Abstract
1,5,7-Triazabicyclo-[4,4,0]-dec-1-ene functionalized mesoporous silica nanoparticle promoted one-pot multicomponent synthesis of 2-amino-4H-chromenes from a phenolic component, different aldehydes and malononitrile was achieved in aqueous media at room temperature. The methodology promises advantages of short reaction times, environmentally benign conditions, high yields, and operational convenience. The catalyst was reused several times without significant loss in activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the increase in environmental awareness worldwide, synthetic organic chemists have become more concerned with adopting ‘green’ protocols in research and development [1]. The most significant measures taken are to avoid the use of hazardous solvents and harmful chemicals, to reduce the formation of by-products as chemical wastes, promoting atom-economical reactions, and reusability of reagents. [2, 3]. Accordingly, implementation of greener methods has become a ubiquitous research topic. Multicomponent reactions (MCRs) have emerged as one of the most potent strategies as a part of ‘green chemistry’ with ample opportunities to assemble different medicinally privileged scaffolds in a single molecule for the synthesis of bioactive pharmacophores [4–7].
Chromenes are an important class of oxygenated heterocycles; among them, the chemistry of 2-Amino-4H-chromene has received great interest over the last few years. The scaffold functions as a significant structural component in naturally occurring and synthetically made drug-like molecules, displaying a broad spectrum of biological activities (Scheme 1). They are endowed with a wide range of pharmaceutical aspects such as antiinfertility [8], anticancer [9, 10], antidepressant, antihypertensive, antitubulin, antiviral and antioxidant qualities [11, 12]. They have potential application in psoriatic and rheumatoid arthritis [13–15]. Chromenes are known to activate potassium channels and inhibit phosphodiesterase IV and dihydrofolate reductases [16–22]. They are the main constituents of many natural products and potentially biodegradable agrochemicals [23, 24].
Several methods have been applied in the synthesis of 2-amino-4H-chromenes from resorcinol, aldehydes and active methylene nitriles using catalysts like piperazine [25], DBU [26], NEt3 [27], Et2NH [28], K2CO3 [29], glycine [30], l-Proline [31], Fe(HSO4)3 [32], CuO–CeO2 nanocomposite [33], nano MgO [34], hydrotalcite [35], Na2CO3/ball milling [36] etc.
Although earlier reported methods are efficient and offer relative advantages with regard to one another, some of them are confronted with drawbacks regarding eco-friendliness of reaction conditions, tedious work-ups, use of toxic and expensive catalysts, high temperature conditions and recyclability of catalysts. However, despite the merits of the reported methods, there is still scope for further development on the environmental and economical impacts of the reaction.
In continuation of our efforts for the development of environmentally benign synthetic methodologies for the production of various biologically important moieties using mesoporous heterogeneous and reusable catalysts [37–43], we wish to report for the first time the use of 1,5,7-triazabicyclo-[4,4,0]-dec-1-ene (TBD), a cyclic guanidine base anchored by mesoporous silica nanoparticles (MSN) [44], in the three-component expeditious synthesis of 2-amino-4H-chromene derivatives in an aqueous medium at room temperature, requiring stirring only (Scheme 2).
Surface functionalization of nanomaterials has emerged as a powerful technique for tuning physical and chemical properties [45–51]. Mesoporous silica usually have a high surface area, covered by silanol groups, which has made the functionalization of the pore surface feasible by covalent bonding with TBD. The high surface area of silica nanoparticles, strong basicity of the well dispersed organobase active sites and reusability are the key factors behind making the catalyst very efficient as evident from the excellent yield of the products in short time frames.
The aim of this work was the synthesis and physical characterization of organobase functionalized mesoporous silica nanoparticles and assessment of its catalytic performance in the synthesis of diverse functionalized 2-amino-4H-chromenes.
Experimental
Preparation of the catalysts
Preparation of mesoporous silica nanoparticle (MSN) and chloropropyl functionalization (CPMSN)
MSN was prepared based on the MCM-41 preparation strategy. The surfactant and structure directing template CTAB (Cetyl Triethyl Ammonium Bromide) was dissolved in 2(M) NaOH at 80 °C. Then TEOS (Tetra Ethyl Ortho Silicate) was added and stirred at the same temperature setting in a condenser for 2 h. The solution was cooled, filtered and dried for 24 h at 100 °C. The surfactant was removed by washing with methanolic HCl at 60 °C for 6 h. Finally, it was filtered and dried at 100 °C for another 6 h to obtain a fine white powder (MSN). The powder was then dispersed in dry toluene and 3-chloropropyltrimethoxysilane was added to it and refluxed for 24 h at 115 °C. The mass was then filtered and washed, dried at 100 °C for 6 h to get the CPMSN (Scheme 3).
TBD substitution over CPMSN
The CPMSN was suspended in dry toluene and 1,5,7-triazabicyclo-[4,4,0]-dec-1-ene (TBD) was added and the mixture was refluxed at 115 °C for 8 h. It was then cooled, filtered and washed well with a solvent mixture of toluene, methanol and water (1:1:1 by volume). Finally the material was dried in air for 12 h to get the final TBD–MSN catalyst (Scheme 4).
General synthesis of 2-amino-4H-chromenes
A mixture of active phenol (1.0 eq), aldehyde (1.0 eq) and malononitrile (1.0 eq) was dissolved in 5 mL water in the presence of 10 mg of MSN–TBD catalyst. The mixture was then stirred for the requisite time. After completion (indicated by TLC, solvent system 2:3 EtOAc/petroleum ether) the reaction mixture was filtered to separate the precipitated product. The crude product was then redissolved in ethanol, the catalyst was filtered out and the filtrate was recrystallized to obtain pure products. Finally, the isolated compounds were characterized by mp, 1H NMR spectroscopy, 13C NMR spectrometry and spectral analysis (C, H, and N).
Results and discussion
Physicochemical characterization of catalyst
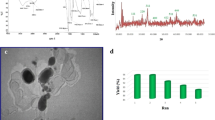
The TBD–MSN catalyst was characterized by High Resolution Transmission Electron Micrograph (HRTEM), FT-IR, Powder X-ray diffraction (XRD) and BET surface area analysis. TEM images of the particles are shown in Fig. 2a, b. Dimensions of the particles was around 100 nm. A perfectly round shaped particle is shown in Fig. 1a. No bulk aggregation was observed and all the particles are of the same size. Each particle contains numerous microsized pores to make the surface spongy as showed in Fig. 1b. The ordered porous morphology ensures high surface area. BET measurement showed the surface area of the MSN to be 676 m2 g−1.
FT-IR spectrum (Fig. 2) of the TBD–MSN exhibits sharp bands at 466, 798, and 1067 cm−1 which attributes to the condensed silica (Si–O–Si) network. Besides, a shoulder band at 956 cm−1 can also be detected in the spectra, corresponding to the stretching vibrations of ≡Si OH bonds. The covalent bonding of TBD moiety is verified from the presence of sharp aliphatic N=C vibrations at 1634 cm−1 and C–H bending and stretching modes at 1324, 1377, 1444, 2948, 2882 cm−1, respectively [52].
Figure 3 represents the small angle X-ray diffraction pattern of TBD–MSN material. In the analysis, a sharp peak appears at 2θ = 0.4° and a small peak at 2θ = 0.7°. These two correspond to Bragg reflections on the 100 and 110 planes, respectively. These peaks are clear indications of mesoporosity, which remains intact even after post-grafting.
Reaction conditions
A very simple protocol was followed in the reaction processes. A mixture of resorcinol, aldehyde and malononitrile was stirred at room temperature in water over TBD–MSN catalyst. Progress was checked by TLC, and after a characteristic reaction time the product was obtained as precipitate in excellent yields. There was no requirement of tedious work-ups and chromatographic separation in any of the cases, and simple filtration sufficed to do the job. Moreover, no other additive was necessary to promote the reaction. For further purification it was recrystallized from hot aqueous ethanol.
However, prior to this generalization, feasibility of the reaction conditions were investigated (Table 1). To recognize the optimization conditions, a representative reaction assembling resorcinol, 4-chlorobenzaldehyde and malononitrile, taken in equimolar proportion (1.0 mmol each), and a series of catalysts (10 mg) as well as solvents (5 mL), including catalyst-free and solvent-free conditions, were employed. As per our ongoing research on eco-friendly solvent mediated reactions, the reactants were initially taken in water and ethanol, respectively, and stirred in absence of any catalyst. Even after a run of 12 h, the desired product was not obtained (Table 1, entries 1, 2). The same proportion of reactants was treated in water with various catalysts like unfunctionalized MSN, silica gel (230–400 mesh), basic Al2O3, amino functionalized MSN, TBD–Silica gel and TBD–MSN. Only 35 % yield was produced with unfunctionalized silica nanoparticles (Table 1, entry 3). Silica gel (30 %) and basic alumina (58 %) also failed to promote the reaction up to the mark (Table 1, entries 4, 5). Moderate yield was obtained with amino propyl functionalized AP–MSN (Table 1, entry 6). However, TBD functionalized silica gel improved the yield, affording 87 % product, but the most satisfactory result in terms of yield as well as reaction time was achieved when TBD–MSN was used in the reaction (Table 1, entries 7 and 8).
The next step was then to explore the effects of solvents on productivity. With the best catalyst, TBD–MSN, different solvents were used keeping other factors intact. Methanol and ethanol were almost comparable in solvation and polarity and thereby also the yield (Table 1, entries 9, 10). Acetonitrile also proved to be a good solvent but was not encouraging in view of green methodology (Table 1, entry 11). Relatively less polar dichloromethane or diethyl ether could not improve the yield significantly (Table 1, entries 12, 13). Even in solvent-free conditions, satisfactory output was not obtained (Table 1, entry 14). The reaction was optimized to be carried out in aqueous medium over TBD–MSN, affording complete conversion in a short time. The marked polarity and high solvation character of water was the key for superior behavior.
As the reaction involved Knoevenegel and Michael reactions as key steps, which are typically base catalyzed reactions, a strong base catalyst resulted in high yields. It is pertinent to mention that the high surface area of MSN upon which the bulky TBD base was grafted definitely had immense influence over the yield.
The conditions thus optimized were applied in the synthesis of diverse functionalized aminochromenes by combining resorcinol, malononitrile and a wide variety of aromatic and heterocyclic aldehydes in order to delineate the approach. Details of the study are given in Table 2. Different functional electron-attracting and electron-donating groups such as nitro, halogen, hydroxy, methoxy and nitrile, substituted for benzaldehydes, were highly compatible under reaction conditions and excellent yields were obtained in each case. Different active phenolic systems such as catechol and naphthols were also equally productive when coupled with malononitrile and 4-cholorobenzaldehyde. The reaction proceeded smoothly with all the substrates within 1.2–2.5 h at room temperature, and that is the distinguishing feature of TBD–MSN catalyst compared to the hitherto reported methods. However, the reactions did not produce satisfactory yields while reacting with aliphatic aldehydes, probably due to their low reactivity.
The advantage of the current simple and benign synthetic methodology may be demonstrated by comparison with various previous reports (Table 3). The published results clearly show that the present ambient and expeditious protocol furnishes quantitative yields in water. Previous catalytic techniques were performed mostly at elevated temperatures and with longer reaction times. They require costly purification workups with hazardous waste formation, disposal of which impedes catalyst recovery.
To obtain further pure crystallized product, the reaction mixture was dissolved in hot ethanol, filtered to separate the catalyst and the filtrate was simply crystallized after concentration, which furnished pure crystals of the products. Thus the method is free from work up, column chromatography or chemical garbage disposal, and hence satisfies the green protocol.
A probable mechanistic pathway has been elucidated in Scheme 3. The initial reaction involves a base catalyzed Knoevenagel reaction between aldeyde and methylene nitrile, which is further attacked by active phenol following the Michael pathway. Finally cyclization and tautomerism leads to the substituted 2-amino-4H-chromene.
Spectroscopic data for some of the characteristic compounds are given as-
(Table 2, entry 13) 2-amino-7-hydroxy-4-(3,4,5-trimethoxyphenyl)-4H-chromene-3-carbonitrile
1H NMR (DMSO-d6, 300 MHz): δ 3.79 (s, 3H, OCH3), 3.81 (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 4.58 (s, 1H, CH), 6.39 (s, 1H, aromatic), 6.47–6.51 (m, 3H, NH2 and 1H aromatic), 6.84–6.89 (m, 3H, aromatic), 9.67 (s, 1H, OH); 13C NMR (DMSO-d6, 100 MHz): δ 30.7, 57.2, 57.5, 59.8, 105.4, 106.2, 111.2, 113.5, 117.8, 128.3, 134.7, 135.8, 151.5, 154.6, 157.8, 178.0; Anal. Calcd. for C19H18N2O5: C, 64.40; H, 5.12; N, 7.91. Found: C, 64.37; H, 5.19; N, 7.83. LCMS m/z: 355.09 (M + 1).
(Table 2, entry 17) 2-amino-7-hydroxy-4-(4-imidazoyl)-4H-chromene-3-carbonitrile
1H NMR (DMSO-d6, 300 MHz): δ 4.58 (s, 1H, CH), 6.34 (s, 1H, aromatic), 6.47 (d, 1H, aromatic), 6.72 (s, 2H, NH2), 6.8 (s, 1H, imidazoyl), 6.94 (d, 1H, aromatic), 7.48 (s, 1H, imidazoyl), 9.6 (s, 1H, OH), 11.8 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz): δ 30.2, 58.6, 104.8, 107.9, 110.6, 117.8, 120.1, 132.0, 134.9, 155.8, 156.8, 177.3; Anal. Calcd. for C13H10N4O2: C, 61.41; H, 3.96; N, 22.04. Found: C, 62.37; H, 3.91; N, 23.05.
Catalyst reusability study
The reusability study of the catalyst was done separately with a larger batch size. Initially 0.5 g resorcinol was used with equivalent amount of 4-chlorobenzaldehyde and malononitrile in presence of 50 mg catalyst. After completion of the reaction, the catalyst was recovered by filtration over Whatmann filter paper, washed several times with ethanol to remove any adhered product, dried at 100 C for 3 h and reused in further cycles. The results are shown in Table 4. The catalyst was so efficient that we could use it 5 times in succession without much change in catalytic activity, as indicated in the change in yield of the product.
Conclusions
In conclusion, one-pot three component synthesis of substituted 2-amino-4H-chromene derivatives has been reported. TBD anchored mesoporous silica nanoparticle was found to be an efficient catalyst due to its applicability to a wide range of starting materials, heterogeneity and high reusability. Moreover, simple work-up, clean procedure, room temperature reaction, short reaction times, and high yields of products distinguishes this procedure from the available methods.
References
P.T. Anastas, J.C. Warner, Green Chemistry, Theory and Practice (Oxford University Press, Oxford, 1998)
V. Polshettiwar, R.S. Varma, Chem. Soc. Rev. 37, 1546 (2008)
K. Tanaka, F. Toda, Chem. Rev. 100, 1025 (2000)
A. Domling, W. Wang, K. Wang, Chem. Rev. 112, 3083 (2012)
E. Ruijter, R.V.A. Orru, Drug Discov. Today: Technol. 10, 1 (2013)
B.B. Toure, D.G. Hall, Chem. Rev. 109, 4439 (2009)
B. Jang, F. Shi, S.-T. Tu, Curr. Org. Chem. 14, 357 (2010)
M. Kidwai, S. Saxena, M.R.K. Khan, S.S. Thukral, Bioorg. Med. Chem. Lett. 15, 4295 (2005)
L.R. Morgan, B.S. Jursic, C.L. Hooper, D.M. Neumann, K. Thangaraj, B. LeBlanc, Bioorg. Med. Chem. Lett. 12, 3407 (2002)
A. Bolognese, G. Correale, M. Manfra, A. Lavecchia, O. Mazzoni, E. Novellino, P. La Colla, G. Sanna, R. Loddo, J. Med. Chem. 47, 849 (2004)
M. Curini, G. Cravotto, F. Epifano, G. Giannone, Curr. Med. Chem. 13, 199 (2006)
P. O’Kennedy, R.D. Thornes, In Coumarins: Biology, Applications and Mode of Action (Wiley, Chichester, 1997)
J. Skommer, D. Wlodkowic, M. Matto, M. Eray, J. Pelkonen, Leuk. Res. 30, 322 (2006)
W. Kemnitzer, S. Kasibhatla, S. Jiang, H. Zhang, J. Zhao, S. Jia, L. Xu, C. Crogan-Grundy, R. Denis, N. Barriault, L. Vaillancourt, S. Charron, J. Dodd, G. Attardo, D. Labrecque, S. Lamothe, H. Gourdeau, B. Tseng, J. Drewe, S.X. Cai, Bioorg. Med. Chem. Lett. 15, 4745 (2005)
H. Gourdeau, L. Leblond, B. Hamelin, C. Desputeau, K. Dong, I. Kianicka, D. Custeau, C. Bourdeau, L. Geerts, S.X. Cai, J. Drewe, D. Labrecque, S. Kasibhatla, B. Tseng, Mol. Cancer Ther. 3, 1375 (2004)
W.P. Smith, L.S. Sollis, D.P. Howes, C.P. Cherry, D.I. Starkey, N.K. Cobley, J. Med. Chem. 41, 787 (1998)
A.G. Martinez, L.J. Marco, Bioorg. Med. Chem. Lett. 7, 3165 (1997)
R.O.S. Kitamura, P. Romoff, M.C.M. Young, M.J. Kato, J.H.G. Lago, Phytochemistry 67, 2398 (2006)
F. Borges, F. Roleira, N. Milhazes, L. Santana, E. Uriarte, Curr. Med. Chem. 12, 887 (2005)
F. Chimenti, B. Bizzarri, A. Bolasco, D. Secci, P. Chimenti, S. Carradori, A. Granese, D. Rivanera, D. Lilli, M.M. Scaltrito, M.I. Brenciaglia, Eur. J. Med. Chem. 41, 208 (2006)
S.J. Mohr, M.A. Chirigos, F.S. Fuhrman, J.W. Pryor, Cancer Res. 35, 3750 (1975)
D.R. Anderson, S. Hegde, E. Reinhard, L. Gomez, W.F. Vernier, L. Lee, S. Liu, A. Sambandam, P.A. Snider, L. Masih, Bioorg. Med. Chem. Lett. 15, 1587 (2005)
G.P. Ellis, A. Weissberger, E.C. Taylor (eds.), The Chemistry of Heterocyclic Compounds Chromenes (Wiley, New York, 1977), p. 11
E.A. Hafez, M.H. Elnagdi, A.G.A. Elagemey, F.M.A.A. El-Taweel, Heterocycles 26, 903 (1987)
Mobinikhaledi, H. Moghanian, F. Sasani, Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 41, 262 (2011)
D.S. Raghuvanshi, K.N. Singh, Arkivoc 10, 305 (2010)
A.M. Shestopalov, Y.M. Emelianov, V.N. Nesterov, Russ. Chem. Bull. Int. Ed. 51, 2238 (2002)
F.K. Behbahani, S. Samaei, Heterocycl. Lett. 5(4), 529 (2015)
A.M. Shestopalov, Y.M. Emelianov, V.N. Nesterov, Russ. Chem. Bull. 51, 2238 (2002)
B. Datta, M.A. Pasha, Ultrason. Sonochem. 19, 725 (2012)
F.K. Behbahani, S. Mehraban, J. Korean Chem. Soc. 59, 284 (2015)
H. Eshghi, S. Damavandi, G.H. Zohuri, Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 41, 1067 (2011)
J. Albadi, A. Razeghi, A. Mansournezhad, Z. Azarian, J. Nanostruct. Chem. 3(85), 1 (2013)
J. Safari, Z. Zarnegar, M. Heydarian, J. Taibah Univ. Sci. 7, 17 (2013)
S.R. Kale, S.S. Kahandal, A.S. Burange, M.B. Gawande, R.V. Jayaram, Catal. Sci. Technol. 3, 2050 (2013)
O.H. Quareaghaj, S. Mashkouri, M.R. Naimi Jamal, G. Kaupp, R. Sci. Adv. 4, 48191 (2014)
B. Karmakar, A. Nayak, B. Chowdhury, J. Banerji, Arkivoc 12, 209 (2009)
G. Postole, B. Chowdhury, B. Karmakar, K. Pinki, J. Banerji, A. Auroux, J. Catal. 269, 110 (2010)
B. Karmakar, B. Chowdhury, J. Banerji, Catal. Commun. 11, 601 (2010)
B. Karmakar, J. Banerji, Tetrahedron Lett. 51, 3855 (2010)
B. Karmakar, A. Sinhamahapatra, A.B. Panda, J. Banerji, B. Chowdhury, Appl. Catal. A: Gen. 392, 111 (2010)
B. Karmakar, J. Banerji, Tetrahedron Lett. 52, 4957 (2011)
B. Karmakar, J. Banerji, Tetrahedron Lett. 52, 6584 (2011)
B. Karmakar, Aust. J. Chem. (2016). doi:10.1071/CH15812
T. Yokoi, Y. Kubota, T. Tatsumi, Appl. Catal. A: Gen. 421, 14 (2012)
W. Xie, M. Fan, Biorg. Technol. 139, 388 (2013)
K. Sarkar, M. Nandi, M. Islam, M. Mubarak, A. Bhaumik, Appl. Catal. A: Gen. 352, 81 (2009)
A. Corma, A. Fuerte, M. Iglesias, F. Sanchez, J. Mol. Catal. A: Chem. 107, 225 (1996)
A. Sakthivel, J. Zhao, G. Raudaschl-Sieber, M. Hanzlik, A.S.T. Chiang, F.E. Kühn, Appl. Catal. A 281, 267 (2005)
K. Ariga, A. Vinu, J.P. Hill, T. Mori, Coord. Chem. Rev. 251, 2562 (2007)
H.M.A. Hassan, E.M. Saad, M.S. Soltan, M.A. Betiha, I.S. Butler, S.I. Mostafa, Appl. Catal. A: Gen. 488, 148 (2014)
J.E.G. Mdoe, Tanz. J. Sci. 37, 156 (2011)
Acknowledgments
B.K. thanks University Grants Commission (New Delhi) for financial support under Minor Research Project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karmakar, B., Nandi, R. A green route towards substituted 2-amino-4H-chromenes catalyzed by an organobase (TBD) functionalized mesoporous silica nanoparticle without heating. Res Chem Intermed 47, 2161–2172 (2021). https://doi.org/10.1007/s11164-016-2755-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2755-9