang A, Somfai P (2016). Angew Chem Int Ed 55:1801–1804
Yang J-M, Li P-H, Wei Y, Tang X-Y, Shi M (2016). Chem Commun 52:346–349
Cera G, Chiarucci M, Mazzanti A, Mancinelli M, Bandini M (2012). Org Lett 14:1350–1353
Lin Ch DH-J, Zhao H, Yan D-F, Liu N-X, Sun H, Wen X, Xu Q-L (2017). Org Biomol Chem 15:3472–3478
Soleymani M, Dashi Khavidaki H (2017). Comp Theor Chem 1112:37–45
Soleymani M (2018). Monatshe Chem Chem Mon 149:2183–2193
Memarian HR, Soleymani M, Sabzyan H, Bagherzadeh M, Ahmadi H (2011). J Phys Chem A 115:8264–8270
Memarian HR, Sabzyan H, Soleymani M, Habibi MH, Suzuki T (2011). J Mol Struc 998:91–98
Geerlings P, De Proft F, Langenaeker W (2003). Chem Rev 103:1793–1874
Ess DH, Jones GO, Houk KN (2006). Adv Synth Catal 348:2337–2361
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002). Tetrahedron 58:4417–4423
Yang W, Mortier WJ (1986). J Am Chem Soc 108:5708–5711
Domingo LR, Aurell MJ, Pêrez P, Contreras R (2002). J Phys Chem A 106:6871–6875
Pêrez P, Domingo LR, Duque-Noreňa M, Chamorro E (2009). J Mol Struct THEOCHEM 895:86–91
Domingo LR, Pérez P, Sáez JA (2013). RSC Adv 3:1486–1494
Chamorro E, Pérez P, Domingo LR (2013). Chem Phys Lett 582:141–143
Eyring H (1935). J Chem Phys 3:107–115
Domingo LR (2014). RSC Adv 4:32415–32428
Da Silva RR, Ramalho TC, Santos JM, Figueroa-Villar JD (2006). J Phys Chem A 110:1031–1040
Lee C, Yang W, Parr RG (1988) Phys. Rev. B 37:785–789
Zhao Y, Truhlar DG (2006). J Phys Chem 110:5121–5129
Barone V, Cossi M (1998). J Phys Chem A 102:1995–2001
Schlegel HB (1982). J Comput Chem 3:214–218
Peng C, Ayala PY, Schlegel HB, Frisch MJ (1996). J Comput Chem 17:49–56
Gonzalez C, Schlegel HB (1989). J Chem Phys 90:2154–2161
Gonzalez C, Schlegel HB (1990). J Phys Chem 94:5523–5527
Reed AE, Curtiss LA, Weinhold F (1988). Chem Rev 88:899–926
Carpenter JE, Weinhold FJ (1988). J Mol Struct 169:41–62
Domingo LR, Perez P, Ortega DE (2013). J Org Chem 78:2462–2471
Parr RG, Szentpaly LV, Liu S (1999). J Am Chem Soc 121:1922–1924
Parr RG, Pearson RG (1983). J Am Chem Soc 105:7512–7516
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, New York
Lecea B, Arrieta A, Roa G, Ugalde JM, Cossio FP (1994). J Am Chem Soc 116:9613–9619
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta Jr JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09, revision E. Gaussian, Inc, Wallingford, p 01
Funding
I am thankful to the Research Council and Office of Graduate Studies of the University of Ayatollah Alozma Borujerdi for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 162 kb)
Rights and permissions
About this article
Cite this article
Soleymani, M. Theoretical study on the [4+2] cycloaddition of 1,3-dimethylindole with 2,6-dimethylquinone. Struct Chem 30, 1173–1184 (2019). https://doi.org/10.1007/s11224-018-1259-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1259-1