Abstract
In this work a new core–shell nanocatalyst, Fe3O4@SiO2/Schiff base of Ni(II), (Fe3O4@SiO2/SB(Ni)), was synthesized and characterized. The Schiff base immobilized on magnetite nanoparticles introduced in this study, is a simple and aliphatic Schiff base. The synthesis steps were meticulously followed. First, magnetite-silica nanoparticles were prepared and functionalized by –NH2 groups using 3-amino propyl triethoxysilane (APTES). Subsequently, the aliphatic Schiff base ligand was immobilized through a condensation reaction between the –NH2 groups on the magnetite-silica and the –C=O groups of acetylacetone. The supported Schiff base complex of Ni(II) on magnetite-silica was synthesized by a reaction between immobilized Schiff base and Ni(II) acetate tetrahydrate salt. Finally, the immobilized Ni(II) Schiff base complex was fully characterized using several techniques including, FT-IR, VSM, XRD, FE-SEM, EDX, TEM, BET, TGA-DTA, and AAS. The magnetically recoverable core–shell nanocatalyst, demonstrated remarkable catalytic activity in the synthesis of 3,4-dihydropyrimidine-2-(1H)-one. This reaction involved three components: an aldehyde, β-keto ester, and urea, and was carried out via the solvent-free Biginelli reaction. The results indicate that the products are synthesized in good to excellent yields (82–91%) within 13–22 min. At the end of the reaction, the nanocatalyst can be easily removed from the reaction mixture using an external magnet and reused several times. The synthesized products were purified and characterized by FT-IR and 1H NMR techniques.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among transition metal complexes, Ni(II) complexes have been extensively used as catalysts for the Biginelli reaction. The Biginelli reaction was first reported in 1893 by the Italian chemist Pietro Biginelli for the synthesis of 3,4 dihydropyrimidinones (DHPM) under highly acidic conditions. This multi-component reaction results from the condensation between a β-ketoester, an aldehyde, and urea in an appropriate solution under reflux conditions [1]. In this reaction, HCl serves as the catalyst. It is noteworthty that 3,4-dihydropyrimidin-2-(1H)-ones (DHPMs) and their derivatives have received much attention due to their important pharmacological and therapeutic properties including antiviral [2], antibacterial [3], anti-inflammatory [4], anticancer [5], antihypertensive [6] in medical and organic chemistry. Numerous methods have been reported for the synthesis of dihydropyrimidine derivatives utilizing a variety of catalysts such as GaCl3 [7], DBU [8], MNP-L-proline-SO3H [9], Li(Glycine)(CF3SO3) [10], MOx-MWCNTs [11], Si-[SbSipim][PF6] [12], KF-modified clay [13], chiral copper(II) complex [14]. However, most of the mentioned catalysts suffer from drawbacks such as long reaction times, formation of toxic by-products, highly acidic conditions, use of hazardous organic solvents, unrecyclable catalysts and low yields. To address these weaknesses, the immobilized catalysts on magnetic nanoparticles (MNPs) serve as suitable options [15,16,17]. These magnetic nanocatalysts are heterogeneous. In comparison between homogeneous and heterogeneous catalysts, homogeneous catalysts are commonly more efficient however, heterogeneous catalysts have some advantages. Heterogeneous catalysts find extensive use due to their ease of recovery and separation, although they exhibit lower reactivity and selectivity compared to homogeneous catalysts [18, 19]. This weakness can also be overcome by immobilizing the catalyst onto magnetic nanoparticles [20]. Magnetic nanoparticles demonstrate high dispersion and reactivity in the reactions owing to their nanometer sizes. Recent advancements in catalysis have led to the synthesis and documentation of diverse catalysts supported on magnetic nanoparticles. [21,22,23,24]. Additionally, MNPs have attracted significant interest due to their unique properties including high surface area, low toxicity and price, easy synthesis and functionalization, separate-ability, and biocompatibility [25,26,27]. Despite these advantages, the use of nanoparticles as catalyst has some disadvantages. One such drawback is highly active surfaces which can lead agglomeration. To mitigate this issue, an organic or inorganic shell can prevent the occurrence of aggregation of magnetic nanoparticles [28,29,30]. Nowadays, catalytic systems based on MNPs find widespread application in chemical reactions. A key advantages of these systems is their easy separation facilitated by the use of a simple external magnet. [31].
Schiff base ligands and complexes are well-established in coordination chemistry and extensive studies have explored various aspects from synthesis to application [32,33,34,35,36]. These compounds find applications as catalysts in diverse reactions, including olefins epoxidation [37], polymerization [38], oxygen activation [39] and, carbon dioxide transformation [40]. However, homogenous metal Schiff base complex catalysts are prone to deactivated due to the formation of dimeric peroxo and-oxo species [41]. To address this limitation, Schiff base ligands and complexes can be immobilized on magnetic cores such as magnetite silica functionalized with –NH2 groups. These magnetic core–shell structures serve as effective catalysts in organic reactions and, at the end of the reaction, the catalyst can be easily extracted from the reaction mixture using an external magnet [42, 43]. Furthermore, the Synthesis of various materials supported on magnetic nanoparticles and their applications as catalysts for the preparation of 3,4-dihydropyrimidin-2-(1H)-ones (DHPMs) has been the subject of several kinds of literature [44,45,46,47].
As part of our ongoing research program on the development of efficient methods for the preparation of biologically active compounds [48,49,50,51,52], In this work, an aliphatic Schiff base complex of Ni(II) that was immobilized on functionalized silica-coated magnetite nanoparticles [(Fe3O4@SiO2/SB(Ni)] was synthesized and fully characterized by various techniques. The synthesized and utilized aliphatic Schiff base in this study serves as a straightforward and economically viable ligand. Despite its low cost and use of inexpensive materials, the Schiff base complex has exhibited remarkable catalytic efficiency as a nano-catalyst with dimensions less than 100 nm. This novel and the very efficient catalyst was used for the green synthesis of 3,4 dihydropyrimidines in solvent free conditions. This method has been reported as a suitable method for the preparation of Biginelli compounds due to the short reaction time, simple purification of the product with high efficiency, and solvent-free condition.
Experimental
Materials and instrumentations
All materials (solvents and chemicals) used in this work were obtained from Merck and Aldrich chemical company and were used without further purification. The melting point of synthetic derivatives was measured using an electrothermal MK3 apparatus. The progress of the reaction was monitored by thin-layer chromatography (TLC) with an aluminum plate and silica gel F254 under a UV lamp. 1H NMR spectra of products were obtained by Bruker Avance spectrometer instrument in DMSO-d6 as the solvent. FT-IR spectra of all samples and catalyst were reported by the Perkin Elmer-550 infrared spectrometer with potassium bromide pellets. X-ray diffraction (XRD) of the catalyst was Specified using X’PertPro (Cu Kα radiation, λ = 1.54 Å) in the region of 2ϴ = 20°–80°. The morphology, particle size, and composition of the catalyst were obtained by Field Emission scanning electron microscopy (FE-SEM, Hitachi S4160) equipped with energy-dispersive X-ray spectrometry (EDX). Transmission electron microscopy (TEM) images were obtained on a Zeiss EM10C with an accelerating voltage of 100 kV.Nickel content in the catalysts was determined by a Perkin-Elmer 2380 atomic absorption spectrophotometer. The magnetic properties of the nanocatalyst were studied by Meghnetis daghigh kavir Co Vibrating sample magnetometer (VSM) at room temperature. Thermal gravimetric analysis patterns (TGA) of the catalyst were performed by a Rheometric Scientific Inc. 1998 thermal analysis apparatus under an N2 atmosphere. BET and BJH curves were used for the measurement of nanocatalyst specific surface area; these curves were obtained by an ISO15901-2 model number.
Preparation of Fe3O4 (magnetite) nanoparticles (MNPs)
MNPs were prepared according to the previous report [53]. Briefly, to a solution of FeCl2.4H2O (15 mmol) in 20 ml of distilled water was added a solution of FeCl3.6H2O (22 mmol) in 30 ml of distilled water. The mixture was stirred vigorously for 15 min. and vigorous stirring continued for 30 min. at 70 °C. Thereupon NH3 25% was added into the solution until the pH was enhanced to 10. The solution was stirred for 2.5 h at 60 °C. The final solid was collected by an external powerful magnet and washed with water and ethanol and dried under vacuum for 8 h at 70 °C.
Preparation of core–shell structure of Fe3O4@SiO2
The silica layer (SiO2) in the core–shell structure of Fe3O4@SiO2 was prepared through some modification of the Stober method [54]. The MNPs powder (0.5 g) was added to 50 ml ethanol and 5 ml water, and then MNPs were dispersed by ultrasonic vibration in a water bath for 15 min. After that, TEOS (0.2 mL) and NaOH (5 ml) (10%W) were added dropwise to the mixture and stirred at room temperature for 1 h. The product was collected using a powerful external magnet, washed with water, and ethanol, and dried under vacuum for 10 h at 80 °C.
Functionalizing of magnetite silica nanoparticles by aminopropyl trieththoxysilane (Fe3O4@SiO2/APTES)
Functionalizing of magnetite silica was carried out according to the previously reported procedure [55]. Briefly, 0.5 g of the Fe3O4@SiO2 was added to a mixture of 0.5 mL aminopropyl trieththoxysilane (APTES) in 12 mL dry toluene. The mixture was dispersed by ultrasonic vibration in a water bath for 15 min. Subsequently, the mixture was refluxed for 24 h at 100 °C. The resulting product was collected using an external magnet, washed with water, toluene, and ethanol, and dried under vacuum for 14 h at 70 °C.
Preparation of nano-Fe3O4@SiO2/Schiff base
The aliphatic Schiff base, supported on MNPs, was synthesized through the condensation reaction between the –NH2 groups on the surface of Fe3O4@SiO2/APTES and the carbonyl group of benzoylaceton. Specifically, Fe3O4@SiO2/APTES (0.3 g) was dispersed in a solution of benzoylaceton (1.2 mmol) in 25 mL ethanol using ultrasonic vibration in a water bath for 15 min. Subsequently, the mixture was refluxed for 24 h at 100 °C. The resulting product was collected, and washed with ethanol, and dried under vacuum for 10 h at 50 °C.
Preparation of nano-Fe3O4@SiO2/SB(Ni)
0.5 g of the Fe3O4@SiO2/Schiff base was dispersed in 20 mL ethanol using ultrasonic vibration in a water bath for 15 min. Next, Ni(II)acetate (1.8 mmol) was dissolved in 20 mL ethanol and added to the mixture of Fe3O4@SiO2/Schiff-base and refluxed for 24 h. The resulting product was collected using a magnet, washed with water and ethanol, and dried under vacuum for 12 h at 100 °C.
preparation of 3,4-dihydropyrimidin-2-(1H)-ones
There are several methods to prepare 3,4-dihydropyrimidin-2-(1H)-ones, but the method used in this paper is green and environmentally friendly method because no solvents are used in this method (solvent free). Briefly, a mixture of aryl aldehyde (1.25 mmol), urea (1.25 mmol), and either ethyl acetoacetate or acetylacetone (1.90 mmol) was combined with nano-(Fe3O4@SiO2/SB(Ni)) (0.005 g) as the catalyst and stirred at 100 °C for 15 min. After completion of the reaction (monitored by TLC analysis), the nanocatalyst was removed using a powerful external magnet. The solid products were then purified and identified using 1H NMR and FT-IR.
5-Ethoxycarbonyl-6-methyl-4-phenyl-3,4-dihydropyrimidine-2(1H)-one (4a) IR (KBr, cm-1): 3244, 3116, 2977, 1725, 1646, 1463, 781. 1H NMR (400 MHz, DMSO-d6): δ 9.20 (s, 1H, NH), 7.74 (s, 1H, NH), 7.30 (d, J = 7.3 Hz, 2H, H-Aro), 7.22 (d, J = 7.5 Hz, 3H, H-Aro), 5.13 (s, 1H, CH), 3.97 (q, J = 7.1 Hz, 2H, CH2), 2.23 (s, 3H, CH3), 1.08 (t, J = 7 Hz, 3H, CH3).
5-Ethoxycarbonyl-6-methyl-4-(2-chlorophenyl)-3,4-dihydropyrimidine-2(1H)-one (4b) IR (KBr, cm−1): 3352, 3113, 2976, 1696, 1640,795. 1H NMR (400 MHz, DMSO-d6): δ 9.26 (s, 1H, NH), 7.69 (s, 1H, NH), 7.39 (d, J = 7.7 Hz, 1H, H-Aro), 7.33–7.21 (m, 3H, H-Aro), 5.62 (s, 1H, CH), 3.88 (q, J = 7.0 Hz, 2H, CH2), 2.29 (s, 3H, CH3), 0.98 (t, J = 7.0 Hz, 3H, CH3).
5-Ethoxycarbonyl-6-methyl-4-(4-chlorophenyl)-3,4-dihydropyrimidine-2(1H)-one (4c) IR (KBr, cm−1): 3243, 3118, 2979, 1700, 1647, 783. 1H NMR (400 MHz, DMSO-d6): δ 9.25 (s, 1H, NH), 7.77 (s, 1H, NH), 7.38 (d, J = 8.1 Hz, 2H, H-Aro), 7.23 (d, J = 7.9 Hz, 2H, H-Aro), 5.12 (s, 1H, CH), 3.97 (q, J = 7.1 Hz, 2H, CH2), 2.23 (s, 3H, CH3), 1.08 (t, J = 7.0 Hz, 3H, CH3).
5-Ethoxycarbonyl-6-methyl-4-(2,4-dichlorophenyl)-3,4-dihydropyrimidine-2(1H)-one (4d) IR (KBr, cm−1): 3358, 3105, 2971, 1697, 1642, 816. 1H NMR (400 MHz, DMSO-d6): δ 9.31 (s, 1H, NH), 7.75 (s, 1H, NH), 7.40 (d, J = 8.6 Hz, 3H, H-Aro), 7.30 (d, J = 8.2 Hz, 1H, H-Aro), 5.58 (s, 1H, CH), 3.88 (q, J = 7.2 Hz, 2H, CH2), 2.28 (s, 3H, CH3), 0.99 (t, J = 7.2 Hz, 3H, CH3).
5-Ethoxycarbonyl-6-methyl-4-(3-nitrophenyl)-3,4-dihydropyrimidine-2(1H)-one (4e) IR (KBr, cm−1): 3350, 3112, 2976, 1696, 1639, 1534, 1345, 796. 1H NMR (400 MHz, DMSO-d6): δ 9.37 (s, 1H, NH), 8.16–8.05,7.66 (m, 4H, H-Aro), 7.89 (s, 1H, NH), 5.29 (d, J = 3.4 Hz, 1H, CH), 3.98 (qq, J = 8.2, 3.7 Hz, 2H, CH2), 2.26 (s, 3H, CH3), 1.09 (t, J = 7.1 Hz, 3H, CH3).
5-Ethoxycarbonyl-6-methyl-4-(4-methylphenyl)-3,4-dihydropyrimidine-2(1H)-one (4f) IR (KBr, cm−1): 3347, 3116, 2995, 1684, 1640, 1504, 1366. 1H NMR (400 MHz, DMSO-d6): δ 9.15 (s, 1H, NH), 7.68 (s, 1H, NH), 7.11 (s, 4H, H-Aro), 5.10 (s, 1H, CH), 3.97 (q, J = 7.2 Hz, 2H, CH2), 2.30–2.21 (m, 7H, CH3), 1.09 (t, J = 7.1 Hz, 3H, CH3).
5-Ethoxycarbonyl-6-methyl-4-(4-methoxyphenyl)-3,4-dihydropyrimidine-2(1H)-one (4g) IR (KBr, cm−1): 3242, 3111, 2955, 1705, 1649, 1460, 788. 1H NMR (400 MHz, DMSO-d6): δ 9.15 (s, 1H, NH), 7.66 (s, 1H, NH), 7.13 (d, J = 8.2 Hz, 2H, H-Aro), 6.86 (d, J = 8.3 Hz, 2H, H-Aro), 5.07 (s, 1H, CH), 3.96 (q, J = 7.0 Hz, 2H, CH2), 3.70 (s, 3H, OCH3), 2.22 (s, 3H, CH3), 1.09 (t, J = 7.1 Hz, 3H, CH3).
5-Ethoxycarbonyl-6-methyl-4-(3-hydroxyphenyl)-3,4-dihydropyrimidine-2(1H)-one (4h) IR (KBr, cm−1): 3374, 3119, 2994, 1687, 1641, 1530, 1351, 885. 1H NMR (400 MHz, DMSO-d6): δ 9.34 (s, 1H, OH), 9.13 (s, 1H, NH), 7.66 (s, 1H, NH), 7.08 (t, J = 7.7 Hz, 1H, H-Aro), 6.73–6.63 (m, 2H, H-Aro), 6.61 (d, J = 8.0 Hz, 1H, H-Aro), 5.04 (s, 1H, CH), 3.98 (q, J = 7.3, 6.9 Hz, 2H, CH2), 2.22 (d, J = 2.9 Hz, 3H, CH3), 1.10 (t, J = 7.1 Hz, 3H, CH3).
5-Ethoxycarbonyl-6-methyl-4-(2,3dimethoxyphenyl)-3,4-dihydropyrimidine-2(1H)-one (4i) IR (KBr, cm−1): 3232, 3106, 2940, 1706, 1645, 1478, 772. 1H NMR (400 MHz, DMSO-d6): δ 9.12 (s, 1H, NH), 7.34 (s, 1H, NH), 6.98 (t, J = 7.9 Hz, 1H, H-Aro), 6.93 (d, J = 8.0 Hz, 1H, H-Aro), 6.71 (d, J = 7.5 Hz, 1H, H-Aro), 5.44 (s, 1H, CH), 3.95–3.87 (m, 2H, CH2), 3.76 (d, J = 14.7 Hz, 6H, OCH3), 2.25 (s, 3H, CH3), 1.02 (t, J = 7.2 Hz, 3H, CH3).
5-Ethoxycarbonyl-6-methyl-4-(2,5dimethoxyphenyl)-3,4-dihydropyrimidine-2(1H)-one (4j) IR (KBr, cm−1): 3250, 3113, 2953, 1706, 1645, 1496, 806. 1H NMR (400 MHz, DMSO-d6): δ 9.12 (s, 1H, NH), 7.27 (s, 1H, NH), 6.90 (d, J = 8.9 Hz, 1H, H-Aro), 6.79 (dd, J = 8.9, 3.1 Hz, 1H, H-Aro), 6.57 (d, J = 3.2 Hz, 1H, H-Aro), 5.42 (m, 1H, CH), 3.91 (qt, J = 8.0, 4.5 Hz, 2H), 3.33 (s, 1H, OCH3), 2.26 (s, 3H, CH3), 1.03 (t, J = 7.1 Hz, 3H, CH3).
5-Acetyl-6-methyl-4-phenyl-3,4-dihydropyrimidine-2(1H)-one (4k) IR (KBr, cm−1):3257, 2929, 1704, 1675, 1453, 705. 1H NMR (400 MHz, DMSO-d6): δ 9.17 (s, 1H, NH), 7.82 (s, 1H, NH), 7.31 (d, J = 7.3 Hz, 2H, H-Aro), 7.24 (s, 3H, H-Aro), 5.25 (s, 1H, CH), 2.27 (s, 3H, CH3), 2.09 (s, 3H, CH3).
5-Acetyl-6-methyl-4-(4-methylphenyl)-3,4-dihydropyrimidine-2(1H)-one (4l) IR (KBr, cm−1): 3362, 3095, 2894, 1688, 1632, 1566, 1350, 861. 1H NMR (400 MHz, DMSO-d6): δ 9.12 (s, 1H, NH), 7.74 (s, 1H, NH), 7.11 (s, 4H, H-Ar), 5.20 (s, 1H, CH), 2.26 (dd, J = 5.9, 2.2 Hz, 6H, CH3), 2.07 (s, 3H, CH3).
Results and discussion
Characterization of Fe3O4@SiO2/SB(Ni) (nanocatalyst)
After the successful synthesis of the nanocatalyst, this compound was fully characterized by various spectroscopic and microscopic techniques including FT-IR, TGA, VSM, XRD, FE-SEM, EDX, TEM and BET and AAS. Step by step synthesis of the nanocatalyst [Fe3O4@SiO2/SB(Ni)] has been shown in Scheme 1.
FT-IR spectra
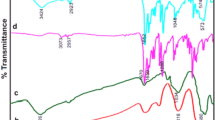
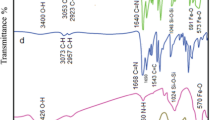
Figure 1 shows the FT-IR spectra of Fe3O4 MNPs (a), Fe3O4@SiO2 core–shell structure (b), magnetite nanoparticles functionalized with –NH2 groups (Fe3O4@SiO2/APTES) (c), Fe3O4@SiO2/Schiff base (d) and Fe3O4@SiO2/SB(Ni) (e). By comparing the FT-IR spectrum of the samples, some points can be mentioned. FT-IR Spectrum of the magnetic Fe3O4 nanoparticles shows a strong absorption at about 579 cm−1 this absorption can be assigned to Fe–O stretching [56]. The broad vibration band which can be seen at about 3400 cm−1 is related to the stretching vibration of O–H groups. This broad stretching can be seen in the FT-IR spectrum of all samples. In the FT-IR spectrum of Fe3O4@SiO2 core–shell (Fig. 1b), the absorption peak seen at about 1017 cm−1is related to the stretching vibrations of Si–O-Si, which indicates the presence of a SiO2layer on the magnetite nanoparticles [57]. In the FT-IR spectrum of Fe3O4@SiO2/APTES (Fig. 1c), in addition to the bands related to magnetite and silica, the bands can be seen at about 1585 cm−1 and in the range of 2800–2900 cm−1. These bands are attributed to N–H bending and C–H stretching, respectively, and prove the functionalization of magnetite-silica core–shell with –NH2 groups[58].In the FT-IR spectrum of Fe3O4@SiO2/Schiff base (Fig. 1d), in addition to the previously observed bands, a specific band can be seen at 1601 cm−1 which can be attributed to the C=N stretching vibrations. This absorption band clearly shows that the Schiff base ligand has been successfully attached to the magnetite-silica core–shell structure. The FT-IR spectrum of nanocatalyst Fe3O4@SiO2/SB(Ni) is seen in Fig. 1e. In this spectrum, the –C=N band has been shifted to 1575 cm−1, actually 26 cm−1 red shift in comparison with –C=N stretching of free immobilized Schiff base can be seen. The successful coordination of Ni(II) metal ion to immobilize Schiff base is proved by this red shift [43].
TGA-DTA curves
The thermal stability of the nanocatalyst was evaluated by TGA-DTA analysis (Fig. 2). As it is clear in the figure, the first stage of weight loss of nanocatalyst has occurred in the temperature range of 100–260 °C, which is related to the removal of water from the surface and inside the cavities of the nanocatalyst. In this step, about 7% of the weight of the sample has been lost. The second step of weight loss has occurred in the temperature range of 260–380 °C and another 8% of the weight has been lost. This step of weight loss can be attributed to the start thermal decomposition of the Schiff base and the immobilized organic part on the core–shell structure. The third step of weight loss has occurred in the temperature range of 380–610 °C and can be related to the thermal oxidation of carbonaceous residue left on the magnetite-silica surface. As can be seen, the total percentage of weight loss is approximately 30%.
VSM analysis
Figure 3 shows two VSM curves of Fe3O4 (a), and [Fe3O4@SiO2/SB(Ni)] (b). The magnetic properties of the samples were measured by a vibrating samplemagnetometer (VSM) at room temperature. The results indicate that the nanoparticles are superparamagnetic because no obvious hysteresis loop was observed in the VSM curve of samples. As seen in Fig. 3 the saturation magnetization values for MNPs (Fig. 3a) and [Fe3O4@SiO2/SB(Ni)] (Fig. 3b) are 57.6 and 45.5 emu g−1 respectively. It should be noted that creation of two layers (silica and Schiff base complex) on the MNPs core, reduced the saturation magnetization values compared to MNPs.
XRD-patterns
The crystal structures of MNPs, Fe3O4@SiO2and [Fe3O4@SiO2/SB(Ni)] were studied by X-ray powder diffraction analysis (XRD)(Fig. 4). As shown in this figure, the presence of silica (SiO2) and metal Schiff base complex layers on the magnetite core does not alter the structure of the magnetite nanoparticles. The XRD patterns for all three structures are consistent with the reference pattern for Fe3O4 [card no. (75–0033)]. All diffraction peaks observed for MNPs (Fig. 4a) appear at 2Ɵ = 30.6349, 33.3579, 35.8325, 38.8982, 43.4576, 57.5545, 63.0626 corresponding to the reflections of (220), (311), (400), (422), (511), (440) and (533) Miller indices planes of MNPs, respectively. The silica layer and Schiff base complex on the MNPs cause a slight shift in the location of the diffraction peaks, so all three samples don’t have the same peak location. The XRD patterns show that all three structures are highly crystalline and cubic. It is also important to note that the modification of MNPs with SiO2 and Schiff base preserves the crystalline cubic inverse spinel structure.
FE-SEM images of Fe3O4@SiO2/SB(Ni)
Figure 5 shows the FE-SEM images of the nanocatalyst. The FE-SEM images of the Fe3O4@SiO2/SB(Ni) nanocatalyst at different magnifications has been displayed. As shown in Fig. 5, the nanocatalyst structure consists of nanoparticles with almost spherical shape that are glued together and have relatively uniform sizes at the nanometer scale.
Particle size distribution histogram
The particle size distribution histogram of [Fe3O4@SiO2/SB(Ni)] nanocatalysts is shown in Fig. 6. As depicted in this figure, the smallest nanoparticles size is approximately 10 nm while, the largest size reaches around 100 nm. However, the estimated average diameter size is approximately 40 ± 5.0 nm with high abundance. The particles are distributed within the range of 10 and 110 nm exhibiting varying frequencies of abundance.
EDX analysis of the Fe3O4@SiO2/SB(Ni)
The energy dispersive X-ray spectroscopy (EDX) shows the existence of various elements in the catalyst structure. As shown in Fig. 7, the nanocatalyst consists of elements that are completely under the expectation in the nanocatalyst structure. These elements include Fe (0–1 kev and 6–7 kev), O (0–1 kev), Si (1–2 kev), C (0–1 kev), N (0–1 kev) and Ni (0–1 kev and 7–8 kev).
TEM image of the Fe3O4@SiO2/SB(Ni)
The structure and size of the [Fe3O4@SiO2/SB(Ni)] complex were analyzed using TEM (Fig. 8). The particles exhibited a nearly spherical shape. Furthermore, TEM image revealed that magnetite-silica (Fe3O4@SiO2) nanocomposite made as support for the Schiff base complex had an almost regular core–shell structure. Some aggregation of the particles was observed in TEM image possibly due to the magnetic property of nanoparticles. These results confirm the successful formation of the desired core–shell structure on the surface of the modified magnetic nanoparticles.
Leaching test of the Fe3O4@SiO2/SB(Ni)
The leaching test demonstrate the necessity of having the catalyst present during the preparation of the desired product. As seen in Fig. 9 the absence of the catalyst (red line) results in product production but the reaction efficiency is very low. In the subsequent experiment (blue line), the catalyst is removed with an external magnet midway through the reaction. As shown in the figure, after removing the catalyst the production process slows down completely. However, in the third reaction (green line), the presence of a small amount of catalyst significantly improves the reaction efficiency.
AAS of the Fe3O4@SiO2/SB(Ni)
Atomic Absorption Spectroscopy (AAS) can help to evaluate how much metal ion has been loaded on the catalyst. For this aim, 0.1 g of nano-catalyst was dissolved in aqua regia (a mixture of HNO3 and HCl with a ratio of 1–3) and the volume reached 250 mL as an unknown solution). To draw a calibration curve and calculation for AAS we prepared a number of solutions with specific concentrations of nickel (II) nitrate (0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0 ppm) To determine the degree chemical absorption of nickel (II) metal ion on the Schiff base in nano-catalyst, diluted 1 mL of the unknown solution with deionized water to a volume of 10 mL and atomic absorption of nickel ion was measured. The data revealed that the amount of nickel ion was 0.771% w/w of the solid catalyst. Also, based on data from AAS, the amount of the nickel ion as an active site loaded on the surface of the catalyst was calculated 0.131 mmol g−1.
BET and BJH spectra of the Fe3O4@SiO2/SB(Ni)
The Brunauer–Emmett–Teller (BET) method is one of the most common methods for measuring porosity and determining the specific surface area of materials, which is based on gas adsorption–desorption. In many applications such as catalysts, pharmaceuticals, nanoabsorbents, food industries, and also in nanostructures such as metal nanoparticles, nanofibers, and nanotubes, accurate measurement of surface area and porosity is very important, and by interpreting the results, useful information about the material is obtained.
According to the type of absorption and desorption spectrum obtained from the nanocatalyst in this work (Fig. 10), it was found that the sample synthesized in this research corresponds to H3-type hysteresis loops. H3-type hysteresis loops do not show a limit to absorption at high relative pressures and are seen in materials with slit-shaped cavities. The relative pressure is low (P/P0 < 0.6), so it is concluded that the pore type is not obvious here [59].
The size distribution of the pores was determined using Barret–Joyner–Halenda (BJH) method. The BJH curve for the nanocatalyst is shown in Fig. 11. All samples were distributed in the mesoporous range (between 2 and 50 nm). According to the diagram, the diameter of the pores, volume, and regional distribution of the pores can be obtained, which gives us very important information about the catalyst and shows the availability of the desired catalyst for better preparation of dihydropyrimidines. The characteristics of the desired nanocatalyst in BET analysis are listed in Table 1.
Catalytic and substrate evaluation in the synthesis of 3,4-dihydropyrimidine derivatives under thermal condition
After the characterization of the [Fe3O4@SiO2/SB(Ni)] catalyst, it was used in organic multicomponent reactions for the synthesis of 3,4-dihydropyrimidine derivatives (Scheme 2).
To determine the optimal reaction conditions, a mixture of benzaldehyde (1.25 mmol), urea (1.25 mmol), ethylacetoacetate (1.90 mmol), and catalyst (85.0 mg) at reflux conditions was used as a model reaction and examined different conditions. The reaction was examined by using several solvents (and solvent-free conditions), temperatures, and amounts of catalyst (Table 2). To evaluate the effect of solvent on the reaction, different solvents were tested, and the solvent-free condition in comparison with various solvents showed excellent yield for the product (entry 6). Also, the quantity of catalyst was optimized, and the results reveal that without the catalyst the reaction did not progress and no product was detected (entry 2), but using [Fe3O4@SiO2/SB(Ni)] catalyst, a satisfactory yield of product was observed. Experiments showed that 85.0 mg of catalyst is the desirable amount (entry 6) and further use of the catalyst did not have affirmative efficacy on the yield or reaction time. Moreover, different temperatures such as 25, 100, and 120 °C were investigated for this reaction that, and the best results were obtained at 100 °C (entry 6), whereas at 25 °C was obtained less product and temperatures above 100 °C had no positive effect on the yield or reaction time. The results reveal that the reaction proceeded in excellent yields in the present 85.0 mg nanocatalyst at 100 °C under solvent-free conditions in 15 min. At the end of the reaction, the catalyst is easily removed from the solution and Used several times without losing the catalytic effect.
The proposed reaction mechanism of 3,4-dihydropyrimidine derivatives
Under optimal conditions, 3,4-dihydropyrimidine-2-(1H)-one derivatives (4a-) were prepared in excellent yields from various aromatic aldehydes, urea and ethylacetoacetate or acetylaceton. As shown in Table 3, aromatic aldehydes with both electron withdrawing and electron-donating groups underwent a multi component reaction resulting in the corresponding products with excellent efficiency.
The proposed reaction mechanism in Scheme 3 appears reasonable for the one-pot, the three-component reaction of urea, ethyl acetoacetate, and aldehyde catalyzed by Fe3O4@SiO2/SB(Ni). The reaction proceeds through two catalytic cycles and a series of protonic shifts can occur through the activated metal center to the substances. In the first catalytic cycle, ethyl acetoacetate is activated (I) by the Fe3O4@SiO2/SB(Ni) metal center to give II. After the first catalytic cycle, the aldehyde is activated by the Fe3O4@SiO2/SB(Ni) metal center to give III. The nucleophilic attack of urea on the activated carbonyl group of III after releasing an H2O molecule affords 1-benzylideneurea (IV). Nucleophilic attack of activated ethyl acetoacetate moiety (II) to 1-benzylideneurea (IV), gives intermediate V, which subsequently through releasing of an H2O molecule, converts to the desired product VI. The Fe3O4@SiO2/SB(Ni) as the catalyst is released from the final product for the next catalytic cycle.
The catalytic activity, for a valid comparison between different catalysts, must be expressed to the number of exposed surface atoms with substrate available. Thus a convenient method to describe catalytic activity is employing a turnover number (TON) equal to the number of reactant molecules converted per minute per catalytic site for given reaction conditions. Indeed, it is an agreeable term, focusing exactly on the catalytic center, as distinct from the classical term “rate of reaction”, which expresses accordingly the consumption of reactants or production of products. The TON and TOF parameters were calculated using reaction data for every product entry and reported in Table 3.
Comparison of the catalytic efciency of MSA-SB@Ni(II) nanocatalyst with other reported catalytic systems
To confirm the advantages of this catalyst, the [Fe3O4@SiO2/SB(Ni)] catalyst is compared with other catalysts reported in the literature for the synthesis of 3,4-dihydropyrimidine derivatives (Table 4). The results demonstrate that the catalyst used in this work is an efficient catalyst in terms of reaction time, because in the presence of this catalyst, the reaction is completed in 15 min with good efficiency.
Recyclability of the catalyst
In this study, we evaluated the reusability and recovery of the magnetic catalyst from an environmentally friendly conditions in green chemistry (Fig. 12). The Biginelli reaction, involving benzaldehyde (1.25 mmol), urea (1.25 mmol), ethyl acetoacetate (1.90 mmol) under solvent–free conditions at 100 °C serve as a model reaction. At the end of the reaction, ethanol was added to the reaction mixture, allowing easily separation of the catalyst using an external magnet. The catalyst was thoroughly washed with ethanol (3 × 20 mL), dried at 60–65 °C for 1 h, and then used in a new reaction cycle. The [Fe3O4@SiO2/SB(Ni)] catalyst can be reused up to four times without significant loss of catalytic activity.
Conclusions
In this study, we introduced a new nanocatalyst called [Fe3O4@SiO2/SB(Ni)] which is green, efficient, and reusable. The nanocatalyst was synthesized in multiple stages. First, magnetic nanoparticles were prepared using the co-precipitation method. Then, silica was coated on the magnetic nanoparticles using the Stober method. Finally, Schiff base–Ni complex was covalently grafted to the surface of magnetic nanoparticles. The new catalyst was confirmed using different techniques such as SEM, EDAX, TGA, XRD, FT-IR, BET and VSM.
To evaluate the performance of the catalyst, it was applied for synthesis of 3,4-dihydropyrimidine derivatives from an urea, β-ketoester, and aromatic aldehydes under solvent-free condition at 100 °C. The excellent advantages of this catalyst include short reaction time, high product yield, great catalytic activity, easy workup of product under solvent-free condition and environmentally secure. Moreover, the catalyst could be simply recovered from the reaction mixture using an external magnet and reused without a noticeable decline in its activity.
The results showed that in the presence of the optimized amount of nanocatalyst (85 mg) all dihydropyrimidine-2-(1H)-one derivatives are synthesized in good to excellent yield (82–91%) in solvent free conditions. These yields are obtained at 100 °C and within 15–25 min. The results also showed that the presence of electron-withdrawing substituents increases the yield of the reaction and the presence of electron-donating substituents decreases the yield. At the end of the reaction, the nanocatalyst was easily recovered by an external magnet and used for several runs without significant difference in its performance. The reusability of the nanocatalyst showed that it can be used up to five times with a yield of 77% and higher for the reaction.
Availability of data and materials
We declare that the all research data related to the article is available in the text of the article and supplementary file.
References
H. Nagarajaiah, A. Mukhopadhyay, J.N. Moorthy, Tetrahedron Lett. 57, 5135 (2016)
R.R. Alavala, U. Kulandaivelu, P. Bonagiri, S. Boyapati, V. Jayaprakash, A.T. Subramaniam, Anti-Infec. Agents 13, 154 (2015)
A.E. Huseynzada, C. Jelch, H.V.N. Akhundzada, S. Soudani, C.B. Nasr, A. Israyilova, F. Doria, U.A. Hasanova, R.F. Khankishiyeva, M. Freccero, RSC Adv. 11, 6312 (2021)
G. Lauro, M. Strocchia, S. Terracciano, I. Bruno, K. Fischer, C. Pergola, O. Werz, R. Riccio, G. Bifulco, Eur. J. Med. Chem. 80, 407 (2014)
M.H. El-Wakil, M. Teleb, M.M. Abu-Serie, S. Huang, G.W. Zamponi, H. Fahmy, Bioorg. Chem. 115, 105262 (2021)
H.M. Marvaniya, P.K. Parikh, D.J. Sen, J. Appl. Pharm. Sci. 1, 109 (2011)
H. Yuan, K. Zhang, J. Xia, X. Hu, S. Yuan, Cogent Chem. 3, 1318692 (2017)
T. Sekhar, P. Thriveni, M. Harikrishna, K. Murali, Asian J. Chem. 30, 1243 (2018)
M. Afradi, N. Foroughifar, H. Pasdar, H. Moghanian, RSC Adv. 6, 59343 (2016)
E. Abbaspour-Gilandeh, S.C. Azimi, A. Mohammadi-Barkchai, RSC Adv. 4, 54854 (2014)
J. Safari, S. Gandomi-Ravandi, J. Mol. Struct. 1074, 71 (2014)
L.-Q. Kang, D.-Y. Jin, Y.-Q. Cai, Synth. Commun. 43, 1896 (2013)
S. Bentahar, M.A. Taleb, A. Sabour, A. Dbik, M. El Khomri, N. El Messaoudi, A. Lacherai, R. Mamouni, Russ. J. Org. Chem. 55, 1423 (2019)
M. Kamali, Int. J. Chem. Tech. Res. 8, 536 (2015)
M. Norouzi, N. Noormoradi, M. Mohammadi, Nanoscale Adv. 5, 6594 (2023)
M. Kazemi, M. Mohammadi, Appl. Organomet. Chem. 34, e5400 (2020)
M. Mohammadi, A. Ghorbani-Choghamarani, S.M. Ramish, J. Mol. Struct. 1292, 136115 (2023)
A. Ghorbani-Choghamarani, M. Mohammadi, T. Tamoradi, M. Ghadermazi, Polyhedron 158, 25 (2019)
F. Ghobakhloo, D. Azarifar, M. Mohammadi, H. Keypour, H. Zeynali, Inorg. Chem. 61, 4825 (2022)
M. Zhu, G. Diao, J. Phys. Chem. C 115, 24743 (2011)
M. Sheykhan, A. Yahyazadeh, L. Ramezani, Mol. Catal. 435, 166 (2017)
S. Akbarpour, A. Bezaatpour, E. Askarizadeh, M. Amiri, Appl. Organomet. Chem. 31, e3804 (2017)
A. Bezaatpour, S. Khatami, M. Amiri, RSC Adv. 6, 27452 (2016)
M. Hajjami, F. Sharifirad, F. Gholamian, Appl. Organomet. Chem. 31, e3844 (2017)
A.H. Lu, E.L. Salabas, F. Schüth, Angew. Chem. Int. Ed. Engl. 46, 1222 (2007)
M. Mohammadi, M. Khodamorady, B. Tahmasbi, K. Bahrami, A. Ghorbani-Choghamarani, J. Ind. Eng. Chem. 97, 1 (2021)
F. Ghobakhloo, M. Mohammadi, M. Ghaemi, D. Azarifar, ACS Appl. Nano Mater. 7, 1265 (2024)
A. Spoială, C.-I. Ilie, L.N. Crăciun, D. Ficai, A. Ficai, E. Andronescu, Appl. Sci. 11, 11075 (2021)
M. Mohammadi, A. Ghorbani-Choghamarani, Res. Chem. Intermed. 48, 2641 (2022)
H. Keypour, J. Kouhdareh, S. Alavinia, K. Rabiei, M. Mohammadi, A. Maryamabadi, S. Babaei, J. Organomet. Chem. 989, 122646 (2023)
M. Mohammadi, A. Ghorbani-Choghamarani, RSC Adv. 12, 26023 (2022)
A.M. Abu-Dief, I.M. Mohamed, Beni-Suef Univ. J. Basic Appl. Sci. 4, 119 (2015)
Z. Asadi, M. Asadi, M. Setoodehkhah, Spectrochim. Acta A Mol. Biomol. 112, 214 (2013)
M. Asadi, M.S. Khah, J. Iran. Chem. Soc. 7, 875 (2010)
K. Mohammadi, M. Asadi, M.S. Khah, H. Sepehrpour, Croat. Chem. Acta (2016). https://doi.org/10.5562/cca2706
K. Mohammadi, M. Asadi, M. Setoodeh Khah, H. Sepehrpour, Croat. Chem. Acta 89, 277 (2016)
B.S. Lane, K. Burgess, Chem. Rev. 103, 2457 (2003)
J.N. Pédeutour, K. Radhakrishnan, H. Cramail, A. Deffieux, Macromol. Rapid Commun. 22, 1095 (2001)
P. Manisankar, A. Gomathi, D. Velayutham, J. Solid State Electrochem. 9, 601 (2005)
A. Ríos-Escudero, M. Villagrán, F. Caruso, J. Muena, E. Spodine, D. Venegas-Yazigi, L. Massa, L. Todaro, J. Zagal, G. Cárdenas-Jirón, Inorganica Chim. Acta 359, 3947 (2006)
C. Heinrichs, W.F. Hölderich, Catal. Lett. 58, 75 (1999)
R.E. Malekshah, B. Fahimirad, A. Khaleghian, Int. J. Nanomed. 15, 2583 (2020)
M. Setoodehkhah, A. Mazraati, M. Moradian, J. Inorg. Organomet. Polym., (2021)
A.R. Kiasat, J. Davarpanah, Res. Chem. Intermed. 41, 2991 (2015)
R. Gurav, A. Gurav, S. Salunkhe-Gawali, S. Jadhav, P. Choudhari, S. Sankpal, S. Hangirgekar, Appl. Organomet. Chem. 36, e6547 (2022)
A. Mobinikhaledi, N. Foroughifar, A. Khajeh-Amiri, React. Kinet. Mech. 117, 59 (2016)
L.V. Chopda, P.N. Dave, ChemistrySelect 5, 5552 (2020)
A. Mazraati, M. Setoodehkhah, M. Moradian, J. Inorg. Organomet. Polym. Mater. 32, 143 (2022)
Z. Karimi-Jaberi, M.S. Moaddeli, M. Setoodehkhah, M.R. Nazarifar, Res. Chem. Intermed. 42, 4641 (2016)
M. Ghanbari, S. Moradi, M. Setoodehkhah, Green Chem. Lett. Rev. 11, 111 (2018)
S. Yazdanseta, K. Yasin, M. Setoodehkhah, M. Ghanbari, E. Fadaee, Res. Chem. Intermed. 48, 1 (2022)
A. Mazraati, M. Setoodehkhah, M. Moradian, J. Cluster Sci. 35, 1 (2024)
F. Nemati, M.M. Heravi, R.S. Rad, Chin. J. Catal. 33, 1825 (2012)
M. Sonmez, M. Georgescu, L. Alexandrescu, D. Gurau, A. Ficai, D. Ficai, E. Andronescu, Curr. Pharm. Des. 21, 5324 (2015)
T. Karimpour, E. Safaei, B. Karimi, Y.I. Lee, ChemCatChem 10, 1889 (2018)
A. Noormohamadi, M. Homayoonfal, M.R. Mehrnia, F. Davar, Ceram. Int. 43, 17174 (2017)
W.F. Elmobarak, F. Almomani, Chemosphere 265, 129054 (2021)
Z. Zhang, W. Zhang, in 2015 International Symposium on Energy Science and Chemical Engineering. (Atlantis Press, 2015), pp. 180.
W. Wang, P. Liu, M. Zhang, J. Hu, F. Xing, Open J. Compos. Mater. 2, 104 (2012)
Z. Ramazani, D. Elhamifar, M. Norouzi, R. Mirbagheri, Compos. B Eng. 164, 10 (2019)
R. Velpula, J. Banothu, R. Gali, R. Deshineni, R. Bavantula, Chin. Chem. Lett. 26, 309 (2015)
Z. Liu, R. Ma, D. Cao, C. Liu, Molecules 21, 462 (2016)
P. Wu, L. Feng, Y. Liang, X. Zhang, B. Mahmoudi, M. Kazemnejadi, Appl. Catal. A-Gen. 590, 117301 (2020)
Acknowledgements
The authors are deeply grateful to the University of Kashan for financial support of this research project.
Funding
All of the sources of funding for the work described in this publication are acknowledged below: University of Kashan (IRAN) was financial support of this research project.
Author information
Authors and Affiliations
Contributions
We confirm that the manuscript has been read and approved by all named authors and the order of authors listed in the manuscript has been approved by all named authors.
Corresponding author
Ethics declarations
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Ethical approval
This declaration is “not applicable”.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abravi, Z., Setoodehkhah, M. & Moradian, M. Synthesis and characterization of Ni(II) complex supported on magnetite-silica nanoparticles and investigation of its catalytic activity in Biginelli reaction under solvent-free conditions. Res Chem Intermed 50, 2067–2090 (2024). https://doi.org/10.1007/s11164-024-05273-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-024-05273-x