Abstract
A fluorescent and colorimetric sensor based on rhodamine 6 g (RD6g) was designed, synthesized, and characterized using microwave irradiation. The sensing behavior of this compound was studied by UV–visible and fluorescence spectroscopy. Sensor RD6g exhibits a high selectivity and an excellent sensitivity and is a dual-responsive colorimetric and fluorescent Hg2+-specific sensor in aqueous buffer solution. Mercury ions give rise to the development of a very fluorescent ring-open amide spirolactam system. The detection limit for Hg2+ was found to be 1.2 × 10−8 M. The binding ratio of RD6g-Hg2+ complex was determined to be 1:1 according to the Job’s plot. The reversibility of RD6g‧Hg2+ complex has been achieved with CN− anions. The test strip based on RD6g was developed, which could be used as a suitable and methodical Hg2+ test kit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mercury, one of the most prevalent toxic metals in the environment, easily passes through biological membranes such as skin, respiratory, and gastrointestinal tissues [1]. It plays a role in the food cycle, just as it exists in certain fish [2]. Even at low concentrations, Hg2+ can cause great harm to human function. It can be deposited in various forms in the brain, liver, kidneys, and digestive system and causes other inflammations even death [3,4,5,6]. Mercury was listed as one of the priority pollutants according to the U.S. EPA [7]. Due to the severe toxicity to human neurological and environmental systems, helpful methods for the detection of Hg2+ are urgently demanded. There are a lot of traditional detection methods for Hg2+, including gas chromatography [8], inductively coupled plasma mass spectrometry (ICP-MS) [9], electrochemical method [10], and atomic absorption spectrometry [11]. However, most of them require tedious sample preparation and complex instrumentation, which limits their practical applications. Fluorescence sensing of Hg2+ has received considerable attention due to the fast response, ‘naked-eye’ detection, low cost, excellent sensitivity, selectivity, and other advantages.
Rhodamine-based compounds have been widely used as chemosensors due to their remarkable spectroscopic properties including high absorption coefficients, high fluorescent quantum yields, and excitation and emission with the visible wavelength region [12]. Under these fascinating properties, various rhodamine-based chemosensors have been widely used for the recognition of the numerous analytes. Rhodamine derivatives are non-fluorescent and colorless, but ring-opening of the corresponding spirolactam produces strong fluorescence emission and pink color [12,13,14]. This property provides an ideal model for constructing OFF–ON fluorescent sensors. The cation-sensing mechanism of most of the rhodamine probes is based on a change from the spirolactam to an open-ring amide, resulting in a color change, highly fluorescent compound [15,16,17,18,19,20]. Thus, most of the sensors comprise of rhodamine core structure, especially for Hg2+ metal ion-based sensors, are of chromogenic and fluorogenic types [16]. There have been many reports of Hg2+ ion probes in recent years [21,22,23,24,25,26]. However, since Hg2+ ions are generally used as quenchers due to the spin–orbit coupling effects, most of the probes have problems such as high detection limit [27], poor water solubility [28], and toxicity of sulfur [29]. Therefore, designing dual-channel probes with naked eye recognition and turn-on fluorescent signals in an aqueous solution has attracted more attention.
Here, we report a new rhodamine-based receptor (RD6g), substituted with phenyl ring on both sides are synthesized (Scheme 1) as a turn-on fluorescent sensor for Hg2+ detection. The rhodamine moiety is incorporated on both the side of the phenyl ring to enhance the chelation sites for Hg2+ and achieve a lower detection limit. The synthesized sensor RD6g demonstrates extremely selective and sensitive fluorescent enhancement after the addition of the Hg2+ because of the chelation enhanced fluorescence phenomenon. In this paper, a suitable, very clean, and simple microwave-assisted synthesis technique is presented to prepare a rhodamine-based sensor. The coordination of the sensor RD6g with Hg2+ ion was rigorously investigated by optical spectroscopy, NMR, IR, high-resolution mass spectral analysis (HRMS), and time-dependent density functional theory (TD-DFT) calculations.
Experimental
Materials and instrumentation
All the reagents and solvents were acquired from Sigma-Aldrich including rhodamine 6 g, hydrazine, isophthalaldehyde, ethanol, acetonitrile, and used without further purification. Microwave-assisted reactions were carried out in a CEM microwave reactor. All absorption and fluorescence spectra were recorded using Agilent Cary 60 UV/Vis’s spectrometer and Cary Eclipse fluorescence spectrophotometer, respectively. 1H-NMR and 13C-NMR spectra were recorded using Bruker 400 MHz spectrometer. For NMR spectra DMSO-d6 was used as a solvent using TMS as an internal standard. High-resolution mass spectrometry (HR-MS) was recorded using Bruker 12 T solaris FT-ICR-MS. The IR spectrum was obtained using the Shimadzu IRAffinity FTIR spectrometer. All the measurements were achieved at room temperature.
Preparation of stock solution
The solutions of metal ions were prepared using nitrates [Co(NO3)2, Zn(NO3)2, Ni(NO3)2, Pb(NO3)2, Cd(NO3)2, Hg(NO3)2, NaNO3, KNO3] or chlorides [CuCl2, FeCl3, FeCl2, CaCl2], and anion species from their tetrabutylammonium salts. All the solutions were prepared in water. Stock solutions of receptor RD6g (1 × 10–3 M), selected salts of cations (1 × 10–3 M), and anions (1 × 10–4 M) were prepared in CH3CN/H2O. Mercury (II) complex solution ([RD6g‧Hg2+]) was prepared by addition of 1.0 equivalent of Hg2+ to the solution of RD6g (20 µM) in Tris–HCl (10 mM, pH = 7.0) buffer containing acetonitrile/water (9:1 v/v) solution. A simple equation (DL = 3σ/S) is used for the determination of detection limit (DL) of RD6g for Hg2+, where σ is the standard deviation and S is the slope. The association constant between mercury (II) and RD6g was estimated from absorption titration data. This was calculated from the Hildebrand equation:
(1/A − A0 = 1/K(Amax − A0)[Mx+]n + (Amax − A0)) Where A0 is the absorbance of sensors RD6g without Hg2+, A is the absorbance of RD6g with Hg2+ and Amax is the absorbance with [Hg2+]n max.
DFT studies
Time-density functional theory (TD-DFT) calculations were employed to elucidate the Hg2+ interactions with RD6g systems. All computations were carried out using Spartan’18 software package. Geometry optimization of the ground state structures was carried out with TD-DFT at the B3LYP level of theory using 6-31G (d, p) basis set in the gas phase and in both simulated CH3CN and water media, using a conductor-like polarizable continuum model (CPCM).
Microwave-assisted synthesis of RD6g
Synthesis of 1: The starting material hydrazide has been prepared according to literature reports [30]. Synthesis of RD6g: A mixture of intermediate 1 (100 mg, 0.219 mmol), isophthalaldehyde (16 mg, 0.219 mmol), and ethanol (2 mL) was placed and stirred in a 10 mL reaction vial, and it was placed in the cavity of a CEM microwave reactor. The closed reaction vessel irradiated according to the parameters described in Table S1. The mixture was allowed to cool to room temperature. The solid was filtered, washed with cold ethanol, and air-dried (80% yield). 1H-NMR (DMSO-d6), δ (ppm): 8.37 (2H, s), 7.92 (2H, d), 7.59 (4H, m, H-Ar), 7.32 (4H, m, H-Ar), 7.03 (2H, d), 6.35 (4H, s), 6.15 (4H, s), 5.07 (4H, m), 3.16 (8H, q, NCH2CH3), 1.81 (12H, s, -CH3), 1.22 (12H, t, NCH2CH3). 13C NMR ((DMSO-d6), δ (ppm): 165.25, 163.95, 151.63, 150.83, 147.36, 144.66, 136.52, 135.37, 134.95, 131.90, 130.77, 129.78, 128.14, 127.32, 126.67, 124.78, 123.72, 122.14, 118.31, 117.79, 104.98, 95.87, 65.27, 37.49, 17.04, 14.20. HRMS (MALDI): m/z Calcd for C60H58N8O4: 955.15; Found: 955.47.
Results and discussion
Microwave-assisted synthesis and design of RD6g
The target sensor RD6g was synthesized using a microwave-assisted organic synthesis method which gave us a good yield (Scheme 1). The process was easy, fast, decreased reaction time, and with no side reactions. The product is characterized and established by 1H-NMR, 13C-NMR, HR-MS analysis (Fig. S3-S5), and it was designed to chelate with metal ions via its carbonyl O and imine N atoms. The sensor RD6g consists of two units of rhodamine moieties and phenyl ring using hydrazide as the linker and coordination sites for Hg2+ ion.
Photophysical studies
The sensing ability of RD6g was investigated by ultraviolet/visible absorption spectroscopy. The compound did not show any absorption band above 400 nm, which is typical for the most prominent ring-closed spirolactone of rhodamine derivatives (Fig.S1). The changes in absorption spectra of RD6g with the supplement of various metal ions examined in acetonitrile–water (9:1; v/v) buffer system. The interaction studies performed with metal ions showed RD6g is very selective and sensitive towards Hg2+. The sensor displays a characteristic peak of ring opened amide form of rhodamine at 530 nm with a shoulder at 490 nm (Fig. 1a). A new absorption band produced at A530 nm, and isosbestic points at 280 and 330 nm, demonstrating that a metal complex was formed. The new absorption band gradually increases and saturated at 2.2 equivalent of Hg2+ ions (Fig. 1b). The change in color from colorless to pink demonstrated the opening of the spirolactam ring upon coordination with Hg2+ ions. The color change noted after the addition of all the metal ions tested was photographed and shown in Fig. 2. As shown in Fig. 3a, a Job’s plot experiment was conducted. The maximum absorbance intensity at 530 nm was appeared with mole fraction of Hg2+ was close to 0.5, which indicated that 1:1 stoichiometry was the most possible binding mode of Hg2+ and RD6g. The association constant (K) between Hg2+ and RD6g was found to be 2.4 × 107 M−1 in CH3CN/H2O (9:1; v/v) buffer solution based on 1:1 stoichiometry.
a UV–Vis absorption spectra of RD6g (10 µM) with 20 µM various cations (Na+, K+, Mg2+, Ca2+, Ni2+, Zn2+, Co2+, Hg2+, Pb2+, Cd2+, Cu2+, Fe2+, and Fe3+) in CH3CN/H2O (9:1 v/v) buffer system. b UV–Vis’s absorption spectra of RD6g (3 µM) with Hg2+ (0–310 µM) in acetonitrile–water (9:1 v/v) buffer solution
Rhodamine derivatives have been described to develop fluorescent sensors for the identification of cations because of a ring-opening and structural change of a sensing compound. To evaluate the optimal conditions for the detection of metal ions, it is necessary to investigate the pH effect on fluorescence response of sensor RD6g. The sensor RD6g exhibits very strong fluorescence at the low pH range (Fig. 3b). When pH˃6.5, there are not obviously fluorescence intensity change, which may be caused by closed structure. Thus, all the optical measurements were conducted in acetonitrile–water (9:1, v/v, 10 mM Tris–HCl buffer, pH of 7) to maintain the ring-closed spirolactam form of the sensor. As expected, the free sensor RD6g exhibited very weak fluorescence above 520 nm (λex,510 nm) in the acetonitrile/water ((9:1); v/v) buffer system. The selectivity of this proposed sensor RD6g was conducted in acetonitrile–water, and the tested cations used were Na+, K+, Mg2+, Ca2+, Ni2+, Cu2+, Cd2+, Zn2+, Co2+, Hg2+, Pb2+, Fe2+, Fe3+ (Fig. 4). The study revealed that the interaction of Hg2+ induced the fluorescent enhancement of RD6g at 560 nm (Fig. 4a). The fluorescence intensity change attributed with the formation of ring-opened spirolactam amide form of sensor RD6g upon binding with Hg2+ ion. The visual color change from colorless to light green examined under 365 nm light in darkness confirmed the formation of a fluorescent ring open form of spirolactam (Figure b, inset). The study also revealed the interaction with copper (II) ion. Like some other similar reported rhodamine-based Hg2+sensors [31,32,33,34,35], the emission intensity of RD6g were also affected in the presence of paramagnetic copper (II) ion. Copper (II) may get involved in the coordination with RD6g and form a ring-opened system. In the fluorescence emission detection of Hg2+ using sensor RD6g, it is very important to avoid a high concentration of copper (II) ion interference using a quinoline-based derivatives [36]. Other tested cations did not have an influence on the fluorescence emission spectrum of the sensor RD6g. The incremental addition of Hg2+ to RD6g enhances fluorescence intensity at 560 nm and gets saturated at 20 equivalents of Hg2+ ion (Fig. 4b). The detection limit of RD6g for Hg2+ was estimated based on the fluorescence titration as 1.2 × 10−8 M.
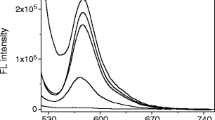
The fluorescence lifetime measurement is used for investigating various properties of excited-state dynamics and molecular interactions. The fluorescence lifetime of the sensor RD6g with Hg2+ and Cu2+ was explored to confirmed photophysical studies of the sensor (Fig. 5). For the sensor RD6g, it was clear that the incorporation of the Hg2+ impacted the lifetime of the corresponding rhodamine derivative. The free sensor RD6g, [RD6g‧Hg2+], and Cu2+complex displayed second exponential characteristics. The [RD6g‧Hg2+] complex showed the longest lifetime followed by the free RD6g and then RD6g with Cu2+ (Table S2). It is obvious that the addition of metal ions can affect the time a rhodamine derivative spends in the excited state before emitting a photon and returning to the ground table. The fluorescence emission and colorimetric sensing method toward Hg2+ was compared with some other described fluorescence emission sensing methods of Hg2+, as listed in Table 1, [37,38,39,40,41,42].
Coordination mechanism of RD6g with Hg2+
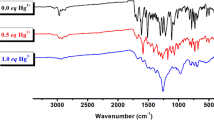
The 1:1 stoichiometry between RD6g and Hg2+ is confirmed by Job’s plot experiment. Likewise, the coordination between RD6g and Hg2+ ion is confirmed by the 1H-NMR titration experiment in DMSO-d6. Figure 6 comprises two distinct spectra, in which the bottom was recorded only for RD6g while the top spectra comprise RD6g with 1.0 equivalents of Hg2+. The sensor RD6g bears a chemical shift at 5.06 ppm for the NH group, which completely disappeared in the addition of 1 equivalent of Hg2+ ion ([RD6g‧Hg2+]) complex. The disappearance of NH signal suggests that due to coordination of Hg2+ion with RD6g delocalized xanthene tautomer of the rhodamine moiety is formed. The partial 1H-NMR spectrum of RD6g shows the signal at δ 8.40 corresponds to N=CH proton which gets slightly shifted (δ 0.02) down filled with the addition of 1 equivalent of Hg2+ ion. The characteristic carbonyl stretching frequency at 1720 cm−1in the IR spectra also slightly shifted to 1718 cm−1 and the intensity of the peak decreased upon coordination with Hg2+(Fig. S6). As a result of the above description, RD6g is most likely to coordinate with Hg2+ via oxygen on the carbonyl group and nitrogen on the hydrazine group. The plausible binding mode of RD6g toward Hg2+ is shown in Scheme 2.
Cyanide detection
Further, it was of great interest to investigate the reversible binding nature of the sensor RD6g. Figure 7a shows the addition of 20 µM of anions to [RD6g‧Hg2+] (1:1) complex of which CN− changes the emission band at 560 nm. Other common anions (Cl−, I−, F−, ClO4−, CH3COO−, HSO4−, PO43−, SCN−, and OH−) did not generate the same results. It indicated that CN− can peel Hg2+ from the coordination region and sensor RD6g is reversible. The process was found to be resulting in a net color change from pink to colorless, which was observed with the naked eye. The absorption profile of RD6g with anions was very similar to the fluorescence emission spectra: again CN−1 ion changes the absorption intensity while other anions showed no significant change (Figure S2). Due to the stability of Hg(CN)2, the [RD6g‧Hg2+] complex could be used as a possible means to detect CN− ion. The response mechanism between the sensor RD6g and Hg2+ in the presence of CN− is shown in Scheme 2.
Filter paper test strip application
To explore the practical application of fluorescent sensor RD6g, test strips were prepared followed by the report procedure [43]. Sensor test strips were fabricated by immersing Whatman filter paper into acetonitrile solution of RD6g (1 mM) for one minute and the test strips were air-dried before use. Then, these test strips holding RD6g were used to detect Hg2+. As shown in Fig. 8, an obvious color change, colorless to green, was observed under the 365 nm UV lamp which confirms the presence of Hg2+. Other potential competitive metal ions did not produce any color changes on the test strips. The significant quenching of fluorescence and disappearance of greenish color in the RD6g + Hg2 system due to regeneration of RD6g was observed in the presence of CN− ions. It suggests that the strong interaction of Hg2+ and CN− ions possibly facilitates the rapture of the RD6g. Hg2+ complex in support of a quenching phenomenon. The results demonstrate that the obtained RD6g.Hg2+ complex can be used as an efficient fluorescent sensor for recognition CN−. Therefore, this study indicated that filter paper test strips of RD6g could easily sense both Hg2+ and CN− colorimetrially and fluorometrically in solid state.
Time-dependent density functional theory (TD-DFT) studies
Time-dependent density functional theory (TD-DFT) calculations were employed to elucidate Hg+2 interactions with the RD6g system. All computations were carried out using the Spartan ‘18 software package. Employing the Spartan ‘18 (TDA) B3LYP functional and the 6-31G(d,p) basis set, sensor RD6g and its respective [RD6g‧Hg2+] complex was optimized in the gas phase (vacuum), and in both simulated CH3CN and water media, using a conductor-like polarizable continuum model (CPCM). Vertical electronic absorption and fluorescence emission data of the said optimized structures were obtained and compared with empirical obtained UV–Vis data. Wavelengths and oscillator strengths (f) for prominent vertical electronic excitations, as well as emission data for both sensor and complex, are highlighted.
For sensor RD6g in the gas phase, the energy gap between the highest occupied molecular orbital (HOMO = − 5.06 eV) and the lowest unoccupied molecular orbital (LUMO = − 1.04 eV) is 4.02 eV. Four computed, prominent vertical electronic transitions are highlighted for RD6g with wavelengths and f values as follows: 271.70 nm (f = 0.1515), 280.85 nm (f = 0.1216), 296.98 nm (f = 0.1775) and 308.15 nm (f = 0.1170). Upon formation of gaseous [RD6g‧Hg2+] complex from RD6g and Hg+2, the frontier energy gap decreased from 4.02 to 1.26 eV, [RD6g‧Hg2+], HOMO = − 8.99 eV, and LUMO = − 7.73 eV). And the four computed, prominent electronic transitions for [RD6g‧Hg2+] complex in vacuum are at: 476.57 nm (f = 0.0418), 526.52 nm (f = 0.0198), 683.02 nm (f = 0.0220), and 1178.95 nm (f = 0.1506). For sensor RD6g in CH3CN, the frontier energy gap is much smaller than in vacuum, ΔE = 3.60 eV (RD6g in CH3CN, HOMO = − 5.28 eV and LUMO = − 1.68 eV) as opposed to 4.02 eV. The four prominent transitions for RD6g in CH3CN are at 294.52 nm (f = 0.4369), 310.29 nm (f = 0.1855), 318.20 nm (0.8064), and 334.48 nm (f = 0.2809). On forming [RD6g‧Hg2+] complex from RD6g and Hg+2 in CH3CN, ΔE (RD6g) changed from 3.60 to 2.47 eV (CH3CN), and the four prominent transitions for [RD6g‧Hg2+] complex in CH3CN are at 339.81 nm (f = 0.2249), 405.75 nm (f = 0.1105), 597.25 nm (f = 0.0449) and 605.03 nm (f = 0.0318). For sensor RD6g in water, the frontier energy gap (ΔE) is = 3.58 eV (HOMO = -5.28 eV and LUMO = − 1.70 eV), which is slightly less than in CH3CN, ΔE = − 3.60 eV. The four prominent transitions for RD6g in water are at 285.36 nm (f = 0.1812), 288.89 nm (f = 0.2487), 305.89 nm (0.1826), and 314.43 nm (f = 0.7889). For [RD6g‧Hg2+] in water, ΔE is slightly larger (2.49 eV, HOMO = − 5.44 eV and LUMO = − 2.95 eV) than in CH3CN, ΔE = 2.47 eV. The four computed, prominent electronic transitions for [RD6g‧Hg2+] in water are at 337.99 nm (f = 0.1860), 402.94 nm (f = 0.0954), 487.73 nm (f = 0.0276), and 589.72 nm (f = 0.0447).
In the gas phase, HOMO of RD6g is found primarily centered on one of the xanthene moieties (Fig. 9), whereas, in both acetonitrile and water (Figs. 10 and 11), HOMO is found throughout both xanthene moieties. In all three media, LUMO of RD6g is found about the spirolactam moiety, including the two imine groups and the carbonyl oxygens but is absent about the ethereal oxygens and part of the connecting phenyl group. However, in these same three media, HOMO of [RD6g‧Hg2+] complex appears to be delocalized over the same position in the two xanthene moieties. And LUMO is found exclusively about the region connecting the two xanthene moieties which include Hg+2, the two carbonyl oxygens, the two imine groups, and part of the phenyl group.
In the gas phase, sensor RD6g is described by three computed, prominent emission wavelengths, one at 360.72 nm (f = 0.2102), a second at 373.17 (f = 0.2792) and a third at 380.51 nm (f = 0.4213); [RD6g‧Hg2+] complex in vacuum is also described by three prominent ones as well: 1061.23 (f = 0.0111), 1178.94 (f = 0.1503) and 1388.50 (f = 0.0184). In CH3CN, sensor RD6g is described by three prominent emission wavelengths at: 392.06 nm (f = 0.0031), 393.50 nm (f = 0.0841) and 410.03 (f = 0070). For [RD6g‧Hg2+] complex in CH3CN, three computed, prominent emission wavelengths are at: 491.57 nm (f = 0.0317), 597.25 nm (f = 0.0450), and 605.03 nm (f = 0.0315). And for sensor RD6g in water, the three prominent emission transitions computed are at: 406.79 nm (f = 0.0153), 407.35 nm (f = 0.0178), and 423.91 nm (f = 0.0614), and for [RD6g‧Hg2+] complex in water, the three prominent emission transitions are at: 487.43 nm (f = 0.0274), 589.25 nm (f = 0.0449), and 596.76 nm (f = 0.0204).
Conclusion
In conclusion, a simple rhodamine-based colorimetric and fluorescent sensor RD6g for the detection of Hg2+ ions has been synthesized using microwave irradiation. The sensor RD6g displayed a highly sensitive fluorescence increment upon the addition of an Hg2+ ion. Clear visible color change from colorless to pink allows RD6g to use as a naked eye sensor. The stoichiometry of the 1:1 complex was recognized for a mole fraction of 0.5. Moreover, the detection limit of the sensor RD6g for Hg2+ was 1.2 × 10–8 M, which indicated that the sensor could be useful as a highly selective and sensitive sensor for detecting Hg2+. Further, the reversible switch of complex [RD6g‧Hg2+] was investigated and attained in the presence of a CN−1 ion. The test strip based on RD6g was developed, which could be used as a suitable and methodical Hg2+ test kit. The design of bis(rhodamine)-based compounds can be further explored to obtain highly selective and sensitive sensors.
References
J. Gutknecht, J. Membr. Biol. 61, 61–66 (1981)
D. Camur, C. Gueler, S.C. Vaizoglu, Toxicol. Ind. Health 32, 1215–1223 (2016)
L.L. Tan, Y. Zhang, H. Qiang, Sens. Actuators B: Chem. 229, 686–691 (2016)
X. Zhu, Y. Yuan, L. Li, Mater. Des. A29, 91–102 (2017)
D. Sun, G. Sun, X. Zhu, Fuel 211, 609–620 (2018)
Y. Yang, S. Rong, W. Ying-Zhe, Sens. Actuator B: Chem. 255, 3479–3487 (2018)
Z. Sun, M. Yang, Y. Ma, I. Li, Cryst. Growth Des. 17, 4326–4335 (2017)
F.X. Han, W.D. Patterson, Y.J. Xia, B.B. Sridhar, Y. Su, Water Air Soil Pollut. 170, 161–171 (2006)
T. Li, S.J. Dong, E. Wang, Anal. Chem. 81, 2144–2149 (2009)
M.H. Mashhadizadeh, I. Sheikhshoaie, Talanta 60, 73–80 (2003)
E.C. Rupp, E.J. Granite, D.C. Stanko, Anal. Chem. 82, 6315–6317 (2010)
Z. Yang, S. Chen, Y. Zhao, P. Zhou, Z. Cheng, Sens. Actuators B 266, 422–428 (2018)
W. Huang, X. Zhu, D. Wua, C. He, X. Hu, C. Duan, Dalton Trans. 45, 10457–10465 (2009)
Q. Wang, L. Jin, W. Wang, T. Hu, C. Chen, J. Lumin. 209, 411–419 (2019)
G. Heo, D. Lee, C.G. Kim, J.Y. Do, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 188, 285–290 (2018)
F. Abebe, P. Perkins, R. Shaw, S. Tadesse, J. Mol. Struct. 1205, 127594 (2020)
F. Abebe, T. Sutton, P. Perkins, R. Shaw, Luminescence 33, 1194–1201 (2018)
F. Abebe, J. Gonzalez, K. Makins-Dennis, R. Shaw, Inorg. Chem. Commun. 120, 108154 (2020)
M. Desi, H. Basu, S. Saha, R. Singhal, S. Kailasa, J. Mol. Liq. 336, 116239 (2021)
M. Kateshiya, G. George, J. Rohit, N. Malek, S. Kailasa, Microchem. J. 158, 105212 (2020)
S.K. Patil, D. Das, Spectrochim. Acta A. Mol. Biomol. Spectrosc. 210, 44–51 (2019)
P.G. Rao, B. Saritha, R.T. Siva, J. Fluoresc. 29, 353–360 (2019)
K. Zhang, J. Zhang, Res. Chem. Intermed. 46, 987–997 (2020)
C. Wang, L. Wang, S. Fang, D. Quin, J. Zhou, G. Yang, S. Jin, H. Duan, Res. Chem. Intermed. 45, 2045–2063 (2019)
J.H. Hu, C. Long, Q.Q. Fu, P.W. Ni, Z.Y. Yin, J. Photochem. Photobiol. A Chem. 379, 105–111 (2019)
R. Ji, A. Liu, S. Shen, X. Cao, F. Li, Y. Ge, RSC Adv. 7, 40829–40829 (2017)
Y. Fang, Y. Zhou, J.Y. Li, Q.Q. Rui, C. Yao, Sens. Actuators B Chem. 215, 350–359 (2015)
K. Song, J. Mo, C. Lu, Spectrochim. Acta A. Mol. Biomol. Spectrosc. 179, 125–334 (2017)
H. Jiqu, Y. Qixia, Spectrochim. Acta A. Mol. Biomol. Spectrosc. 149, 487–493 (2015)
A. Leite, A. Silva, L. Cunha-Silvs, B. Castro, P. Gameiro, M. Rangel, Dalton Trans. 42, 6110–6118 (2013)
G. Li, L. Bai, F. Tao, A. Deng, L. Wang, Analyst 143, 5395–5403 (2018)
H.Y. Lee, K.M.K. Swamy, J.Y. Jung, G. Kim, J. Yoon, Sens. Actuators B 182, 530–537 (2013)
S. Shyamsivappan, A. Saravanan, N. Vandana, T. Surash, S. Surash, R. Nandhakumar, P. Mohan, ACS Omega 42, 27245–27253 (2020)
N. Wanichacheva, O. Hanmeng, S. Kraithong, K. Sukrat, J. Photochem. Photobiol. A Chem. 278, 75–81 (2014)
A. Maity, A. Sil, S. Nad, S.K. Patra, Sens. Actuators B 255, 299–308 (2018)
C.B. Bai, P. Xu, J. Zhang, R. Qiao, M.Y. Chen, M.Y. Mei, B. Wei, C. Wang, L. Zhang, S.S. Chen, ACS Omega 4, 14621–14625 (2019)
M. Bahta, N. Ahmed, J. Photochem. Photobiol. A Chem. 391, 112354 (2020)
A. Kumar, R. Ananthakrishnan, G. Jana, P. Pratim, P.K. Chattaraj, S. Nayak, S.K. Ghosh, ChemistrySelect 4, 4810–4819 (2019)
Y. Yuan, X. Chen, Q. Chen, G. Jiang, H. Wang, J. Wang, Anal. Biochem. 585, 113403 (2019)
A. Shaily, N. Kumar, Ahmed. Ing. Eng. Chem. Res. 56, 6358–6368 (2017)
Y. Zhang, C. Zhang, Y. Wu, B. Zhao, L. Wang, B. Song, RCS Adv. 9, 23382–23389 (2019)
M. Hong, Y. Chen, Y. Zhang, D. Xu, Analyst 144, 7351–7358 (2019)
R. Qiao, W.Z. Xiong, C.B. Bai, J.X. Liao, L. Ahang, Supramol. Chem. 30, 911–917 (2018)
Acknowledgements
This work was supported by the National Science Foundation’s Division of Chemistry under Grant [2100629] and Morgan State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aduroja, O., Shaw, R. & Abebe, F. A bis(rhodamine 6G)-based fluorescent sensor for Hg2+: microwave-assisted synthesis, photophysical properties, and computational studies. Res Chem Intermed 48, 1847–1861 (2022). https://doi.org/10.1007/s11164-022-04704-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04704-x