Abstract
A series of novel organic dyes (ICZA1, ICZA2, ICZA3, ICZA4) with D-π-A structural configuration incorporating indolo[3,2,1-jk]carbazole moiety as donor (D) unit, thiophene as π-linker and 2-cyanoacrylic acid as acceptor unit were investigated using density functional theory (DFT) and time-dependent DFT (TD-DFT) methods. Indolo[3,2,1-jk]carbazole-based D-π-A dyes composed of different acceptor groups were designed. By modulating acceptor unit, the efficiency of D-π-A dye-based dye-sensitized solar cells (DSSCs) can be further improved. In the present work, four novel push-pull organic dyes only differing in electron acceptor, have been designed based on the experimental literature value of IC-2. In order to further improve the light harvesting capability of indolo[3,2,1-jk]carbazole dyes, the acceptor influence on the dye performance were examined. The NLO property of the designed dye molecules can be derived as polarizability and hyperpolarizability. The calculated value of ICZA2 dye is the best candidate for NLO properties. Furthermore, the designed organic dyes exhibit good photovoltaic performance of charge transfer characteristics, driving force of electron injection, dye regeneration, global reactivity, and light harvesting efficiency (LHE). From the calculated value of ICZA4 dye, it has been identified as a good candidate for DSSCs applications. Finally, it is concluded that the both ICZA2 and ICZA4 dyes theoretically agrees well with the experimental value of IC-2 dye. Hence, the dyes ICZA2 and ICZA4 can serve as an excellent electron withdrawing groups for NLO and DSSCs applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
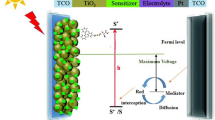
In 1991, Gratzel and coworkers have reported that dye-sensitized solar cells (DSSCs) are promising candidate for photovoltaic performance [1]. The working process of DSSCs requires that these dyes upon light absorption, photo-induced excited electron is then injected into the conduction band of semiconductor TiO2 anode in a femtosecond lifetime by the anchoring group. Further, the presence of redox electrolyte regenerates the oxidized sensitizer. The oxidized dye is then neutralized to ground state by the \( {I}^{-}/{I}_3^{-} \) redox system [2]. There are two major categories sensitizers commonly used as (1) metal complexes and (2) metal-free organic dyes. Although N719 Ru(II)-polypyridyl photosensitizers have shown the highest performance of DSSCs exceeding 11.8% [3], in 2011, zinc porphyrin-based dye has the best power conversion efficiency (PCE) of DSSCs up to 12.3% [4]. Recently, Gratzel and Oxford University research teams independently developed a solid-state DSSCs PCE exceeding 15%, further creating a new record [5, 6].
Due to this, metal-free organic dyes and natural dyes have attracted considerable attention for their tunable electronic structure, absorption spectra and electrochemical properties, ease of molecular tailoring, and low cost processes [7]. Recent literature reports indicate achievement of PCE 13.1% using pure organic sensitizer C281, such as high PCE has been reached with a metal-free organic dye in DSSCs [8]. In 2017, Kar et al. using N,N′-dialkylaniline based (NDI 6) dye has reached the best PCE of DSSCs in 19.24%. [9]. Several organic dyes such as coumarin [10, 11], polyene [12], indoline [13, 14], triphenylamine (TPA) [15], carbazole [16], tetrahydroquinoline [17], and phenothiazine [18] have been investigated for DSSCs and showed good photovoltaic performance. For example, a typical TPA-based dye (TPC1) reported by H. Tian and coworkers used in DSSCs as the sensitizer exhibits an impressive PCE of 5.3% [19]. Other major advantages of metal-free organic dyes are their tunable absorption and photochemical properties through suitable molecular design [20]. The most traditional organic efficient sensitizers are generally configuration made of electron donor (D), π-spacer, and electron acceptor (A) [21,22,23]. This D-π-A architecture produces an effective intramolecular charge transfer (ICT) from D to A during photoexcitation process [24].
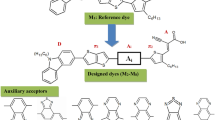
In the study of Chunhua Luo in 2014, [25] indolo[3,2,1-jk]carbazole sensitized photovoltaic device exhibited a high PCE of 3.68%, photocurrent (9.78 mA cm−2), and photovoltage (0.66 V) measured under illumination of AM1.5G simulated solar light (100 mW cm−2) at room temperature. Additionally, the acceptor groups of organic dyes play an important role onto the semiconductor surface and enhance efficiency photovoltaic cell performance, namely PC, PN, PMN, and PR [26]. Different acceptor groups were employed in order to further increase the absorption spectra of the organic sensitizer. In the current study, the optoelectronic properties of D-π-A organic dyes with different electron acceptor groups have been studied using density functional theory (DFT) and time-dependent DFT (TD-DFT) approach in order to good sensitizing properties for DSSCs. Hence, in the present work, the NLO property of the designed dye molecules was analyzed through the static polarizability and first hyperpolarizability. The influence of photovoltaic properties of the DSSCs based on electron acceptor was under investigation.
Theoretical setup
The ground state geometries of these molecules were fully optimized without any symmetry constrains. Full geometry optimized structure were confirmed to be at its local minimum (no imaginary frequency modes) energy surface was found. The optimization of ground state structure are performed in DFT [27, 28] method with Becke’s three-parameter and Lee-Yang-Parr (B3LYP) [29] hybrid functional using 6-31G(d, p) basis set on all atoms.
The TD-DFT calculations were performed to calculate the UV-Vis optical absorption spectra. In general, different exchange-correlation (XC) functionals for charge transfer (CT) excitations often show significant effects. To select suitable functional, the optical absorption spectra of IC-2 by different XC functionals, including B3LYP, CAM-B3LYP [30], and WB97XD, were calculated using [31] TD-DFT method. From three functionals, the absolute values of 468, 393, and 385 nm were compared to experimental absorption spectra 382 nm. It gives errors of 86, 11, and 03 nm, as shown in Table 1. From Fig. 1, TD-DFT optical absorption spectra of IC-2 was calculated at three hybrid functionals using in CH2Cl2 solution. While taking as reference value for further newly designed dye molecules, the three optical absorption wavelengths, with the help of TD-WB97XD functional with 6-31G(d,p) basis sets, were performed.
Therefore, TD-WB97XD functional with 6-31G (d,p) basis set were chosen for combining the conductor-like polarizable continuum model (CPCM) [32] in dichloromethane (CH2Cl2) solution to predict the optical absorption properties of designed dye molecules. All the calculations were performed with Gaussian 09 package [33]. The optical absorption wavelengths are obtained by using Gausssum [34].
Results and discussion
Screening of the electron withdrawing groups
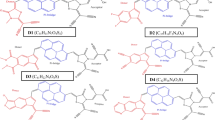
The electron withdrawing groups play an important role in D-π-A organic structure and further influence the PCE of DSSCs performance. The acceptor molecules were collected from literature survey. In the previous study, the acceptor molecules are used in highly efficient organic DSSCs application. In this way, these molecules will be effected by the indolo[3,2,1-jk]carbazole-based dye derivatives. A series of metal-free organic dyes ICZA1-ICZA4 with D-π-A structure were designed in order to screen excellent electron withdrawing groups compared to IC-2. Structure of indolo[3,2,1-jk]carbazole was shown in Fig. 2. In order to explore considerably highly efficient indolocarbazole derivative dyes, tailoring of molecular design for D-π-A using different electron withdrawing groups were designed (ICZA1-ICZA4) as shown in Fig. 3. B3LYP/6-31G(d,p) were used for the optimization of indolo[3,2,1-jk]carbazole and four newly designed efficient sensitizers. Optimized geometric structures were shown in Fig. S0 (Supporting information). From the geometric structures explore that the electron acceptor effect of ICZA1-ICZA4 have shown coplanar structures, which might be favorable for the photo-induced electron CT and to broaden the optical absorption wavelength.
Intramolecular charge transfer effects
In DSSCs, the energy levels and corresponding orbitals distribution of frontier molecular orbitals (FMOs) are closely related to the intramolecular charge transfer (ICT) from electron donor to acceptor group of metal-free organic sensitizers [35]. It has a great effect on the optical absorption wavelength. Figure 4 shows the orbital spatial distribution of highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs) of the studied dye molecules. The HOMOs and LUMOs energy levels are very important aspects to explain efficient charge separation between donor and acceptor groups.
A strong D-π-A molecular structure has the character of the HOMOs are mainly contained on the donor parts to the π-conjugated spacer and LUMOs are mainly distributed on the electron acceptor moieties (anchoring group) when absorbed the photon in all dye sensitizers. The electron acceptor effect on the electronic properties by using various acceptor segments was investigated. The molecular orbitals (MO) contributions of HOMOs, LUMOs, and energy gap (Eg) are listed in Table 2.
Frontier molecular orbitals
The FMOs contribution is a very significant factor in determining the charge separated states of dye sensitizers [36]. In newly designed dye molecules, the HOMOs, LUMOs energy levels and their Eg values are among the most significant properties dominating the dye performance in photovoltaic devices. The smaller Eg values play an important role in broadening the visible absorption region, which improves the photoelectric properties of the dyes [37, 38]. The LUMOs are π*-orbitals of all the dye sensitizers are above the semiconductor conduction band edge (CBE) of TiO2 (−4.0 eV) surface, and the HOMOs energy levels of π-orbitals are sufficiently below the redox potential of the \( {I}^{-}/{I}_3^{-} \) liquid electrolyte (−4.8 eV) system [39]. It is recommended that all the dye sensitizer should be capable of electron injection into the CBE of semiconductor and could be recombination process of electrons from the redox electrolyte. The HOMOs and LUMOs energies were calculated using B3LYP/6-31G(d,p) level of theory. The FMOs isodensity contour plots of IC-2, ICZA1-A4 were shown in Fig. 5. Four different structures of electron acceptor dyes were investigated. The HOMOs and LUMOs of the energy levels, with the increasing order of ICZA3 < ICZA2 < ICZA1 < ICZA4 and ICZA2 < ICZA4 < ICZA3 < ICZA1, respectively. The Eg values are between 2.63 and 3.31 eV. These results reveal that the sensitizers that have smaller Eg values show several characteristics beneficial to higher light harvesting efficiency (LHE) in the performance of DSSCs.
Global reactivity descriptors
The ionization potential (IP) and electron affinity (EA) of organic dyes give information about the charge-injection and charge transport character of the designed dye molecules [40]. The Eg between HOMOs and LUMOs for a molecule is an important parameter to determine electronic transport properties of DSSCs. The global chemical reactivity descriptors of molecules, chemical hardness (η), and softness (S) have been defined on the basis of EHOMO and ELUMO [41, 42].
By using Koopman’s theorem [43] for closed-shell compounds of η and S can be defined as follows:
Softness is a property of designed dye molecules that measures the extent of chemical reactivity.
where IP and EA can be obtained as IP = −EHOMO and EA = −ELUMO.
All the calculated values of η and S of the designed dye molecules were listed in Table 3. From the table identified that the large HOMOs-LUMOs gap as a hard molecule and small HOMOs-LUMOs gap as a soft molecule while considering η. The stability of a molecule and its reactivity can be related to chemical η, which means that the dye molecules with least HOMOs LUMOs gaps have more reactive.
It is well identified that the higher the value of EA, the higher can be the electron transport ability. It is interesting to note that lower HOMOs-LUMOs gap of ICZA2 and ICZA4 shows lower IP values of 5.66 and 5.48 eV and higher EA values of 2.84 and 2.63 eV, respectively. This indicates that both the ICZA2 and ICZA4 dyes can be the best candidates for both hole and electron transport material compared with IC-2.
Non-linear optical study
The non-linear optical study (NLO) response of an isolated molecule in an electric field (E) can be represented as static polarizability (α), anisotropic polarizability (Δα), and first hyperpolarizability (β) of the designed dye molecules were calculated using the following equation [44]:
The third rank tensor of β can be described by 3 × 3 × 3 matrix.
where αxx, αyy & αzz polarizability tensor components, βzyy, βzzz, βxxx, βxyy, βxzz, βxxy, βyyyandβyzz magnitude of the first hyperpolarizability tensor components. These constraints contribute in the non-linearity of the designed dye molecules.
The highest molecular α and β exhibit good sensitizing property upon photoexcitation because it tends to decrease the aggregates formation on the surface of TiO2 leads to better conversion efficiency. The calculated values were summarized in Table S1 and S2 (Supporting information). The α is directly proportional to the dipole moment and the β is inversely proportional to the vertical transition energy [45]. It has been observed that the α values of ICZA2 (231 a.u.), ICZA3 (193 a.u.), and ICZA4 (218 a.u.) are higher than that of the other molecules when compared to IC-2 (186 a.u.), except the value of ICZA1 (181 a.u.). The substitution effect of strong electron withdrawing groups of ICZA2 and ICZA4 dyes enhanced the α. The ICZA2 dye has the maximum value of α as 3.424 × 10−23 e.s.u. when compared with IC-2 as 2.764 × 10−23 e.s.u. The highest value of β, which is a measure of NLO activity of the molecular system, is related with the ICT, resulting from the electron cloud movement, through the acceptor framework of electron [46]. Accordingly, ICZA2 dye with minimum transition energy (2.92 eV obtained from TD-DFT calculation) exhibits the maximum β value of 11.424 × 10−30 e.s.u. A higher value of the β is important for active NLO performance and the present results indicate that the ICZA2 possess larger β value particularly which can be used for NLO applications.
Spectral analysis
The simulated optical absorption spectra of IC-2 and ICZA1-ICZA4 in dichloromethane solution were displayed in Fig. 6. It is obvious that all the dye sensitizers lie in the entire visible region of 410 nm. The calculated excitation wavelength, oscillator strength, LHE, and major orbitals transitions of IC-2, ICZA1-ICZA4 were shown in Table 4. The maximum absorption peak of ICZA4 is 423 nm and exhibits a large redshift of 38 nm compared to IC-2 (385 nm), which is consistent with the smaller Eg, while the presence of ICZA1 (366 nm) was blue-shifted 19 nm compared to IC-2. The longer absorption wavelength was assigned to ICT between the donor and electron acceptor moieties. All the absorption spectra also belonged to n-π* transitions. In Table 4, vertical excitation energies (E) were changed in decreasing order, ICZA4 > ICZA2 > ICZA3 > ICZA1, showing that there is a redshift when passing from ICZA4-ICZA1. We have also calculated the LHE is one of the important parameter which determines the efficiency of DSSCs.
LHE (λ) can be calculated according to the following [47]:
where f represents oscillator strength of the dye sensitizer related to the λmax.
In Eq. 7, highest f and hence higher LHE were found for ICZA2. λmax has the highest value for ICZA4 dye sensitizer for which LHE (1.65 a.u.). Therefore, according to LHE, ICZA4 dye should be the best sensitizer compared to IC-2 (1.40 a.u.), which has beneficial for larger short-circuit current density (JSC). In all the studied dye molecules, the dominant absorption band has associated with HOMO-LUMO transition.
These results show that all dye sensitizers have only one band in the visible region (λmax > 350 nm). This also indicates that the ICZA4 could harvest more light at the longer-wavelength region, which can be helpful to further increase in the PCE of corresponding DSSCs.
Overall efficiency of DSSCs
As we know, the overall efficiency of DSSCs device is mainly determined byJSC, open-circuit photovoltage (Voc), the fill factor (FF), and the intensity of the incident light (PIN), it can be expressed as the following Eq. 8 [48]:
In DSSCs, Voc can be described by the following [49]:
where unit charge is q, thermal energy is KT, nC is the conduction band (CB) of the electron number, NCB is the accessible density of CB states, and Eredox is the oxidation potential of the liquid electrolyte.
TheVOC is determined by energy difference between the CBE and redox electrolyte. Usually, the solution \( {I}^{-}/{I}_3^{-} \) is used as the redox potential, so we take it as a constant. ∆CBE is an important factor of Voc and can be followed by [50]:
The outermost level concentration of dye sensitizer isγ, μnormal is the dipole moment of individual molecular perpendicular to the boundary condition of the semiconductor and ε0, ε it will be constants.
It is apparent that a large μnormal will lead to greater extent shift of CBE which will result in largerVoc. As shown in Table 4, ICZA1-ICZA4 values identified that the dipole moments are 6.52, 10.83, 8.98, and 12.06 Debye. The dyes ICZA2 and ICZA4 have the largest dipole moment, leading to largerVOC. All four dyes were highly compared to IC-2 (10.35 Debye), except the values of ICZA1 and ICZA3. Among these dyes, ICZA4 can be the outstanding performance for improved efficiency of DSSCs.
TheJSC in DSSCs can be determined by the following [51]:
where LHE at a given wavelength of λmax. ΦINJ is the electron injection efficiency and charge collection efficiency isηcoll. Hence, it is reasonable to assume that ηcoll is a constant. Another way of growing JSCis to improve the electron injection rate of free energyΔGinject. ΦINJis related to the driving force ΔGinjectof electrons injecting from excited state of molecule to the semiconductor CBE of TiO2.
ΔGinject can be described by the following [52]:
where \( {E}_{OX}^{dye^{\ast }} \) oxidation potential of the excited state of dye sensitizer and \( {E}_{CB}^{TiO_2} \) CBE of TiO2 in the reduction potential energy surface.
\( {E}_{OX}^{dye^{\ast }} \)can be calculated by Eq. 13 [53,54,55]:
where \( {E}_{OX}^{dye} \) reduction potential of the ground state of dye, while E is a vertical excitation energy corresponding toλmax. According to Islam investigation, when ΔGinject > 0.2 eV, the electron injection efficiency (ΦINJ) is almost equal to one [56]. As shown in Tables 4 and 5, the absolute values of ΔGinject for ICZA1-ICZA4 are much greater than 0.2 eV. So, it can be predicted that these sensitizers have driving force for the fast ΔGinjectof excited state electrons into CBE of TiO2.
JSC is also influenced by the regeneration efficiency of sensitizer (ηreg), which can be determined by the driving force of regeneration ΔGreg. It can be calculated by Eq. 14 [57]:
According to the survey of Robson, the regeneration process of the dye can significantly influence the efficiency of DSSCs [58]. The ICZA3 dye having larger driving forces of regeneration can cause the improvement of ηreg. Finally, ICZA2 and ICZA4 will be the promising sensitizers due to their good PCE in DSSCs.
Conclusion
In summary, we have demonstrated that the dyes ICZA1-A4 can be transformed into suitable sensitizers especially in the higher molar extinction coefficient and longer-wavelength visible region of the solar spectrum. Photovoltaic properties of these D-π-A systems with different acceptor groups have been investigated by DFT and TD-DFT method. These dyes exhibit higher molar extinction coefficient and broad electronic absorption properties of DSSCs. Overall, a new type of ICZA1-A4 acceptor moieties were changed to different anchoring modes which can be successfully employed for DSSCs. In comparison with IC-2, the different acceptor segment of dyes ICZA2-ICZA4 have exhibited a higher absorption, LHE, smaller Eg, and obvious redshifts, for obtaining the improved PCE. The NLO property of the designed dye molecules were calculated at static polarizability and first hyperpolarizability values. It shows that the ICZA2 molecule possess better NLO performance. In particular, these results demonstrated that ICZA2 and ICZA4 acceptor moieties are promising electron withdrawing groups for high performance of DSSCs. Finally, to conclude, the promising D-π-A method has provided evidences with the electron withdrawing groups of ICZA2 and ICZA4 dyes for the further development of highly efficient metal-free organic dye sensitizer in practical application of DSSCs.
References
O’Regan B, Gratzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740
Feng J, Jiao Y, Ma W, Nazeeruddin MK, Grätzel M, Meng S (2013) First principles design of dye molecules with ullazine donor for dye sensitized solar cells. J Phys Chem C 117:3772–3778
Nazeeruddin MK, De Angelis F, Fantacci S, Selloni A, Viscardi G, Liska P, Ito S, Takeru B, Gr¨atzel M (2005) Combined experimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. J Am Chem Soc 127:16835–16847
Yella A, Lee HW, Tsao HN, Yi C, Chandiran AK, Nazeeruddin MKE, Diau WG, Yeh CY, Zakeeruddin SM, Grätzel M (2011) Porphyrin-sensitized solar cells with cobalt (II/III)–based redox electrolyte exceed 12 percent efficiency. Science 334:629–634
Burschka J, Pellet N, Moon SJ, Humphry-Baker R, Gao P, Nazeeruddin MK, Grätzel M (2013) Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 499:316–319
Liu M, Johnston MB, Snaith HJ (2013) Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 501:395–398
Li H, Koh TM, Hagfeldt A, Grätzel M, Mhaisalkar SG, Grimsdale AC (2013) New donor–π–acceptor sensitizers containing 5H-[1, 2, 5] thiadiazolo [3, 4-f] isoindole-5, 7 (6H)-dione and 6 H-pyrrolo [3, 4-g] quinoxaline-6, 8 (7 H)-dione units. Chem Commun 49:2409–2411
Yao Z, Wu H, Li Y, Wang J, Zhang J, Zhang M, Wang P (2015) Dithienopicenocarbazole as the kernel module of low-energy-gap organic dyes for efficient conversion of sunlight to electricity. Energy Environ Sci 8:3192–3197
Kar S, Roy JK, Leszczynski J (2017) In silico designing of power conversion efficient organic lead dyes for solar cells using todays innovative approaches to assure renewable energy for future. NPJ Computational Materials 3:1
Seo KD, Song HM, Lee MJ, Pastore M, Anselmi C, De Angelis F, Kim HK (2011) Coumarin dyes containing low-band-gap chromophores for dye-sensitized solar cells. Dyes Pigments 90:304–310
Han L, Wu H, Cui Y, Zu X, Ye Q, Gao J (2014) Synthesis and density functional theory study of novel coumarin-type dyes for dye sensitized solar cells. J Photochem Photobio A 290:54–62
Hara K, Kurashige M, Ito S, Shinpo A, Suga S, Sayama K, Arakawa H (2003) Novel polyene dyes for highly efficient dye-sensitized solar cells. Chem Commun 2:252–253
Rudolph M, Yoshida T, Miura H, Schlettwein D (2014) Improvement of light harvesting by addition of a long-wavelength absorber in dye-sensitized solar cells based on ZnO and indoline dyes. J Phys Chem C 119:1298–1311
Sobuś J, Karolczak J, Komar D, Anta JA, Ziółek M (2015) Transient states and the role of excited state self-quenching of indoline dyes in complete dye-sensitized solar cells. Dyes Pigments 113:692–701
Prakasam M, Anbarasan PM (2016) Second order hyperpolarizability of triphenylamine based organic sensitizers: a first principle theoretical study. RSC Adv 6:75242–75250
Wang ZS, Koumura N, Cui Y, Miyashita M, Mori S, Hara K (2009) Exploitation of ionic liquid electrolyte for dye-sensitized solar cells by molecular modification of organic-dye sensitizers. Chem Mater 21:2810–2816
Chen R, Yang X, Tian H, Sun L (2007) Tetrahydroquinoline dyes with different spacers for organic dye-sensitized solar cells. J Photochem Photobiol A Chem 189(2):295–300
Tian H, Yang X, Chen R, Pan Y, Li L, Hagfeldt A, Sun L (2007) Phenothiazine derivatives for efficient organic dye-sensitized solar cells. Chem Commun 36:3741–3743
Tian H, Yang X, Chen R, Zhang R, Hagfeldt A, Sun L (2008) Effect of different dye baths and dye-structures on the performance of dye-sensitized solar cells based on triphenylamine dyes. J Phys Chem C 112:11023–11033
Kitamura T, Ikeda M, Shigaki K, Inoue T, Anderson NA, Ai X, Lian TQ, Yanagida S (2004) Phenyl-conjugated oligoene sensitizers for TiO2 solar cells. Chem Mater 16:1806–1812
Liang M, Chen J (2013) Arylamine organic dyes for dye-sensitized solar cells. Chem Soc Rev 42:3453–3488
Ooyama Y, Harima Y (2012) Photophysical and electrochemical properties, and molecular structures of organic dyes for dye-sensitized solar cells. Chem Phys Chem 13:4032–4080
Mishra A, Fischer MKR, Bäuerle P (2009) Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew Chem Int Ed 48:2474–2499
Mohr T, Aroulmoji V, Ravindran RS, Müller M, Ranjitha S, Rajarajan G, Anbarasan PM (2015) DFT and TD-DFT study on geometries, electronic structures and electronic absorption of some metal free dye sensitizers for dye sensitized solar cells. Spectrochimica Acta Part A: Mol Biomol Spect 135:1066–1073
Luo C, Bi W, Deng S, Zhang J, Chen S, Li B, Chu J (2014) Indolo [3, 2, 1-jk] carbazole derivatives-sensitized solar cells: effect of π-bridges on the performance of cells. J Phys Chem C 118:14211–14217
Ganesan P, Rajadurai VS, Sivanadanam J, Ponnambalam V, Rajalingam R (2013) Effect of electron withdrawing anchoring groups on the optoelectronic properties of pyrene sensitizers and their interaction with TiO2: a combined experimental and theoretical approach. J Photochem and Photobio A: Chem 271:31–44
Klimeš J, Michaelides A (2012) Perspective: advances and challenges in treating Van der Waals dispersion forces in density functional theory. J Chem Phys 137:120901
Ruiz VG, Liu W, Zojer E, Scheffler M, Tkatchenko A (2012) Density-functional theory with screened Van der Waals interactions for the modeling of hybrid inorganic-organic systems. Phys Rev Lett 108:146103
Hertwig RH, Koch W (1997) On the parameterization of the local correlation functional. What is Becke-3-Lyp? Chem Phys Lett 268:345–351
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phy Let 393:51–57
Chai JD, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin, AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A.1. Gaussian, Inc., Wallingford
O'boyle NM, Tenderholt AL, Langner KM (2008) Cclib: a library for package-independent computational chemistry algorithms. J Comput Chem 29:839–845
Hodge PA, Power GA, Rabjohns M (1997) Synthesis of poly(anthracene-2,6-diyl) and a copolymer containing alternately anthracene-2,6-diyl and p-phenylene units. Chem Commun 1:73–74
Jungsuttiwong S, Tarsang R, Sudyoadsuk T, Promarak V, Khongpracha P, Namuangruk S (2013) Theoretical study on novel double donor-based dyes used in high efficient dye-sensitized solar cells: the application of td-dft study to the electron injection process. Org Electron 14:711–722
He J, Wu W, Hua J, Jiang Y, Qu S, Li J, Tian H (2011) Bithiazole-bridged dyes for dye-sensitized solar cells with high open circuit voltage performance. J Mater Chem 21:6054–6062
Mathew S, Imahori H (2011) Tunable, strongly-donating perylene photosensitizers for dye-sensitized solar cells. J Mater Chem 21:7166–7174
Fitri A, Benjelloun AT, Benzakour M, McHarfi M, Hamidi M, Bouachrine M (2014) Theoretical investigation of new thiazolothiazole-based D-Pi-a organic dyes for efficient dye-sensitized solar cell. Spectrochim Acta Part A 124:646–654
Parr RG, Szentpaly LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924
Happ B, Winter A, Hager MD, Schubert U (2012) Photogenerated avenues in macromolecules containing Re (I), Ru (II), Os (II), and Ir (III) metal complexes of pyridine-based ligands. Chem Soc Rev 41:2222–2255
Chattaraj PK, Maiti B, Sarkar U (2003) Philicity: a unified treatment of chemical reactivity and selectivity. J Phys Chem A 107:4973–4975
Koopmans T (1933) Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica 1:104–113
Senthilkumar P, Nithya C, Anbarasan PM (2014) Quantum chemical investigations on the effect of dodecyloxy chromophore in 4-amino stilbene sensitizer for DSSCs. Spectrochimica Acta Part A: Mol Biomol Spec 122:15–21
Janjua MRSA, Khan MU, Bashir B, Iqbal MA, Song Y, Naqvi SAR, Khan ZA (2012) Effect of π-conjugation spacer (C C) on the first hyperpolarizabilities of polymeric chain containing polyoxometalate cluster as a side-chain pendant: a DFT study. Comput Theor Chem 994:34–40
Arivazhagan M, Jeyavijayan S (2011) Vibrational spectroscopic, first-order hyperpolarizability and HOMO, LUMO studies of 1,2-dichloro-4-nitrobenzene based on Hartree–Fock and DFT calculations. Spectrochim Acta A 79:376–383
Peach MJ, Benfield P, Helgaker T, Tozer DJ (2008) Excitation energies in density functional theory: an evaluation and a diagnostic test. J Chem Phys 128:044118
Chattopadhyay D, Lastella S, Kim S, Papadimitrakopoulos F (2002) Length separation of zwitterion-functionalized single wall carbon nanotubes by GPC. J Am Chem Soc 124:728–729
Marinado T, Nonomura K, Nissfolk J, Karlsson MK, Hagberg DP, Sun L, Hagfeldt A (2009) How the nature of triphenylamine-polyene dyes in dye-sensitized solar cells affects the open-circuit voltage and electron lifetimes. Langmuir 26:2592–2598
Rühle S, Greenshtein M, Chen SG, Merson A, Pizem H, Sukenik CS, Zaban A (2005) Molecular adjustment of the electronic properties of nanoporous electrodes in dye-sensitized solar cells. J Phys Chem B 109:18907–18913
Chen SL, Yang LN, Li ZS (2013) How to design more efficient organic dyes for dye-sensitized solar cells? Adding more sp2-hybridized nitrogen in the triphenylamine donor. J Power Sources 223:86–93
Katoh R, Furube A, Yoshihara T, Hara K, Fujihashi G, Takano S, Tachiya M (2004) Efficiencies of electron injection from excited N3 dye into nanocrystalline semiconductor (ZrO2, TiO2, ZnO, Nb2O5, SnO2, In2O3) films. J Phys Chem B 108:4818–4822
Preat J, Jacquemin D, Michaux C, Perpète EA (2010) Improvement of the efficiency of thiophene-bridged compounds for dye-sensitized solar cells. Chem Phys 376:56–68
Zhang J, Kan YH, Li HB, Geng Y, Wu Y, Su ZM (2012) How to design proper π-spacer order of the D-π-A dyes for DSSCs? A density functional response. Dyes Pigments 95:313–321
Ding WL, Wang DM, Geng ZY, Zhao XL, Xu WB (2013) Density functional theory characterization and verification of high-performance indoline dyes with D–A–π–A architecture for dye-sensitized solar cells. Dyes Pigments 98:125–135
Islam A, Sugihara H, Arakawa H (2003) Molecular design of ruthenium (II) polypyridyl photosensitizers for efficient nanocrystalline TiO2 solar cells. J Photochem Photobiol A 158:131–138
Daeneke T, Mozer AJ, Uemura Y, Makuta S, Fekete M, Tachibana Y, Spiccia L (2012) Dye regeneration kinetics in dye-sensitized solar cells. J Am Chem Soc 134:16925–16928
Robson KCD, Hu K, Meyer GJ, Berlinguette CP (2013) Atomic level resolution of dye regeneration in the dye-sensitized solar cell. J Am Chem Soc 135:1961–1971
Acknowledgements
The authors are thankful to the learned referees for their useful and critical comments, which can improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Highlights
• Four novel indolo[3,2,1-jk]carbazole-based dyes are designed by modifying electron acceptor.
• The lower Eg and larger absorption spectra will favors to light harvesting process.
• The better ICZA2-ICZA4 electron acceptor can be ascribed to enhanced LHE compare to IC-2.
Electronic supplementary material
ESM 1
(DOC 409 kb)
Rights and permissions
About this article
Cite this article
Ammasi, A., Ponnusamy Munusamy, A. Highly efficient organic indolocarbazole dye in different acceptor units for optoelectronic applications—a first principle study. Struct Chem 29, 967–976 (2018). https://doi.org/10.1007/s11224-018-1073-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1073-9