Abstract
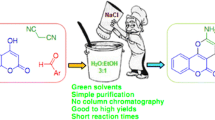
10-Methyl-7-aryl-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8-dione derivatives are significant class of compounds and this is critical to develop methods in water using commercially available and non-toxic catalysts. In this paper, an efficient method is introduced for the synthesis of 10-methyl-7-aryl-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8-dione derivatives. For the synthesis of the desired products, a multicomponent reaction was designed and performed between 4-hydroxycoumarin, an aldehyde, 6-methyl-2H-pyran-2,4(3H)-dione, and ammonium acetate. The products are obtained under green conditions in water in the presence of a catalytic amount of L-proline (10 mol%). The advantage of this method is no need to any toxic solvent, which is critical from the environmental viewpoint. A possible mechanism was suggested, which confirms the role of L-proline in the reaction as the catalyst.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multicomponent reactions are of high significance, due to their unique advantages and properties, including high atom efficiency, fast reaction time, high yield, and the most significant, the removal of multi-steps for the synthesis of the products, especially in the synthesis of compounds with complex structures and in some cases containing several fused heteroatom containing cyclic compounds [1, 2]. This type of reaction is useful for the synthesis of compounds with complex chemical structures in high isolated yields via fast and facile methods [3]. A problem with the organic synthesis is the need to use organic solvents, which are environmental pollutants. Therefore, several efforts have been focused on performing the reactions in water [4].
Coumarin and its derivatives are of high significance in organic chemistry, due to their advantageous properties, which made them applicable in several applications including solar cells dyes [5], flashlamp-pumped dye lasers [6], fluorescence properties [7], and light emitting diodes [8]. In addition, coumarin derivatives have wide uses in biological applications, including anti-Alzheimer [9], antidiabetic [10], serine protease inhibitors [11], anticancer [12, 13], and antibiotic [14] applications. Regarding the wide uses of coumarin derivatives in various areas of applications on the one hand, and the advantages of multicomponent reactions on the other hand, several multicomponent reactions have been developed, in which coumarin is used as starting material for the synthesis of the products [15,16,17,18,19,20]. Pyrano[3,2-c:5,6-c']dichromene-6,8-diones[21], spiro[indoline-3,4′-pyrano[3,2-c]chromene]-3′-carbonitriles [22], dihydro-pyrimido [4,3-d] coumarins [23], and 6Hchromeno[4,3-b]quinolin-6-ones [24] are only a number of examples, in which coumarin and other reagents are participated in multicomponent reactions for the synthesis of compounds with complex structures. Regarding the environmental issues, this is critical to perform the reactions in water [25,26,27,28]. Regarding the significance of this class of compounds, there is a challenge to develop novel synthesis methods, which are efficient, fast, and environmentally friendly. The use of commercially available catalysts and the ability of performing the reactions in water are of the advantages, which could be attractive for their synthesis in industry. In addition, the use of micelles for performing the organic reactions in aqueous phase is a successful way [29,30,31,32,33,34,35].
In this paper, an efficient one-pot method is introduced for the synthesis of 10-methyl-7-aryl-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8-dione derivatives. Following our ongoing projects in the used of multicomponent reactions in homogeneous [36] and heterogeneous metal catalyzed [37, 38], and transition metal free [39, 40] catalyzed reactions for the synthesis of coumarin derivatives [41], we hereby report the synthesis of the desired products via an efficient multicomponent reactions. The products are synthesized from the reaction of 4-hydroxycoumarin, 6-methyl-2H-pyran-2,4(3H)-dione, aldehydes and ammonium acetate in the presence of L-proline as catalyst in water under reflux conditions. The synthesis of the products is presented in Scheme 1.
Results and discussion
In this paper, 10-methyl-7-aryl-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8-dione derivatives are synthesized via the multicomponent reaction of 4-hydroxycoumarin, 6-methyl-2H-pyran-2,4(3H)-dione, aldehydes and ammonium acetate. For obtaining the products in the highest isolated yields, the reaction conditions were first optimized. For this purpose, the reaction of benzaldehydes with 4-hydroxycoumarin, 6-methyl-2H-pyran-2,4(3H)-dione, and ammonium acetate was selected as a model reaction and performed in different reaction conditions. It could be observed in Table 1 that the desired products are obtained in all solvents. However, the best isolated yield of the product was observed in water. On the other hand, studying the effect of temperature on the reaction performance showed that reflux conditions are the best temperature for this reaction. In lower temperatures, lower yields of the products were obtained. In addition, several catalysts were used to increase the efficiency of the reaction. It could be observed that L-proline is the best catalyst for the reaction. Therefore, performing the reaction in water in the presence of 10 mol% of L-proline reflux conditions was selected as the best reaction conditions.
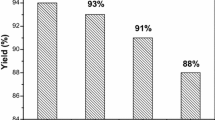
Having the optimized reaction conditions in hand, the generality and scope of the reaction was studied by using several benzaldehyde derivatives as starting materials. For this purpose several benzaldehyde derivatives containing various functional groups were used and reacted to 4-hydroxycoumarin, 6-methyl-2H-pyran-2,4(3H)-dione, and ammonium acetate in water to form the corresponding 10-methyl-7-aryl-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8-diones. The structure and the isolated yields of the products are presented in Table 2. It could be observed that all the substrates have successfully led to the synthesis of the desired products in very good isolated yields. The products are obtained in 88–95% yield. Electron donating groups, such as nitro, carbaldehyde, bromo, and chloro and electron withdrawing groups, including methoxy and dimethoxy groups on benzaldehyde are used as substrates and in all cases, the products are successfully obtained. It could be observed that terephthalaldehyde has participated in the reaction from both aldehyde sites. The results proved the generality of the method for the synthesis of several 10-methyl-7-aryl-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4 -e]pyridine-6,8-dione derivatives from the corresponding starting materials. The advantageous results encouraged us to try a scale up reaction by using large amounts of the starting materials in 10 mmol scale. The scale up result is presented in Table 2, entry 9. The product is obtained in a very good isolated yield, which clearly proves the potential ability of the method in industrial large scale synthesis.
The performance of the reaction was monitored by using various organocatalysts in the reaction. The results of the comparison of the yield of the reaction in the presence of L-proline with other organocatalysts clearly proved the high activity and efficacy of L-proline in the reaction performance. It could be observed in Table t that the desired products were obtained in the presence of other applied organic compounds as catalysts in the reaction. However, the best isolated yield was obtained in the presence of L-proline as catalyst in the reaction. Based on these results, L-proline was used as the optimal catalyst of the reaction.
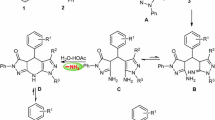
Based on the efficiency of the method for the synthesis of 10-methyl-7-aryl-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8-diones, a possible mechanism was proposed and suggested for the reaction. The suggested mechanism is presented in Scheme 2. It could be observed that the reaction of 4-hydroxycoumarin with ammonium acetate leads to the formation of 4-amino-2H-chromen-2-one (6) intermediate. 6-Methyl-2H-pyran-2,4(3H)-dione separately reacts to the aldehyde to form (E)-3-benzylidene-6-methyl-2H-pyran-2,4(3H)-dione (7). The reaction of compounds 6 and 7 leads to the formation of 4-amino-3-((4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)(phenyl)methyl)-2H-chromen-2-one (8), which finally converts to the desired 10-methyl-7-aryl-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8-dione (5). Based on the proposed mechanism, which is presented in Scheme 2, the presence of L-proline catalyst is critical for the activation of the intermediates, and therefore for the synthesis of the products. The role of the catalyst could be correlated to its acidic nature, which could efficiently activate the carbonyl groups in aldehydes and in the intermediate 7.
To demonstrate the superiority of this method over the methods performed, we compared the yield of the reaction of 2-aminobenzyl alcohol and phenyl isothiocyanate under the optimized reaction conditions in this paper with previous works (Table 3). This method provides excellent yields (up to 95%) in propriety time.
Conclusion
In conclusion, 10-methyl-7-aryl-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8-dione derivatives were synthesized in an efficient method. The method involves the multicomponent reaction of 4-hydroxycoumarin, an aldehyde, 6-methyl-2H-pyran-2,4(3H)-dione, and ammonium acetate. The reaction is best performed in water in the presence of a catalytic amount of L-proline (10 mol%). The scope of the reaction was evaluated by using various substrates and in all cases, the products were obtained in high isolated yields (87–95%). The advantage of this method is no need for any toxic solvent, which is critical from the environmental viewpoint. In addition, the catalyst is commercially available and non-toxic, which makes it appropriate for application in large scale industrial synthesis. For this purpose, a model reaction was performed using 10 mmol of the substrates and the desired product was obtained in 81% yield. A possible mechanism was suggested, which confirms the role of L-proline in the reaction as the catalyst. Based on the advantageous results, this method could be applied for the synthesis of novel compounds with complex structure and/or more synthesis steps.
Experimental
General remarks
All the chemicals, solvents, and the reagents were purchased from Merck, Sigma, Fluka, and Aldrich and were used in the reactions as received without any further purification. The reaction performance was followed by TLC places, which were purchased from Sigma (fluorescent indicator). The structure of the products was confirmed by 1H (500 MHz), 13C NMR (125 MHz), FT-IR, MASS, and elemental analysis. 1H (500 MHz) and 13C NMR (125 MHz) spectra were recorded on a Bruker Avance spectrometer in DMSO, d6 solution. The values of the resulting spectra of the products are reported in ppm in reference with tetramethylsilane (TMS) as an internal standard. In 1H NMR spin coupling multiplicities are reported as s for singlet, b for broad peaks, d for the doublet, t for the triplet, q for the quartet, dd for a doublet of the doublet, or m for multiplet and overlapping spin systems. Values for apparent coupling constants (J) are reported in Hz. FT-IR spectra were recorded on a Shimadzu FT-IR 550 spectrometer using KBr disks. Mass spectra of the samples were recorded on an Agilent Technology (HP) mass spectrometer operating at an ionization potential of 70 eV. Elemental analyses of the products were performed with an elementar analysen system GmbH VarioEL CHNS mode.
Synthesis of 10-methyl-7-aryl-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8-dione derivatives
4-Hydroxycoumarin (324 mg, 2 mmol) and ammonium acetate (1.05 g, 5 mmol) were mixed together and stirred in water under reflux conditions. After 15 min, 6-methyl-2H-pyran-2,4(3H)-dione (252 mg, 2 mmol), an aldehyde (2 mmol) and L-proline (10 mol%) were added the mixture was stirred for more 1 h. The reaction performance was monitored by TLC. After the reaction was completed, the product separated by extraction using ethyl acetate and purified by recrystallization from ethanol.
Spectral data of the products
10-methyl-7-phenyl-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8-dione (5a)
M.P: 228–230 °C; FT-IR (KBr, ν, cm−1): 3402, 3221, 2920, 1685, 1628, 1601, 1535, 1441, 1357, 1229, 1179, 1137, 1085, 853, 760, 696. 1H NMR (250 MHz, DMSO-d6): δ 2.22 (s, 3H), 5.94 (s, 1H), 6.13 (s, 1H), 7.05 (d, J = 7.50 Hz, 1H), 7.11–7.38 (m, 6H), 7.62 (t, J = 7.50 Hz, 1H), 8.03 (d, J = 7.50 Hz, 1H), 11.82 (s, 1H); 13C NMR (63 MHz, DMSO-d6): δ 20.6, 37.3, 97.3, 102.6, 102.7, 103.6, 116.2, 118.2, 124.5, 125.3, 127.1, 127.9, 129.6, 133.5, 140.2, 143.6, 153.3, 154.5, 163.1, 165.1, 167.8, 169.8; Anal. calcd for C22H15NO4: C 73.94, H 4.23, N 3.92; Found: C 73.93, H 4.26, N 3.94; MS, m/z: 357 (M+).
10-methyl-7-(2-nitrophenyl)-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8- dione (5b)
M.P: 270–272 °C; FT-IR (KBr, ν, cm−1): 3409, 3240, 2915, 1682, 1624, 1592, 1522, 1442, 1359, 1292, 1179, 1135, 1087, 997, 862, 774. 1H NMR (250 MHz, DMSO-d6): δ 2.15 (s, 3H), 6.04 (s, 1H), 6.52 (s, 1H), 7.50 (s, 1H), 7.24–7.59 (m, 6H), 7.72 (d, J = 7.50 Hz, 1H), 7.95 (d, J = 7.50 Hz, 1H), 12.00 (s, 1H); 13C NMR (63 MHz, DMSO-d6): δ 20.6, 34.9, 95.8, 100.5, 101.8, 102.7, 103.6, 116.1, 118.0, 124.4, 125.0, 125.7, 128.8, 130.1, 133.4, 133.9, 134.7, 151.0, 153.6, 163.6, 166.8, 169.8; Anal. calcd for C22H14N2O6: C 65.67, H 3.51, N 6.96; Found: C 65.69, H 3.54, N 6.94; MS, m/z: 402 (M+).
10-methyl-7-(3-nitrophenyl)-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8- dione (5c)
M.P: 245–247 °C; FT-IR (KBr, ν, cm−1): 3352, 3210, 2916, 1680, 1623, 1595, 1523, 1438, 1348, 1291, 1178, 1137, 1089, 992, 873, 771. 1H NMR (250 MHz, DMSO-d6): δ 2.20 (s, 3H), 6.07 (s, 1H), 6.14 (s, 1H), 7.34–7.62 (m, 6H), 7.80 (s, 1H), 8.02 (d, J = 6.50 Hz, 1H), 12.08 (s, 1H); 13C NMR (63 MHz, DMSO-d6): δ 20.6, 37.3, 96.0, 101.7, 102.5, 109.3, 116.0, 118.2, 122.5, 124.5, 125.3, 131.1, 133.7, 135.3, 135.9, 143.5, 149.4, 153.4, 154.1, 163.8, 167.5, 170.4; Anal. calcd for C22H14N2O6: C 65.67, H 3.51, N 6.96; Found: C 65.62, H 3.59, N 6.91 MS, m/z: 402 (M+).
7-(2,4-dichlorophenyl)-10-methyl-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8- dione (5d)
M.P: 250–252 °C; FT-IR (KBr, ν, cm−1): 3412, 3242, 2911, 1682, 1622, 1595, 1534, 1435, 1315, 1291, 1127, 1106, 1079, 997, 855, 750. 1H NMR (250 MHz, DMSO-d6): δ 2.17 (s, 3H), 5.93 (s, 1H), 6.06 (s, 1H), 6.98–7.58 (m, 6H), 7.96 (d, J = 7.00 Hz, 1H), 11.98 (s, 1H), 13C NMR (63 MHz, DMSO-d6): δ 20.6, 36.9, 96.8, 101.1, 101.9, 109.3, 116.3, 118.0, 124.3, 124.9, 128.4, 130.1, 131.9, 132.7, 133.3, 135.0, 138.6, 153.2, 153.4, 163.4, 166.8, 169.4; Anal. calcd for C22H13Cl2NO4: C 61.99, H 3.07, N 3.29; Found: C 61.95, H 3.05, N 3.23; MS, m/z: 425 (M+).
7-(4-methoxyphenyl)-10-methyl-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8- dione (5e)
M.P: 250–252 °C; FT-IR (KBr, ν, cm−1): 3409, 3219, 2924, 1678, 1624, 1592, 1534, 1432, 1344, 1281, 1178, 1108, 1075, 996, 891, 752. 1H NMR (250 MHz, DMSO-d6): δ 2.19 (s, 3H), 5.84 (s, 1H), 6.09 (s, 1H), 6.80 (d, J = 8.50 Hz, 1H), 6.95 (d, J = 8.50 Hz, 1H), 7.13–7.37 (m, 2H), 7.61 (t, J = 7.00 Hz, 1H), 8.02 (d, J = 7.00 Hz, 1H), 11.75 (s, 1H); 13C NMR (63 MHz, DMSO-d6): δ 20.6, 36.6, 56.4, 97.6, 101.1, 102.8, 109.3, 115.0, 116.2, 118.2, 124.5, 124.8, 125.3, 129.0, 131.8, 133.5, 153.3, 154.5, 158.8, 162.9, 165.1, 167.8, 169.7; Anal. calcd for C23H17NO5: C 71.31, H 4.42, N 3.62; Found: C 71.35, H 4.45, N 3.69; MS, m/z: 387 (M+).
7-(3,4-dimethoxyphenyl)-10-methyl -7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8- dione (5f)
M.P: 283–285 °C; FT-IR (KBr, ν, cm−1): 3486, 3186, 2922, 1682, 1628, 1596, 1534, 1465, 1344, 1285, 1162, 1143, 1080, 994, 869, 755. 1H NMR (250 MHz, DMSO-d6): δ 2.20 (s, 3H), 3.60 (s, 3H), 3.70 (s, 3H), 5.85 (s, 1H), 6.09 (s, 1H), 6.56 (d, J = 8.00 Hz, 1H), 6.62 (s, 1H), 6.81 (d, J = 8.00 Hz, 1H), 7.30–7.37 (m, 2H), 7.60 (t, J = 7.50 Hz, 1H), 8.02 (d, J = 7.50 Hz, 1H), 11.80 (s, 1H); 13C NMR (63 MHz, DMSO-d6): δ 20.6, 37.0, 56.9, 57.0, 97.6, 102.7, 112.6, 113.1, 116.2, 118.2, 119.9, 124.5, 125.3, 129.0, 132.5, 133.4, 148.6, 150.1, 153.4, 154.4, 162.9, 165.0, 167.7, 169.6; Anal. calcd for C24H19NO6: C 69.06, H 4.59, N 3.36; Found: C 69.10, H 4.63, N 3.39; MS, m/z: 417 (M+).
7-(4-bromophenyl)-10-methyl-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8- dione (5 g)
M.P: 229–231 °C; FT-IR (KBr, ν, cm−1): 3373, 3193, 2923, 1681, 1623, 1597, 1528, 1438, 1358, 1278, 1179, 1135, 1074, 848, 752, 699. 1H NMR (250 MHz, DMSO-d6): δ 2.20 (s, 3H), 5.90 (s, 1H), 6.11 (s, 1H), 7.00 (d, J = 7.50 Hz, 1H), 7.37–7.42 (m, 5H), 7.61 (t, J = 7.50 Hz, 1H), 8.02 (d, J = 7.50 Hz, 1H), 11.90 (s, 1H); 13C NMR (63 MHz, DMSO-d6): δ 20.6, 36.9, 96.7, 102.2, 102.6, 103.0, 116.1, 118.2, 120.0, 124.5, 125.3, 130.4, 132.4, 133.6, 139.3, 140.0, 153.4, 154.3, 163.4, 164.8, 167.6, 170.0; Anal. calcd for C22H14BrNO4: C 60.57, H 3.23, N 3.21; Found: C 60.61, H 3.26, N 3.25; MS, m/z: 436 (M+).
7'-(1,4-phenylene)bis(10-methyl-7,12-dihydro-6H,8H-chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8- dione) (5 h)
M.P: 257–259 °C; FT-IR (KBr, ν, cm−1): 3403, 3233, 2921, 1679, 1626, 1596, 1528, 1440, 1343, 1223, 1178, 1159, 1083, 850, 760, 680. 1H NMR (250 MHz, DMSO-d6): δ 2.17 (s, 6H), 5.87 (s, 2H), 6.07 (s, 2H), 6.85–6.94 (m, 3H), 7.36–7.60 (m, 7H), 8.02 (d, J = 7.5 Hz, 2H), 11.78 (s, 1H); 13C NMR (63 MHz, DMSO-d6): δ 20.6, 37.0, 97.4, 102.8, 116.2, 118.2, 124.6, 125.3, 127.9, 133.5, 137.5, 141.5, 147.5, 154.5, 162.9, 165.2, 167.8, 169.6, 173.9; Anal. calcd for C38H24N2O8: C 71.69, H 3.80, N 4.40; Found: C 71.73, H 3.76, N 4.34; MS, m/z: 636 (M+).
References
R.P. Gore, A.P. Rajput, Drug Invent. Today 5(2), 148 (2013)
T. Zarganes-Tzitzikas, A.L. Chandgude, A. Dömling, Chem. Rec. 15(5), 4 (2015)
S. Brauch, S.S. van Berkel, B. Westermann, Chem. Soc. Rev. 42(12), 4948 (2013)
B.H. Lipshutz, S. Ghorai, Green Chem. 16(8), 3660 (2014)
K. Hara, Z.-S. Wang, T. Sato, A. Furube, R. Katoh, H. Sugihara, Y. Dan-oh, C. Kasada, A. Shinpo, S. Suga, J. Phys. Chem. B 109, 32 (2005)
G. Reynolds, K.H. Drexhage, Optics Commun. 13(3), 222 (1975)
D. Ray, P. Bharadwaj, Inorg. Chem. 47(7), 2252 (2008)
M.-A. Tehfe, J. Lalevée, S. Telitel, J. Sun, J. Zhao, B. Graff, F. Morlet-Savary, J.-P. Fouassier, Polymer 53(14), 2203 (2012)
L. Piazzi, A. Cavalli, F. Colizzi, F. Belluti, M. Bartolini, F. Mancini, M. Recanatini, V. Andrisano, A. Rampa, Bioorg. Med. Chem. Lett. 18(1), 423 (2008)
S.O. Lee, S.Z. Choi, J.H. Lee, S.H. Chung, S.H. Park, H.C. Kang, E.Y. Yang, H.J. Cho, K.R. Lee, Arch. Pharmacal Res. 27(12), 1207 (2004)
L. Pochet, R. Frédérick, B. Masereel, Curr. Pharm. Des. 10(30), 3781 (2004)
A. Thakur, R. Singla, V. Jaitak, Eur. J. Med. Chem. 101, 476 (2015)
T. Nasr, S. Bondock, M. Youns, Eur. J. Med. Chem. 76, 539 (2014)
J. Tao, S. Hu, M. Pacholec, C.T. Walsh, Org. Lett. 5(18), 3233 (2003)
O.M. Singh, N.S. Devi, J. Org. Chem. 74(8), 3141 (2009)
M.N. Khan, S. Pal, S. Karamthulla, L.H. Choudhury, New J. Chem. 38(10), 4722 (2014)
Z. Chen, J. Bi, W. Su, Chin. J. Chem. 31(4), 507 (2013)
M.M. Heravi, M. Zakeri, S. Pooremamy, H.A. Oskooie, Synth. Commun. 41(1), 113 (2010)
A. Yahya-Meymandi, H. Nikookar, S. Moghimi, M. Mahdavi, L. Firoozpour, A. Asadipour, P.R. Ranjbar, A. Foroumadi, J. Iran. Chem. Soc. 14(4), 771 (2017)
S. Samai, G.C. Nandi, R. Kumar, M. Singh, Tetrahedron Lett. 50(50), 7096 (2009)
Z. Heydari, S. Bahadorikhalili, P.R. Ranjbar, M. Mahdavi, Appl. Organomet. Chem. 32(12), e4561 (2018)
S.E. Sadat-Ebrahimi, S.M. Haghayegh-Zavareh, S. Bahadorikhalili, A. Yahya-Meymandi, M. Mahdavi, M. Saeedi, Synth. Commun. 47(24), 2324 (2017)
M. Matache, C. Dobrota, N.D. Bogdan, I. Dumitru, L.L. Ruta, C.C. Paraschivescu, I.C. Farcasanu, I. Baciu, D.P. Funeriu, Tetrahedron 65(31), 5949 (2009)
G. Rahimzadeh, S. Bahadorikhalili, E. Kianmehr, M. Mahdavi, Mol. Diversity 21(3), 597 (2017)
H. Göksu, B. Çelik, Y. Yıldız, F. Şen, B. Kılbaş, Chem. Sel. 1(10), 2366 (2016)
T. Demirci, B. Çelik, Y. Yıldız, S. Eriş, M. Arslan, F. Sen, B. Kilbas, RSC Adv. 6(80), 76948 (2016)
H. Göksu, Y. Yıldız, B. Çelik, M. Yazıcı, B. Kılbaş, F. Şen, Chem. Sel. 1(5), 4452 (2016)
İ Esirden, E. Erken, M. Kaya, F. Sen, Catal. Sci. Technol. 5(9), 4452 (2015)
R. Saha, S.K. Ghosh, A. Ghosh, I. Saha, K. Mukherjee, A. Basu, B. Saha, Res. Chem. Intermed. 39(2), 631 (2013)
P. Sar, A. Ghosh, A. Scarso, B. Saha, Res. Chem. Intermed. 45(12), 6021 (2019)
M.H. Mondal, S. Malik, S. De, S.S. Bhattacharyya, B. Saha, Res. Chem. Intermed. 43(3), 1651 (2017)
P. Sar, A. Ghosh, B. Saha, Res. Chem. Intermed. 41(10), 7775 (2015)
A. Ghosh, P. Das, D. Saha, P. Sar, S.K. Ghosh, B. Saha, Res. Chem. Intermed. 42(3), 2619 (2016)
A. Ghosh, R. Saha, K. Mukherjee, S.K. Ghosh, P. Sar, S. Malik, B. Saha, Res. Chem. Intermed. 41(5), 4873 (2015)
P. Sar, A. Ghosh, D. Ghosh, B. Saha, Res. Chem. Intermed. 41(8), 5565 (2015)
M.H. Sayahi, S.J. Saghanezhad, S. Bahadorikhalili, M. Mahdavi, Appl. Organomet. Chem. 33(1), e4635 (2019)
S. Bahadorikhalili, A. Ashtari, L. Ma’mani, P.R. Ranjbar, M. Mahdavi, Appl. Organomet. Chem. 32(4), e4212 (2018)
S. Bahadorikhalili, H. Arshadi, Z. Afrouzandeh, L. Ma’mani, New J. Chem. 44(21), 8840 (2020)
S. Bahadorikhalili, G. Rahimzadeh, E. Kianmehr, S. Ansari, H. Hamedifar, M. Mahdavi, Chem. Sel. 4(1), 100 (2019)
Z. Ghiyasabadi, S. Bahadorikhalili, M. Saeedi, M. Karimi-Niyazagheh, S.S. Mirfazli, J. Heterocycl. Chem. 57(2), 120 (2020)
M.H. Sayahi, S. Bahadorikhalili, S.J. Saghanezhad, M.A. Miller, M. Mahdavi, Res. Chem. Intermed. 46(1), 491 (2020)
M. Molnar, J. Klenkar, T. Tarnai, Synth. Commun. 47(11), 1040 (2017)
G. Rezanejade Bardajee, A. Ghaedi, S. Hekmat, G. Abarashi, M. Mahdavi, T. Akbarzadeh, J. Sulfur Chem. 38(5), 519 (2017)
H. Eshghi, A. Khojastehnezhad, F. Moeinpour, M. Bakavoli, N. Zeinabi, S. Allameh, Res. Chem. Intermed. 41(10), 7915 (2015)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sayahi, M.H., Shamkhani, F., Mahdavi, M. et al. Efficient synthesis of chromeno[4,3-b]pyrano[3,4-e]pyridine-6,8-dione derivatives via multicomponent one-pot reaction under mild reaction conditions in water. Res Chem Intermed 47, 4101–4112 (2021). https://doi.org/10.1007/s11164-021-04519-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04519-2