A mechanophysical model for crack growth kinetics computation on stress corrosion fracture of modified 06G2BA and 08KhMCHA pipe steels is adequately expressed through the plane stress-strain state dα/dt and dJ/dt ratios that are dependent on the strain crack tip rate. The crack growth accelerated by an aggressive environment occurs under static and cyclic loading due to transient dissolution and repassivation processes at the crack tip. Such accelerations are divided into three categories, determined by the strain rate: mechanical cracking (fatigue crack and stationary plastic crack), corrosion-accelerated mechanical cracking (corrosion fatigue and corrosion-accelerated plastic crack), and sulfide stress corrosion fracture. Metallographic studies revealed the change in the crack nucleation and propagation mechanisms, from transcrystalline to intercrystalline, related to the viscoplastic and brittle structure of steel specimens cyclically loaded and simultaneously affected by a corrosive environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Basic Concepts. Pipe steels of oil and gas pipelines, underground sewer systems, etc., after long service in a corrosive environment typical of water-oil-gas mixtures, are prone to strain aging that brings about sulfide stress corrosion fracture of the pipe metal with subsequent failure of industrial and main pipelines [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. The existing scientific research, design, and engineering developments to extend the safe service life of structural steel constructions, particularly their carrying capacity controlled by the extent of crack resistance [1,2,3,4,5,6,7, 13, 14, 16], reveal contradictions and uncertainties. The absence of quantitative, scientifically substantiated practical recommendations to choose optimum modifications of pipe steels, the need for a comprehensive and systems approach to the nature and mechanisms of corrosion-accelerated mechanical failure, and the establishment of optimum methods to evaluate cracking kinetics in such multistressed constructions confirm the topical feature of this problem.
Sulfide stress corrosion fracture (SSCF) is an important challenge for metallophysicists and engineers. Thus, after revealing such damages in oil and gas pipelines and reactor vessel steels, experts from Karpenko Lviv Institute of Physics and Mechanics, Paton Institute of Electric Welding, and Pisarenko Institute of Problems of Strength of the National Academy of Sciences of Ukraine, National Technical University of Ukraine ‘Igor Sikorsky Kyiv Polytechnic Institute’, and others were involved in studying the SSCF nature and mechanism. From the physicomechanical point of view, the basic SSCF effect on structural steels was found to be a rapid crack growth at dynamic loads, greatly accelerated by a corrosive environment. Low-cycle loads significantly increase the crack growth rate, simultaneously reducing the limiting stress intensity factor value compared to static loading [2,3,4,5,6,7, 14, 16]. Moreover, crack growth rates obtained in low-strain rate experiments are much higher than in SSCF experiments at constant loads and higher stress levels [9,10,11,12,13,14,15,16]. Such factors suggest that the corrosion crack growth is more likely dependent on plastic strains at the crack tip than on the stress itself, especially in the presence of a strong catalyst, viz.
Maximum tensile stresses in the vicinity of the crack tip are known to be defined by mechanical fracture conditions [2, 12, 15]. Thus, experiments would be necessary to examine the plane strain fracture, which could be used to get a reliable critical stress value. If SSCF depends on the plastic strain rate in the vicinity of the crack tip, its experiments should take into account additional assumptions to extend the deformable body mechanics concepts for explaining high crack growth rates in low strain rate experiments.
The mechanical characteristics of the structures in long-term service are significantly changed, which is caused by metal fatigue due to its hydrogen sulfide degradation and strain aging [1, 5, 16]. Therefore, practical-scientific results for these specific corrosion damages (SSCF) are much needed to become the basis for the development of a computation-experimental method to assess the residual life of engineering equipment given the strain aging of pipe steel during long-term operation in chemical corrosion-affected environments of industrial facilities.
From the aforesaid, an increase in the safe service life of engineering structures, such as pipelines, remains a very pressing problem, and its solution is of great industrial importance for Ukraine.
This study aims to investigate and elaborate a mechanophysical model based on elastoplastic fracture mechanics concepts to evaluate corrosion crack growth kinetics in low strain rate experiments on compact specimens in the form of a thin (5 mm thick) precracked plate.
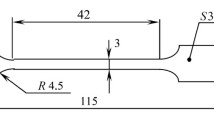
Experimental Procedure and Materials. The crack growth kinetics of modified 08KhMChA and 06G2BA pipe steels were studied on 5 mm thick compact specimens (Fig. 1).
As shown by earlier experimental studies, the critical stress intensity factor KIe in plane strain tested after ASTM E399-78 was less than 4.2 MPa∙m1/2 [15, 16]. Before the tests, the specimens underwent solid solution treatment at 1050°C for 2 h, followed by water quenching. The specimen surfaces were treated with 500-600 grit sandpaper and degreased with vinegar. The crack growth kinetics were evaluated using an Instron 1521 testing machine (UK), having a built-in device with a chamber filled with a NACE solution (5% NaCl solution and 0.5% CH3COOH saturated with hydrogen sulfide). The concentration was set experimentally to get pH 4. Low strain rate experiments were performed at four strain rates (tension), varied within 6.0-200 nm/s at 24°C and 8 MPa. The crack length was measured using a TV camera with a video recorder and time counter. The block diagram of a data processing system is presented in Fig. 2.
Results and Discussion. Intercrystalline cracking of ferritic-pearlitic steel with austenite impurities is treated as the stage of active corrosion cracking, and the model of protective film breakdown is accepted in scientific practice [4, 5, 16]. By the film breakdown model for modified 08KhMChA steel, intergranular cracks grow according to the following steps: strain-induced protective film breakdown on the metal surface, prevailing anodic dissolution in the chromium- and carbon-depleted zone adjacent to the grain boundary, and protective film formation as a result of repassivation. Thus, film fracture mechanics and electrochemical mechanisms responsible for dissolution and repassivation rates govern the crack growth rate. In the computations, the film breakdown rate at the crack tip Vs is assumed to be proportional to the crack tip strain rate. This rate is defined with J-integral as follows:
From recommendations [14], we can assume that the film dissolution rate at the crack tip Ir is dependent on the initial dissolution I0 and repassivation t-n rates
Then, the average dissolution rate \({I}_{r}{\prime}\) will be equal to
Substituting Eq. (1) into (3), we obtain the average dissolution rate or crack growth rate
Equation (4) shows that the crack growth rate can be expressed by the linear log(da/dt) - log(dJ/dt) relation with the slope n. An intermediate conclusion can be drawn that the crack growth rate data for low strain rate experiments are best expressed through dJ/dt and confirm the above mechanophysical model.
A similar computation of the crack growth rate under cyclic loading of 304 stainless steel was carried out in [16]. Under cyclic loading with the stress ratio R and frequency f, Vs is proportional to (1 - R)/(1 + R)∆K2f; then the crack growth rate will be equal to
Thus, the crack growth rate should be expressed by the linear log(da/dt) - log(f) relation with the slope n (here, the slope to the curve axis and the tangent to the crack growth rate vs f curve are meant).
Crack growth data for SSCF-sensitive 08KhMChA steel and less SSCF-sensitive solution-annealed 06G2BA steel under cyclic loading in a NACE solution at 60°C are presented in Fig. 3.
Effect of frequency on the crack growth rate for 08KhMChA steel hardened by know-how technology under sinusoidal cyclic loading in a NACE environment at 40-60°C [15]: (1) intercrystalline crack growth, (2) transcrystalline crack growth, (3) fatigue in air [(1) SSCF under cyclic loading, (2) corrosion fatigue].
The growth of a transcrystalline corrosion-fatigue crack for solution-annealed steel is noticeably accelerated compared to the crack growth in the air; the corresponding curve slope is, on average ≈ 0.6-0.7. For 08KhMChA steel, the crack growth rate increases significantly at loading frequencies less than 10–3 and is accompanied by the transition from the transcrystalline to the intercrystalline cracking mechanism. The curve of intercrystalline cracking at very low frequencies has a slope of ≈ 0.3-0.4; at high frequencies, the curve has a very flat slope. As is seen from Eq. (5), the crack growth vs. frequency is defined by the repassivation rate at the crack tip. The repassivation rate for transcrystalline cracking is higher than that of the intercrystalline one, which proceeds along the chromium and carbon- depleted zone adjacent to grain boundaries. The transition of the cracking mechanism from transcrystalline to intercrystalline can be explained by the competition between transcrystalline corrosion fatigue and intercrystalline SSCF that differently depends on the frequency of cyclic loading.
Comparison of the crack growth under cyclic loading with that under static one demonstrated [16] a complete analogy in both cases, which may be based on the above physicomechanical model in which the crack growth acceleration by the environment compared to mechanical cracking dependent on the strain rate and repassivation rates is explained by the corrosion factor effect on crack wedging (the Rebinder effect) [2,3,4].
The mechanical subcritical crack growth under static loading is known as the stationary plastic crack growth [3, 14, 16]. The stationary plastic crack growth at plane strain is described by the parameter Tz [14]
Moreover, under the condition close to the plane stress state, Eq. (6) demonstrates a valid approximation as long as the crack growth rate is low, as well as in the tests of single-type specimens from the material of the higher Tz (08KhMChA steel). By transforming Eq. (6) to a differential form, we obtain the crack growth rate as a function of time
Equation (7) shows that the crack growth is expressed by a linear log(da/dt) - log(dJ/dt) relation with the slope equal to unity, similar to the fatigue crack growth under cyclic loading. The fracture process covers the nucleation of micropores, their growth, and coalescence at the crack tip on its stationary plastic growth. The environment can adversely affect this active surface, as in corrosion fatigue, even when the repassivation rate at the crack tip is relatively high. As is seen in Fig. 3, specimens tested at 200 nm/s and 60°C in NACE exhibit faster transcrystalline plastic crack growth than specimens tested in air at 24°C. This phenomenon can be termed corrosion-accelerated plastic cracking or spalling affected by an aggressive environment.
Based on the above considerations, all the data on the crack growth of compact specimens, including plastic transcrystalline crack growth in air and NACE, are depicted in Fig. 4.
Computation of the crack growth rate vs. J-integral time derivative under cyclic loading [15, 16]: (1) plastic cracks, (2) corrosion-accelerated plastic cracking, (3) SSCF cracks [(1) plastic transcrystalline crack growth, (2) mixed crack growth (intercrystalline and transcrystalline); (3) intercrystalline crack growth].
This relation corresponds to the crack growth for 08KhMChA steel under cyclic loading, including the transition from the transcrystalline mechanism to the intercrystalline one with a strain rate decrease at the crack tip. Thus, the corrosion-accelerated plastic crack and SSCF under static loading correspond to fatigue, corrosion fatigue, and SSCF under cyclic loading, respectively. In other words, the crack growth under dynamic (cyclic or static) loading in a corrosive environment is divided into three categories concerning the crack tip strain rate: mechanical cracking (n = 1, fatigue or stationary plastic crack), corrosion-accelerated mechanical cracking (n = 0.6-0.7), corrosion fatigue or corrosion-accelerated plastic crack, and SSCF (n = 0.2-0.3). Corrosion-accelerated mechanical cracking and SSCF are the same phenomena under dynamic loading but differently accelerated by the environment. For corrosion-accelerated mechanical cracking with a high repassivation rate (n = 0.7-0.8), the crack cannot grow without slip steps formed due to dynamic loading. On the other hand, at a very low repassivation rate (n = 0.2-0.3), the crack can grow spontaneously by repeating a certain anodic dissolution followed by the slip step formation at the crack tip, even under static loading [14, 16]. This cracking is termed SSCF.
A comparison of maximum growth rates at intercrystalline SSCF demonstrates that the crack growth rate in low strain rate experiments is more than 7-8 times higher than that under cyclic loading. This difference is too great to be explained by the stress state, static and cyclic loading features, or temperature differences. It can be explained by differences in the chemical reaction rate inside the crack since the dissolution and repassivation transient rates can be determined by the diffusion of oxygen, sulfur, and corrosion products dissolved in NACE within the crack.
Thus, though the rate of slip step formation is the same for both processes, the wider crack growth rate in low- strain rate experiments should be higher than that of the sharp crack under cyclic loading. It should be noted that the crack sharpness or opening under static loading can also be computed from the da/dt and dJ/dt ratios. Further studying this phenomenon would require additional investigations to elaborate the theory of strains at the crack tip, particularly at a plane stress state, in view of corrosive environment effects.
The above results of mechanophysical studies were supplemented with metallographic results from fracture fractograms obtained with a JEOL scanning electron microscope (Japan), as shown in Figs. 5-8.
As seen in Figs. 5 and 6, the intercrystalline crack is formed and further propagates along hypoeutectoid ferrite and pearlite boundaries. Moreover, the transcrystalline crack extends through the viscoplastic zone; then, its growth is facilitated in the brittle zone in the presence of hardened upper bainite and residual cementite grains.
Data in Figs. 7 and 8 demonstrate the change in the crack growth mechanism from transcrystalline to intercrystalline. Notably, in both cases, the crack nucleation originates directly from nonmetallic inclusions, particularly ferric and manganous sulfides (Fig. 8).
Conclusions
-
1.
The mechanophysical model to compute the crack growth kinetics on stress corrosion fracture for modified 06G2BA and 08KhMChA pipe steels was elaborated and adequately expressed through the plane stress-strain state dα/dt and dJ/dt ratios dependent on the crack tip strain rate. The crack growth was accelerated by an aggressive environment under static and cyclic loading due to the transient processes of dissolution and repassivation at the crack tip. Such accelerations were divided into three categories determined by the strain rate: mechanical cracking (fatigue crack and stationary plastic crack), corrosion-accelerated mechanical cracking (corrosion fatigue and corrosion- accelerated plastic crack), and sulfide stress corrosion fracture.
-
2.
Metallographic studies demonstrated the change in the crack nucleation and propagation mechanisms, from transcrystalline to intercrystalline, associated with the viscoplastic and brittle structure of steel specimens cyclically loaded and simultaneously affected by a corrosive environment.
References
A. G. Gumerov, N. M. Yamaleev, and G. V. Zhuravlev, Metal Crack Resistance of Pipes of Oil Pipelines [in Russian], Nedra-Business Center LLC, Moscow (2001).
V. D. Makarenko, Reliability of Oil and Gas Field Systems [in Russian], TsNTI, Chelyabinsk (2006).
V. D. Makarenko, S. I. Grachev, and N. N. Prokhorov, Welding and Corrosion of Oil and Gas Pipelines in Western Siberia [in Russian], Naukova Dumka, Kyiv (1996).
V. D. Makarenko, S. Yu. Maksymov, S. I. Bilyk, et al., Corrosion Fracture of Sewage Systems of Ukraine [in Ukrainian], NUBiP of Ukraine, Kyiv (2021).
M. I. Samoilenko, Functional Reliability of Pipelines of Transport Systems [in Ukrainian], KhNAMG, Kharkiv (2009).
I. V. Orynyak and V. V. Rozgonyuk, “Life, durability, and reliability of pipelines. Review of modern approaches and problems of standard coverage in Ukraine” [in Ukrainian], Naft Gaz Prom, No. 4, 54-57 (2003).
N. G. Nasonkina, E. G. Antonenko, and A. S. Tryakiva, “Analysis of damage to water supply and sewerage networks” [in Ukrainian], Such Prom Tsyv Bud, 15, No. 1, 2334 (2019).
Y. Y. Meshkov, “On the possibility of stable equilibrium of sharp cracks at their initiation in metals” [in Russian], Metallofizika, Issue 23, 5-12 (1989)
G. S. Pisarenko and V. A. Strizhalo, Experimental Methods in Mechanics of Deformable Solid Body [in Russian], Naukova Dumka, Kiev (1986).
T. Kawakubo and M. Hishida, “Elastic-plastic fracture mechanics analysis on environmentally accelerated cracking of stainless steel in high temperature water,” J Eng Mater Technol, 107, 240-245 (1985).
“Method for determination of crack resistance parameters ASTM E399-78 (for compact specimens and specimens with a central notch),” J Eng Mater Technol, 107, 107-116 (2005).
F. P. Ford and M. J. Silverman, “Effect of loading rate on environmentally controlled cracking of sensitized 304 stainless steel in high purity water,” Corrosion, 36, 597-603 (1980).
T. Kawakubo and M. Hishida, “Crack initiation and growth analysis by direct optical observation during SSRT in high temperature water,” Corrosion, 40, 120-126 (1984).
J. D. Landes and J. A. Begley, “A fracture mechanics approach to creep crack growth,” in: Mechanics of Crack Growth, ASTM STP 590, American Society for Testing and Materials, Philadelphia, pp. 128–148 (1976).
ASTM Designation E647-81, Annual Book of ASTM Standards, ASTM, Philadelphia (1982).
N. E. Nanneman, “Models for the intergranular stress corrosion cracking of welded type 304 stainless steel piping in boiling water reactors,” in: Proc. of Seminar on Countermeasures for BWR Pipe Cracking, Session 1, EPRI (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Problemy Mitsnosti, No. 5, pp. 39 – 46, September – October, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Makarenko, V.D., Pobeda, S.S., Makarenko, Y.V. et al. Elastoplastic Fracture Mechanics Approach to the Crack Growth Rate Computation of Modified Pipe Steels. Strength Mater 55, 937–944 (2023). https://doi.org/10.1007/s11223-023-00584-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11223-023-00584-7