Specific features of the stress-corrosion fracture of specimens of Kh70 pipe steel are analyzed under the conditions simulating the influence of various combinations of stress-corrosion factors. For the evaluation of the susceptibility of steels to stress-corrosion cracking (SCC), we propose to use a dimensionless coefficient Ks equal to the ratio of the relative narrowing of the specimen in air to its relative narrowing in a solution. We introduce the following evaluation criterion: steel is susceptible to SCC if the coefficient Ks is equal to or greater than 1.6.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of underground pipelines is the most efficient and safest method for the long-distance transportation of oil and gas [1, 2]. For their protection, it is customary to use anticorrosion insulation and electrochemical protection but it is impossible to completely suppress the influence of the ambient medium [1, 3]. Thus, the phenomenon of stress-corrosion cracking (SCC) proves to be one of potential threats for their safe operation [2,3,4,5]. In the course of long-term operation under the indicated conditions, irreversible processes run in the metal even in the case where all technological requirements and conditions are satisfied. This lowers the possibility of subsequent safe operation of the pipeline [6]. Stress corrosion cracking develops under the simultaneous action of mechanical stresses and the complex influence of the ambient medium and metallurgical factors [2].
At present, extensive investigations of this phenomenon are carried out in different countries. In particular, the influence of stresses, hydrogen, and their synergism on the dissolution of steel at the crack tip in media whose pH values are close to neutral was quantitatively evaluated in [7,8,9,10,11,12,13], the effect of cathodic polarization on the mechanism of SCC was analyzed in [14, 15], and a conceptual model for the description of SCC under the conditions of cathodic protection in solutions whose pH values are close to neutral was proposed in [16]. The role of carbon dioxide and sulfate-reducing bacteria in the initiation of SCC was clarified in [17,18,19]. It was emphasized that there are no evident relationships between the failures of pipelines caused by SCC for the pH values close to neutral (or higher) and the chemical compositions or microstructures of the pipes [17]. At the same time, it was discovered [9] that, in solutions with almost neutral рН values, the pipes made of Kh70 steel with bainitic microstructure are highly susceptible to SCC, whereas the pipes made of Kh70 steel with ferritic matrix are weakly susceptible to SCC.
Under the laboratory conditions, the susceptibility of the metal to SCC is determined according to the results of the tests carried out under various conditions (U-bend, C-ring, bent beam, and slow strain-rate tensile tests) [20, 21]. These results make it possible to predict the susceptibility of this kind of steel to the analyzed type of corrosion in a given medium. On the basis of the results of electrochemical investigations, it is possible to determine the mechanism of SCC [22, 23].
In what follows, we propose both a methodical approach and a criterion for the evaluation of the susceptibility of pipe steels to SCC under the conditions of complex influence of various factors.
We tested samples of Kh70 pipe steel of controlled rolling taken from pipes 1420 mm in diameter with wall thicknesses of 15.7 and 17.5 mm after operation. The chemical composition of steel was as follows (wt.%): 0.095 C, 1.39 Mn, 0.255 Si, 0.005 S, 0.017 P, 0.032 Al, 0.04 Ni, 0.03 Mo, 0.004 Ti, 0.05 V, 0.027 Nb, and 0.04 Cr.
The mechanical properties of the base metal are as follows: the yield strength varies within the range 498–513 MPa (≥ 441 MPa according to the requirements); the ultimate rupture strength is equal to 600–603 MPa (≥ 588 MPa according to the requirements); the relative elongation is as large as 21.5–24.2% (the norm is ≥ 20%), and the impact toughness of V-notched specimens (KCV–15 ) constitutes 224–227 J/cm2 (the norm is ≥ 78.4 J/cm2). The ratio σy /σu, which is also specified by the SNiP 2.05.06, varies within the range 0.83–0.87 (≤ 0.9 according to the requirements). The role of working solution is played by a model soil electrolyte (NS4 solution) with the following composition (g/liter): 0.037 KCl + 0.559 NaHCO3 + 0.008 CaCl2 + 0.089 MgSO4 ; рН 8 [24].
The tests were performed at a small strain rate according to GOST 9.901-1. Plane specimens (Fig. 1) were stretched in an AIМА-5-1 tensile testing machine at a rate of 10–6 sec–1 under corrosion potentials equal to –0.75, –1, and – 2 V (relative to a silver–silver-chloride reference electrode).
The cross-sectional area of the working part of the specimens in the initial state was 9 mm2. The potential was set and controlled by using a PI-50-1.1 potentiostat and a PR-8 programmer. In the course of the tests, we measured the level of stresses, the elongation of the specimen ∆L, and the time to fracture τ . After fracture, we determined the cross-sectional areas of the specimens.
Results and Discussion
For the investigation of the susceptibility of metal to corrosion cracking, it is customary to use the method of slow-strain-rate tests [e.g., (10–3–10–6 )sec–1] under the simultaneous influence of corrosive media and induced potentials [25]. For almost two days, the specimen undergoes either ductile fracture or cracking depending on the susceptibility of the metal. After the tests, we record either the time to fracture of the specimens or, more often, the time prior to the initiation of the first crack. However, it is sometimes difficult to detect the time of crack initiation. Moreover, in this case, the cross-sectional area of specimens after the tests and the character of fracture, which are important characteristics of stress corrosion cracking, are not analyzed.
The plasticity of the metal depends on the relative elongation and narrowing: the higher the values of these characteristics, the greater the plasticity of the material. For steel, the value of relative narrowing may vary from 40 to 65% [26]. In the solution, these values are definitely lower than in air. Moreover, for various combinations of the corrosion factors, one may expect to get different types of fracture of the specimens, namely, ductile, ductile with brittle cleavage, or brittle.
To evaluate the susceptibility of the material to SCC, the researchers use the coefficients of decrease in the ultimate strength, cross-sectional area, and elongation [9, 16, 24]. The susceptibility of pipe steel to SCC under the influence of various combinations of stress-corrosion factors was investigated in [27,28,29,30]. In what follows, by using the experimental results, we develop a methodology and propose a criterion for the evaluation of the susceptibility of pipe steel to SCC under the complex action of various factors.
For this purpose, we use a dimensionless coefficient Ks that takes into account the variations of the plastic properties of the metal in corrosive media and in air. This coefficient is determined as the ratio of the relative narrowing in air to the relative narrowing in the solution
where Ψair and Ψsol are the values of relative narrowing in air and in the solution, respectively, S0 is the cross-sectional area of the specimens prior to testing, mm2 , and \( {S}_1^{\mathrm{air}} \) and \( {S}_1^{\mathrm{sol}} \) are, respectively, the crosssectional areas at the sites of fracture after testing in air and in a solution, mm2.
In analyzing possible values of the cross-sectional areas of the specimens after tests, it is possible to distinguish the following two limit states:
-
if \( {S}_1^{\mathrm{sol}}\to {S}_1^{\mathrm{air}}, \) then \( {\Psi}_1^{\mathrm{sol}}\to {\Psi}_1^{\mathrm{air}},{K}_s\to 1, \) the process is accompanied by ductile fracture, and the susceptibility to SCC is low;
-
if \( {S}_1^{\mathrm{sol}}\to {S}_0, \) then Ψsol → 0, Ks → ∞ , the process is accompanied by brittle fracture, and the susceptibility to SCC is high.
Thus, the values of the coefficient Ks may theoretically vary from 1 to infinity. In practice, in order to predict possible ways of development of SCC, it is necessary to introduce its threshold value above which steel becomes susceptible to SCC under the analyzed conditions.
In our tests, we modeled the action of various combinations of stress-corrosion factors: potentials, complete immersion into the solutions and periodic wetting, accumulated cyclic stresses, the presence of coatings with different transient resistances on the surface, and the presence of local corrosion centers. Some specimens were subjected to cycling in the elastic region within the range of ultimate stresses (0.4–0.8)σy at a frequency of 10 Hz for 105 cycles. The other specimens were not treated (in what follows, they are called specimens in the intact state). To accelerate the initiation of stress-corrosion cracks, we simulated a local corrosion center (LCC), i.e., prepared a V-notch whose depth varied from 0.25 to 0.3 mm on one surface of the specimen.
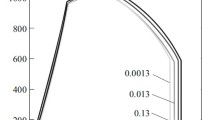
We analyzed the duration of the period of elevated levels of groundwater in the spring–autumn period on the territory of Ukraine and established the following periodicity of wetting in a cycle: 50 min in the solution, and 10 min in air. Since SCC occurs on cathodically protected pipelines, we studied specimens both with and without polymeric coatings. It was shown that the difference between the curves of stress-corrosion fracture of steel is significant (Fig. 2). This is reflected in the properties of steel (see Table 1).
Curves of corrosion-mechanical fracture of the specimens of Kh70 pipe steel for different combinations of stress-corrosion factors under testing conditions: (1) in air; (2) in the intact state with a layer of polymeric primer, – 0.75 V; periodic wetting by the solution; (3) in the intact state without coating but with a local corrosion center; (4) after cycling with a layer of an epoxy coating, – 1 V; periodic immersion into the solution; (5) in the intact state without coating, – 1 V; periodic wetting by the solution, (6) after cycling without coating, – 2 V; complete immersion into the solution.
In particular, in air, the specimens shrink near the site of fracture characterized by the ductile fracture surface. In the fracture curve, we detect a section of ductile plastic deformation. The fracture curves plotted at a potential of – 0.75 V for a specimen with a layer of polymeric primer both under the conditions of periodic wetting by a solution (curve 2) and in air are similar but the time to fracture decreases and the cross-sectional area increases by about 5%. The specimens with LCC placed in the solution and kept at the corrosion potential (curve 3) and in the intact state without coating subjected to periodic wetting by the solution at a potential of – 1 V (curve 5) failed directly under the maximum load, which was accompanied by the decrease in the cross-sectional area and in the coefficient Ks as compared with the same characteristics of fracture of the specimens in air. A similar character of fracture was also typical of a specimen without coating subjected to cycling in the solution under a potential of – 2 V (curve 6) but with a noticeable decrease in the cross-sectional area and in the coefficient Ks.
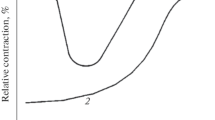
By analyzing the appearance of specimens after fracture (Fig. 3), we can distinguish at least two groups characterized by fundamentally different types of their fracture surfaces. Thus, specimens from the first group with numbers 2–7 underwent plastic deformation in the course of the tests, which led to formation of the socalled “neck”; in this case, fracture was ductile and the coefficient Ks varied from 1.04 to 1.56.
Images of the fracture surfaces of the specimens of Kh70 pipe steel after stress-corrosion tests in air under different conditions (а) and their susceptibility to SCC (b). Numbers 1–15 correspond to the numbers of specimens in Table 1.
In the fracture surfaces of specimens 8–15, we visually detect a smaller fraction of plastic deformation corresponding to a decrease in the relative elongation, an increase in relative narrowing and, hence, an increase in the coefficient Ks up to 1.6–3.5.
Thus, the analyzed steel is susceptible to SCC under the complex influence of different factors if the value of the coefficient Ks is not smaller than 1.6. This criterion is used as basic in the procedure of investigation of the susceptibility of pipe steel to SCC by simulating the action of internal and external factors under the laboratory conditions [31].
Conclusions
For the evaluation of the susceptibility of pipe steel to SCC under various conditions, we propose to use a dimensionless coefficient Ks equal to the ratio of the relative narrowing of a specimen in air to its relative narrowing in a solution. On the basis of the analysis of the array of experimental data, we propose to introduce the following criterion: Steel is regarded as susceptible to SCC if the value of the coefficient Ks is not smaller than 1.6. By using this criterion, it is possible to predict the susceptibility of pipe steel to SCC according to the data of laboratory investigations by simulating the action of various combinations of stress-corrosion factors. The test results form a basis of the “Procedure of investigation of the susceptibility of pipe steel to stress-corrosion cracking based on the simulation of internal and external factors under the laboratory conditions” [31].
References
F. Y. Cheng, “Pipeline Engineering,” in: Y. F. Cheng (editor), Pipeline Engineering, Encyclopedia of Life Support System (EOLSS), Developed under the Auspices of the UNESCO, EOLSS, Oxford (2010).
V. G. Antonov, А. G. Arabei, V. N. Voronin, I. А. Dolgov, М. М. Kantor, Z. Knosziński, and Yu. P. Surkov, Stress-Corrosion Cracking of the Pipes of Gas Mains [in Russian], Nauka, Moscow (2006).
M. Baker, Stress Corrosion Cracking Study: Final Report for OPS TTO8. Integrity Management Program, Calgary (2005).
R. N. Parkins, “A review of stress corrosion cracking of high pressure gas pipelines,” in: Corrosion 2000, Paper No. 363, NACE International, Houston, (2000).
Canadian National Energy Board. Report of Public Inquiry Concerning Stress Corrosion Cracking on Canadian Oil and Gas Pipelines, MH-2-95 (1996).
E. A. Spiridovich, Elevation of the Reliability of Gas Mains under the Conditions of Stress Corrosion Cracking [in Russian], Candidate-Degree Thesis (Engineering), Nizhnii Novgorod (2014).
G. A. Zhang and Y. F. Cheng, “Micro-electrochemical characterization of corrosion of welded X70 pipeline steel in near-neutral pH solution,” Corros. Sci., 51, Issue 8, 1714–1724 (2009).
X. Tang and Y. F. Cheng, “Micro-electrochemical characterization of the effect of applied stress on local anodic dissolution behavior of pipeline steel under near-neutral pH condition,” Electrochim. Acta, 54, 1499–1505 (2009).
Z. Y. Liu, X. G. Li, C. W. Du, G. L. Zhai, and Y. F. Cheng, “Stress corrosion cracking behavior of X70 pipe steel in an acidic soil environment,” Corros. Sci., 50, 2251–2257 (2008).
D. Hardie, E. A. Charles, and A. H. Lopez, “Hydrogen embrittlement of high strength pipeline steels,” Corros. Sci., 48, Issue 12, 4378–4385 (2006).
J. Capelle, J. Gilgert, I. Dmytrakh, and G. Pluvinage, “Sensitivity of pipelines with steel API X52 to hydrogen embrittlement,” Int. J. Hydrogen Energy, 33, 7630–7641 (2008).
T. Michler and J. Naumann, “Microstructural aspects upon hydrogen environment embrittlement of various bcc steels,” Int. J. Hydrogen Energy, 35, 821–832 (2010).
T. Neeraj, R. Srinivasan, and J. Li, “Hydrogen embrittlement of ferritic steels: observations on deformation microstructure nanoscale dimples and failure by nanovoiding,” Acta Mater., 60, 5160–5171 (2012).
Z. F. Wang and A. Atrens, “Initiation of stress corrosion cracking for pipeline steels in a carbonate–bicarbonate solution,” Metall. Mater. Trans. A, 27, 2686–2691 (1996).
Z. Y. Liu, G. L. Zhai, X. G. Li, and C. W. Du, “Effect of deteriorated microstructures on stress corrosion cracking of X70 pipeline steel in acidic soil environment,” J. Univ. Sci. Technol. Beijing, 15, 707–713 (2008).
Z. Y. Liu, X. G. Li, and Y. F. Cheng, “Mechanistic aspect of near-neutral pH stress corrosion cracking of pipelines under cathodic polarization,” Corros. Sci., 55, 54–60 (2012).
B. Y. Fang, A. Atrens, J. Q. Wang, E. H. Han, Z. Y. Zhu, and W. Ke, “Review of stress corrosion cracking of pipeline steels in “low” and “high” pH solutions,” J. Mater. Sci., 38, 127–132 (2003).
T. M. Ahmed, S. B. Lambert, A. Plumtree, and R. Sutherby, “Cyclic crack growth rates of X-60 pipeline steel in a neutral dilute solution,” Corrosion, 53, 581–590 (1997).
L. Niu and Y. F. Cheng, “Corrosion behavior of X-70 pipe steel in near-neutral pH solution,” Appl. Surf. Sci., 253, 8626–8631 (2007).
NACE TM-0198-2004, Slow Strain Rate Test Method for Screening Corrosion Resistant Alloys (CRAs) for Stress-Corrosion Cracking in Sour Oil-field Service, NACE International, Houston, Texas, USA (2004), pp. 1–17.
W. B. Lisagor, “Environmental cracking-stress corrosion,” in: Corrosion Tests and Standards, ASTM International, Baltimore (2005), pp. 289–301.
Z. Y. Liu, X. G. Li, and Y. F. Cheng, “Mechanistic aspect of near-neutral pH stress corrosion cracking of pipelines under cathodic polarization,” Corros. Sci., 55, 54–60 (2012).
M. Javidi and S. Bahalaou Horeh, “Investigating the mechanism of stress corrosion cracking in near-neutral and high pH environments for API 5L X52 steel,” Corros. Sci., 80, 213–220 (2014).
K. Z. Szklarska-Smialowska, Z. Xia, and R. B. Rebak, “Technical note: stress corrosion cracking of Х-52 carbon steel in dilute aqueous solutions,” Corrosion, 50 (5), 334–338 (1994).
GOST 9.901.1-89, Unified System of Corrosion and Aging Protection. Metals and Alloys. General Requirements to the Methods of Testing for Corrosion Cracking [in Russian], Izd. Standartov (1999).
G. S. Pisarenko (editor), Strength of Materials. A Textbook for Institutions of Higher Education [in Russian], Vyshcha Shkola, Kyiv (1979).
L. І. Nyrkova, N. О. Hapula, A. O. Rybakov, S. O. Osadchuk, and S. L. Mel’nychuk, A Method of Testing for the Susceptibility of Pipe Steels to Stress Corrosion Cracking under the Influence of Variable Wetting [in Ukrainian], Patent for Invention No. 107381 MPK (2014.01) G01N 17/00 G01N 3/00 G01N 3/08 (2006.01) G01N 3/20 (2006.01), Publ. on 10.12.2014, Byul. No. 2.
L. I. Nyrkova, S. L. Mel’nychuk, S. O. Osadchuk, A. O. Rybakov, and N. О. Darahanova, “Development of a methodical approach to the investigation of stress corrosion cracking with regard for the complex influence of factors,” Rozv. Rozrob. Naft. Gaz. Rodov., No. 2 (63), 59–65 (2017).
L. I. Nyrkova, “Analysis of the influence of combinations of stress-corrosion factors on the stress-corrosion cracking of pipe steel for near-neutral рH values,” Visn. NTU “KhPI” , No. 16 (1238), 12–16 (2017).
L. I. Nyrkova, S. L. Mel’nychuk, S. O. Osadchuk, and A. O. Rybakov, “Corrosion cracking of Kh70 pipe steel for potentials close to the maximum protective potential,” Fiz.-Khim. Mekh. Mater., 54, No. 4, 110–115 (2018); English translation: Mater. Sci., 54, No. 4, 567–572 (2019).
Procedure of Investigation the Susceptibility of Pipe Steel to Stress-Corrosion Cracking Based on the Simulation of Internal and External Factors under the Laboratory Conditions [in Ukrainian], Paton Institute of Electric Welding, Ukrainian National Academy of Sciences, Kyiv (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 55, No. 5, pp. 14–20, September–October, 2019.
Rights and permissions
About this article
Cite this article
Nyrkova, L.І., Osadchuk, S.О., Rybakov, А.О. et al. Methodical Approach and a Criterion for the Evaluation of the Susceptibility of Pipe Steel to Corrosion Cracking. Mater Sci 55, 625–632 (2020). https://doi.org/10.1007/s11003-020-00352-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-020-00352-x