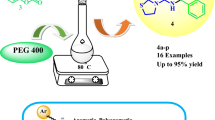
Abstract
Efficient and green reactions of different aromatic aldehydes, 3-amino-1-phenyl-1H-pyrazol-5(4H)-one, and substituted 3-aminopyrazoles for the synthesis of pyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one derivatives have been reported through deamination cyclization reactions. This is a new route for the generation of pyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one compounds. According to the reported literature, these kinds of compounds have important optical properties, and have potential application prospects. Other advantages of this process are simple operation, easy separation, and a wide range of substrates. Best of all, this method possesses an environmentally benign procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a non-toxic, low-cost, readily available and environmentally benign solvent, water has been widely used in organic synthesis [1,2,3,4,5,6]. The results show that the reactivity and selectivity of reactions in water are superior to those in organic solvents. In addition, because most of the organic compounds are not soluble in water, this can easily separate them from aqueous phase. Of course, this phenomenon is very important in industrial scale-up processes to replace the use of hazardous, explosive and flammable organic solvents.
Pyrazolo[3,4-b]pyridine and its derivatives are the important heterocyclic compounds possessing a broad range of biological activities, such as antimicrobial [7], antiviral [8], anti-inflammatory [9], and other properties [10,11,12,13]. They are also used as serotonin re-uptake inhibitors [14], CCK agonists [15] and vasodilators [16]. Importantly, their structural analogy to purine bases means that these compounds are also important constituents of DNA and RNA nucleosides [17]. Pyrazolo[3,4-b:4′,3′-e]pyridine derivatives are fused pyrazolo[3,4-b]pyridine compounds. Research has showed that these compounds often have optical properties [18,19,20,21]. Other publications have reported that these compounds also exhibit antibacterial [22] and moderate antifungal [23] activities. Some methods for the synthesis of fused pyrazolo[3,4-b]pyridine derivatives have been reported. For example, Green et al. [24] reported a multi-step reaction for the synthesis of dipyrazolo[3,4-b:3′,4′-d]pyridin-3-ones from arylhydrazine with low yields. Fused pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidines could be prepared by a solvent-free microwave-assisted reaction of heterocyclic o-aminonitriles and cyanopyridines in the presence of tBuOK as catalyst [25]. Spiro[indoline-pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine]trione derivatives were gained by a three-component condensation reaction of barbituric acids, 1H-pyrazol-5-amines and isatins in aqueous media [26]. Jiang and co-authors also reported domino reactions of arylglyoxals and pyrazol-5-amines to synthesize the fused pyrazolo[3,4-b]pyridine derivatives [27]. In view of the importance of these compounds, here we report an efficient method for the synthesis of pyrazolo[3,4-b:4′,3′-e]pyridine derivatives in water via deamination cyclization reactions.
It can be found that aromatic aldehydes, aminopyrazole, and ketones with active methylene are the key reagents for the synthesis of pyrazolo[3,4-b]pyridine derivatives by dehydration cyclization reactions. For instance , Chen et al. [28] reported a procedure for the synthesis of pyrazolo[3,4-b]pyridine derivatives by one-pot three-component condensation of aminopyrazoles, aldehydes, and cycloketones in water using carbonaceous material as a solid acid catalyst. The one-pot three-component reaction between aldehyde, amino pyrazole, and 1,3-cyclohexanedione in polyethylene glycol (PEG)-400 medium was reported by Karnakar and co-authors, and a variety of pyrazolo[3,4-b] pyridine derivatives were obtained in excellent yields [29]. Zhang et al. [30] studied a multi-component reaction of aldehyde, 5-amino-3-methyl-1-phenylpyrazole and Meldrum acid or dimedone to give pyrazolo[3,4-b]pyridinone derivatives. The reaction of isatin, aminopyrazole, and alkyl cyanoacetate can also synthesize these compounds [31]. In 2011, the three-component reaction of aldehyde, acyl acetonitrile, and electron-rich amino heterocycles (including aminopyrazole and aminouracils) in ionic liquid was reported by Huang and co-authors [32]. In this research, we reported a novel process for the preparation of fused pyrazolo[3,4-b]pyridine derivatives from aromatic aldehydes, 3-amino-1-phenyl-2-pyrazolin-5-one, and aminopyrazole through deamination cyclization reactions.
Experimental
All reagents were purchased from the Merck and Sigma-Aldrich and used without further purification. Melting points were determined on an XT-5 microscopic melting-point apparatus and were uncorrected. IR spectra were recorded on a FT Bruker Tensor 27 spectrometer. 1H NMR and 13C NMR spectra were obtained from solutions in DMSO-d6 with Me4Si as internal standard using a Bruker-400 spectrometer. HRMS spectra were obtained with a Bruker microTOF-Q 134 instrument.
General procedure for the synthesis of pyrazolo[3,4-b:4′,3′-e]pyridine derivatives
The mixture of aromatic aldehydes (1 mmol), 3-amino-1-phenyl-1H-pyrazol-5(4H)-one (1 mmol), substituted 3-aminopyrazoles (1 mmol), H2O (6 mL), HOAc (2 mL) was placed in a 50-mL reaction flask and stirred at 80 °C forabout 5–7 h (monitored by TLC). After completion of the reactions, the mixture was cooled to room temperature and the product was isolated from water. Then, compounds 4a–x were recrystallized from DMF.
4-(4-Chlorophenyl)-2-phenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4a)
Yellow solid, yield, 95%, m.p. > 280°C; IR (KBr) (ν, cm−1): 3440 (NH), 3170 (NH), 1699 (C=O), 1596, 1498, 1395, 1316, 1209, 1095, 1014, 956, 896, 832, 792, 752, 687, 692, 640, 596; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 13.71 (s, 1H, NH), 11.39 (s, 1H, NH), 8.13 (s, 1H, N=CH), 7.88–7.85 (m, 4H, ArH), 7.61 (d, J = 8.4 Hz, 2H, ArH), 7.47 (t, J = 7.6 Hz, 2H, ArH), 7.24-7.20 (m, 1H, ArH); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 159.4 (C=O), 158.6, 154.2, 144.1, 137.9, 135.2, 135.1, 132.7, 131.5, 129.4, 129.0, 128.6, 128.4, 125.3, 119.8, 117.9, 112.6, 103.5; HRMS (ESI-TOF) m/z calculated for C19H12ClN5NaO: 384.0628, found [M + Na]+: 384.0631.
4-(3,4-Dichlorophenyl)-2-phenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4b)
Yellow solid, yield: 93%, m.p. > 280°C; IR (KBr) (ν, cm−1): 3412 (NH), 3219 (NH), 1666 (C=O), 1592, 1552, 1499, 1416, 1363, 1310, 1296, 1205, 1179, 1138, 1031, 962, 902, 832, 804, 756, 733, 682, 644, 544; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 13.74 (s, 1H, NH), 11.44 (s, 1H, NH), 8.15 (s, 1H, N=CH), 8.09 (s, 1H, ArH), 7.86 (s, 2H, ArH), 7.80 (s, 2H, ArH), 7.47 (s, 2H, ArH), 7.23 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 159.2 (C=O), 158.5, 154.2, 142.4, 137.8, 134.9, 133.2, 133.0, 132.8, 131.3, 130.8, 130.8, 129.4, 125.4, 119.8, 112.5, 103.6; HRMS (ESI-TOF) m/z calculated for C19H11Cl2N5NaO: 418.0238, found [M + Na]+: 418.0250.
4-(3,4-Dimethylphenyl)-2-phenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4c)
Yellow solid, yield: 92%, m.p. > 280°C; IR (KBr) (ν, cm−1): 3335 (NH), 3158 (NH), 1648 (C=O), 1588, 1498, 1385, 1316, 1295, 1207, 1094, 1065, 957, 908, 804, 750, 718, 690, 655, 576; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 13.66 (s, 1H, NH), 11.29 (s, 1H, NH), 8.10 (s, 1H, N=CH), 7.89 (d, J = 7.2 Hz, 2H, ArH), 7.62 (s, 1H, ArH), 7.55 (d, J = 7.6 Hz, 1H, ArH), 7.46 (t, J = 7.6 Hz, 2H, ArH), 7.28 (d, J = 7.6 Hz, 1H, ArH), 7.20 (t, J = 6.8 Hz, 1H, ArH), 2.30 (s, 6H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 159.6 (C=O), 158.9, 154.1, 146.1, 138.8, 138.1, 136.3, 135.4, 131.8, 130.4, 129.6, 129.3, 128.3, 125.1, 119.7, 112.7, 103.4, 19.9 (CH3), 19.8 (CH3); HRMS (ESI-TOF) m/z calculated for C21H17N5NaO: 378.1331, found [M + Na]+: 378.1339.
4-(2-Methoxyphenyl)-2-phenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4d)
Yellow solid, yield: 90%, m.p. 225–227°C; IR (KBr) (ν, cm−1): 3412 (NH), 3267 (NH), 1655 (C=O), 1593, 1497, 1460, 1386, 1317, 1301, 1255, 1206, 1047, 1025, 960, 894, 860, 807, 754, 692, 658; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 13.60 (s, 1H, NH), 11.22 (s, 1H, NH), 7.90 (s, 1H, N=CH), 7.86 (d, J = 7.6 Hz, 2H, ArH), 7.53–7.45 (m, 4H, ArH), 7.21 (d, J = 8.4 Hz, 2H, ArH), 7.09 (t, J = 7.6 Hz, 1H, ArH), 3.74 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 159.4 (C=O), 158.6, 157.2, 154.0, 142.4, 138.1, 135.5, 131.6, 131.4, 129.4, 125.1, 122.0, 120.5, 119.5, 113.4, 112.0, 105.1, 55.9 (OCH3); HRMS (ESI-TOF) m/z calculated for C20H15N5NaO2: 380.1123, found [M + Na]+: 380.1127.
4-(4-Methoxyphenyl)-2-phenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4e)
Yellow solid, yield: 94%, m.p. 256–257°C; IR (KBr) (ν, cm−1): 3413 (NH), 3123 (NH), 1660 (C=O), 1594, 1514, 1496, 1401, 1310, 1259, 1171, 1024, 963, 833, 801, 762, 687, 650, 592; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 13.63 (s, 1H, NH), 11.27 (s, 1H, NH), 8.12 (s, 1H, N=CH), 7.86 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 4H, ArH), 7.48 (t, J = 7.6 Hz, 2H, ArH), 7.22 (t, J = 7.6 Hz, 1H, ArH), 7.12 (d, J = 8.8 Hz, 2H, ArH), 3.87 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 159.7 (C=O), 159.0, 154.2, 145.7, 138.0, 135.4, 132.7, 129.4, 125.1, 125.0, 119.7, 114.0, 112.6, 103.1, 55.8 (OCH3); HRMS (ESI-TOF) m/z calculated for C20H15N5NaO2: 380.1123, found [M + Na]+: 380.1136.
4-(2,5-Dimethoxyphenyl)-2-phenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4f)
Yellow solid, yield: 95%, m.p. 250–252°C; IR (KBr) (ν, cm−1): 3350 (NH), 3112 (NH), 1652 (C=O), 1594, 1498, 1400, 1360, 1297, 1276, 1223, 1153, 1048, 956, 908, 805, 756, 731, 692, 651, 577; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 13.66 (s, 1H, NH), 11.28 (s, 1H, NH), 7.97 (s, 1H, N=CH), 7.91 (d, J = 6.4 Hz, 2H, ArH), 7.47 (s, 2H, ArH), 7.20 (s, 1H, ArH), 7.12–7.05 (m, 3H, ArH), 3.75 (s, 3H, OCH3), 3.69 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 159.4 (C=O), 158.6, 154.0, 153.1, 151.2, 142.3, 138.1, 135.7, 129.4, 125.1, 122.7, 119.6, 117.3, 116.1, 113.5, 112.9, 105.1, 56.3 (OCH3), 55.9 (OCH3); HRMS (ESI-TOF) m/z calculated for C21H17N5NaO3: 410.1229, found [M + Na]+: 410.1235.
4-(3,4-Dimethoxyphenyl)-2-phenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4 g)
Yellow solid, yield: 96%, m.p. > 280°C; IR (KBr) (ν, cm−1): 3449 (NH), 3293 (NH), 1693 (C=O), 1590, 1517, 1399, 1309, 1264, 1194, 1139, 1029, 954, 901, 869, 790, 752, 694, 640, 596; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 13.63 (s, 1H, NH), 11.28 (s, 1H, NH), 8.20 (s, 1H, N=CH), 7.89 (d, J = 6.4 Hz, 2H, ArH), 7.52 (s, 1H, ArH), 7.47 (t, J = 8.8 Hz, 3H, ArH), 7.21 (t, J = 6.4 Hz, 1H, ArH), 7.14 (d, J = 8.4 Hz, 1H, ArH), 3.87 (s, 3H, OCH3), 3.85 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 159.7 (C=O), 159.0, 154.2, 150.8, 148.4, 145.9, 138.1, 135.6, 129.4, 125.1, 124.0, 119.8, 115.0, 112.6, 111.6, 103.2, 56.1 (OCH3), 56.0 (OCH3); HRMS (ESI-TOF) m/z calculated for C21H17N5NaO3: 410.1229, found [M + Na]+: 410.1241.
4-(Benzo[d][1, 3]dioxol-5-yl)-2-phenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4 h)
Yellow solid, yield: 95%, m.p. > 280°C; IR (KBr) (ν, cm−1): 3312 (NH), 3116 (NH), 1648 (C=O), 1584, 1500, 1438, 1395, 1289, 1247, 1207, 1103, 1031, 963, 931, 905, 800, 750, 692, 653, 581; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 13.64 (s, 1H, NH), 11.29 (s, 1H, NH), 8.12 (s, 1H, N=CH), 7.90 (d, J = 7.2 Hz, 2H, ArH), 7.47–7.43 (m, 3H, ArH), 7.37 (d, J = 8.0 Hz, 1H, ArH), 7.19 (t, J = 6.8 Hz, 1H, ArH), 7.07 (d, J = 8.4 Hz, 1H, ArH), 6.13 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 159.6 (C=O), 158.9, 154.2, 149.2, 147.4, 145.5, 138.0, 135.4, 129.3, 126.4, 125.4, 125.1, 119.8, 112.7, 111.6, 108.5, 103.3, 102.0 (OCH2O); HRMS (ESI-TOF) m/z calculated for C20H13N5NaO3: 394.0916, found [M + Na]+: 394.0918.
4-(2-Fluorophenyl)-5-methyl-2-phenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4i)
Yellow solid, yield: 93%, m.p. 276–278°C; IR (KBr) (ν, cm−1): 3448 (NH), 3205 (NH), 1701 (C=O), 1594, 1496, 1401, 1385, 1297, 1237, 1215, 1103, 994, 840, 802, 755, 686, 605; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 13.41 (s, 1H, NH), 11.37 (s, 1H, NH), 7.87 (d, J = 7.2 Hz, 2H, ArH), 7.63–7.57 (m, 2H, ArH), 7.46 (t, J = 7.6 Hz, 2H, ArH), 7.42–7.35 (m, 2H, ArH), 7.20 (t, J = 7.2 Hz, 1H, ArH), 2.02 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 159.3 (C=O), 158.2, 155.0, 143.2, 139.3, 137.9, 131.9 (t, JCF = 8.0 Hz), 129.4, 125.2, 124.4, 120.9 (d, JCF = 16.2 Hz), 119.5, 115.7 (d, JCF = 21.2 Hz), 111.6, 105.0, 14.4 (CH3); HRMS (ESI-TOF) m/z calculated for C20H14FN5NaO: 382.1080, found [M + Na]+: 382.1097.
4-(5-Bromo-2-methoxyphenyl)-5-methyl-2-phenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4j)
Yellow solid, yield: 94%, m.p. > 280°C; IR (KBr) (ν, cm−1): 3389 (NH), 3131 (NH), 1668 (C=O), 1593, 1493, 1401, 1345, 1298, 1254, 1182, 1130, 1025, 938, 809, 757, 689, 619; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 13.26 (s, 1H, NH), 11.22 (s, 1H, NH), 7.82 (d, J = 8.0 Hz, 2H, ArH), 7.67 (dd, J1 = 9.2 Hz, J2 = 2.8 Hz, 1H, ArH), 7.54 (d, J = 2.4 Hz, 1H, ArH), 7.46 (t, J = 7.6 Hz, 2H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.16 (d, J = 8.8 Hz, 1H, ArH), 3.70 (s, 3H, OCH3), 1.96 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 159.4 (C=O), 158.2, 156.2, 154.9, 143.4, 141.3, 138.0, 133.4, 132.9, 129.4, 125.1, 124.4, 119.5, 113.7, 111.8, 104.9, 56.3 (OCH3), 14.2 (CH3); HRMS (ESI-TOF) m/z calculated for C21H16BrN5NaO2: 472.0385, found [M + Na]+: 472.0373.
4-(3-Methoxyphenyl)-5-methyl-2-phenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4k)
Yellow solid, yield: 91%, m.p. 255–256°C; IR (KBr) (ν, cm−1): 3419 (NH), 3113 (NH), 1698 (C=O), 1593, 1496, 1428, 1385, 1348, 1298, 1262, 1215, 1181, 1098, 1036, 993, 856, 809, 755, 701, 687, 669; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 13.29 (s, 1H, NH), 11.23 (s, 1H, NH), 7.83 (d, J = 7.6 Hz, 2H, ArH), 7.47–7.40 (m, 3H, ArH), 7.19 (t, J = 7.2 Hz, 1H, ArH), 7.12 (s, 1H, ArH), 7.08 (dd, J1 = 8.0 Hz, J2 = 3.6 Hz, 2H, ArH), 3.80 (s, 3H, OCH3), 1.99 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 159.5 (C=O), 158.8, 158.3, 155.0, 146.5, 143.5, 138.1, 134.3, 129.3, 129.1, 125.0, 122.1, 119.5, 115.4, 114.9, 111.5, 104.2, 55.7 (OCH3), 15.3 (CH3); HRMS (ESI-TOF) m/z calculated for C21H17N5NaO2: 394.1280, found [M + Na]+: 394.1291.
4-(3,4-Dimethoxyphenyl)-5-methyl-2-phenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4l)
Yellow solid, yield: 93%, m.p. 237-239°C; IR (KBr) (ν, cm−1): 3350 (NH), 3133 (NH), 1681 (C=O), 1601, 1492, 1401, 1317, 1301, 1260, 1237, 1185, 1140, 1022, 856, 799, 756, 689, 618, 511; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 13.23 (s, 1H, NH), 11.16 (s, 1H, NH), 7.82 (d, J = 7.2 Hz, 2H, ArH), 7.46 (s, 2H, ArH), 7.20–7.18 (m, 2H, ArH), 7.08 (s, 2H, ArH), 3.86 (s, 3H, OCH3), 3.78 (s, 3H, OCH3), 2.06 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 159.7 (C=O), 155.1, 149.9, 148.1, 143.6, 138.2, 129.4, 124.9, 123.0, 119.5, 114.2, 111.6, 111.1, 104.1, 56.1 (OCH3), 56.0 (OCH3), 15.7 (CH3); HRMS (ESI-TOF) m/z calculated for C22H19N5NaO3: 424.1386, found [M + Na]+: 424.1388.
4-(2-Fluorophenyl)-5-methyl-2,7-diphenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4m)
Yellow solid, yield: 92%, m.p. > 280°C; IR (KBr) (ν, cm−1): 3438 (NH), 1659 (C=O), 1592, 1497, 1425, 1377, 1349, 1299, 1226, 1087, 1029, 860, 800, 758, 690, 640; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 11.78 (s, 1H, NH), 8.23 (d, J = 8.0 Hz, 2H, ArH), 7.85 (d, J = 8.0 Hz, 2H, ArH), 7.66–7.63 (m, 2H, ArH), 7.57 (t, J = 8.0 Hz, 2H, ArH), 7.45 (dd, J1 = 16.0 Hz, J2 = 8.0 Hz, 3H, ArH), 7.40 (t, J = 7.2 Hz, 1H, ArH), 7.33 (t, J = 7.2 Hz, 1H, ArH), 7.22 (t, J = 7.2 Hz, 1H, ArH), 2.06 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 160.7 (C=O), 158.7, 158.2, 157.5, 152.5, 144.8, 140.1, 139.1, 137.6, 132.1 (d, JCF = 8.2 Hz), 132.0 (d, JCF = 2.1 Hz), 129.6, 129.4, 126.3, 125.4, 124.4 (d, JCF = 3.2 Hz), 121.2, 120.3 (d, JCF = 16.1 Hz), 119.59, 115.8 (d, JCF = 21.0 Hz), 113.4, 105.7, 14.4 (CH3); HRMS (ESI-TOF) m/z calculated for C26H18FN5NaO: 458.1393, found [M + Na]+: 458.1399.
4-(3-Fluorophenyl)-5-methyl-2,7-diphenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4n)
Yellow solid, yield: 93%, m.p. > 280°C; IR (KBr) (ν, cm−1): 3443 (NH), 1657 (C=O), 1593, 1498, 1437, 1377, 1349, 1300, 1211, 1089, 1011, 978, 810, 787, 755, 723, 686, 648, 570; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 11.69 (s, 1H, NH), 8.20 (d, J = 8.0 Hz, 2H, ArH), 7.82 (d, J = 8.0 Hz, 2H, ArH), 7.59-7.51 (m, 3H, ArH), 7.47–7.38 (m, 5H, ArH), 7.29 (t, J = 7.6 Hz, 1H, ArH), 7.20 (t, J = 7.6 Hz, 1H, ArH), 1.99 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 163.0 (C=O), 160.6, 158.7, 157.6, 152.4, 145.5, 144.8, 139.1, 137.6, 134.6 (d, JCF = 8.4 Hz), 130.1 (d, JCF = 8.4 Hz), 129.5, 129.3, 126.1, 120.9, 119.5, 125.3, 117.0 (d, JCF = 21.0 Hz), 116.3 (d, JCF = 20.7 Hz), 113.1, 104.9, 15.2 (CH3); HRMS (ESI-TOF) m/z calculated for C26H18FN5NaO: 458.1393, found [M + Na]+: 458.1388.
4-(3-Chlorophenyl)-5-methyl-2,7-diphenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4o)
Yellow solid, yield: 93%, m.p. > 280°C; IR (KBr) (ν, cm−1): 3425 (NH), 1660 (C=O), 1591, 1497, 1401, 1298, 1220, 1087, 955, 786, 755, 720, 685, 646, 610, 556; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 11.68 (s, 1H, NH), 8.19 (d, J = 8.0 Hz, 2H, ArH), 7.82 (d, J = 8.0 Hz, 2H, ArH), 7.66 (s, 1H, ArH), 7.62 (d, J = 7.2 Hz, 1H, ArH), 7.56-7.49 (m, 4H, ArH), 7.44 (t, J = 8.0 Hz, 2H, ArH), 7.29 (t, J = 7.2 Hz, 1H, ArH), 7.20 (t, J = 7.2 Hz, 1H, ArH), 1.97 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 158.8 (C=O), 157.6, 152.5, 145.3, 144.8, 139.2, 137.7, 134.5, 132.9, 130.0, 129.8, 129.5, 129.4, 128.7, 126.2, 125.4, 120.9, 119.6, 119.5, 113.2, 105.0, 15.4 (CH3); HRMS (ESI-TOF) m/z calculated for C26H18ClN5NaO: 474.1098, found [M + Na]+: 474.1113.
4-(2,4-Dichlorophenyl)-5-methyl-2,7-diphenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4p)
Yellow solid, yield: 92%, m.p. > 280°C; IR (KBr) (ν, cm−1): 3443 (NH), 1672 (C=O), 1592, 1496, 1373, 1347, 1290, 1220, 1104, 857, 801, 720, 618; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 11.86 (s, 1H, NH), 8.23 (d, J = 8.0 Hz, 2H, ArH), 7.87–7.83 (m, 3H, ArH), 7.65–7.60 (m, 2H, ArH), 7.57 (t, J = 7.6 Hz, 2H, ArH), 7.46 (t, J = 7.6 Hz, 2H, ArH), 7.33 (t, J = 7.6 Hz, 1H, ArH), 7.22 (t, J = 7.6 Hz, 1H, ArH), 2.01 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 158.5 (C=O), 157.5, 152.6, 144.7, 142.0, 139.1, 137.5, 135.2, 133.3, 132.4, 131.1, 129.6, 129.5, 129.2, 127.6, 126.4, 125.5, 121.1, 119.5, 113.1, 105.6, 14.2 (CH3); HRMS (ESI-TOF) m/z calculated for C26H17Cl2N5NaO: 508.0708, found [M + Na]+: 508.0725.
4-(3-Bromophenyl)-5-methyl-2,7-diphenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4q)
Yellow solid, yield: 93%, m.p. > 280°C; IR (KBr) (ν, cm−1): 3447 (NH), 1663 (C=O), 1590, 1497, 1422, 1377, 1346, 1296, 1220, 1167, 1071, 1031, 948, 862, 806, 784, 754, 706, 685, 645, 609; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 11.68 (s, 1H, NH), 8.19 (d, J = 8.0 Hz, 2H, ArH), 7.83–7.80 (m, 3H, ArH), 7.75 (d, J = 8.0 Hz, 1H, ArH), 7.57 (d, J = 7.6 Hz, 1H, ArH), 7.53–7.42 (m, 5H, ArH), 7.27 (t, J = 7.2 Hz, 1H, ArH), 7.20 (t, J = 7.2 Hz, 1H, ArH), 1.97 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 158.7 (C=O), 157.6, 152.4, 145.2, 144.8, 139.1, 137.6, 134.6, 132.4, 132.3, 130.1, 129.4, 129.4, 129.0, 126.1, 125.3, 121.3, 120.8, 119.6, 113.1, 104.9, 15.4 (CH3); HRMS (ESI-TOF) m/z calculated for C26H18BrN5NaO: 518.0592, found [M + Na]+: 518.0598.
5-Methyl-2,7-diphenyl-4-(4-(trifluoromethyl)phenyl)-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4r)
Yellow solid, yield: 95%, m.p. 252-254°C; IR (KBr) (ν, cm−1): 3424 (NH), 1667 (C=O), 1590, 1499, 1402, 1347, 1323, 1224, 1166, 1129, 1066, 1020, 805, 756, 687, 610; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 11.79 (s, 1H, NH), 8.23 (d, J = 7.6 Hz, 2H, ArH), 7.92 (d, J = 8.0 Hz, 2H, ArH), 7.83 (dd, J1 = 11.2 Hz, J2 = 8.8 Hz, 4H, ArH), 7.58 (t, J = 7.6 Hz, 2H, ArH), 7.46 (t, J = 7.2 Hz, 2H, ArH), 7.36 (t, J = 7.2 Hz, 1H, ArH), 7.22 (t, J = 7.6 Hz, 1H, ArH), 2.02 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 158.8 (C=O), 157.6, 152.6, 145.5, 144.9, 139.1, 137.6, 136.8, 130.8, 130.1, 129.8, 129.6, 129.4, 126.4, 126.1, 125.4, 125.0, 124.9, 123.4, 121.2, 119.6, 113.1, 104.9, 15.4 (CH3); HRMS (ESI-TOF) m/z calculated for C27H18F3N5NaO: 508.1361, found [M + Na]+: 508.1373.
4-(4-Hydroxyphenyl)-5-methyl-2,7-diphenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4s)
Yellow solid, yield: 96%, m.p. > 280°C; IR (KBr) (ν, cm−1): 3416 (NH), 3120 (–OH), 1650 (C=O), 1591, 1516, 1495, 1399, 1344, 1308, 1269, 1231, 1169, 911, 807, 760, 715, 692, 602; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 11.53 (s, 1H, NH), 9.86 (s, 1H, OH), 8.21 (d, J = 7.6 Hz, 2H, ArH), 7.83 (d, J = 8.0 Hz, 2H, ArH), 7.53 (t, J = 7.6 Hz, 2H, ArH), 7.47–7.40 (m, 4H, ArH), 7.29 (t, J = 7.2 Hz, 1H, ArH), 7.20 (t, J = 7.2 Hz, 1H, ArH), 6.92 (d, J = 8.8 Hz, 2H, ArH), 2.08 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 159.2 (C=O), 159.0, 158.0, 152.6, 148.3, 145.2, 139.3, 137.9, 132.0, 129.4, 129.3, 126.0, 125.1, 122.5, 120.9, 119.6, 114.8, 113.4, 104.7, 15.8 (CH3); HRMS (ESI-TOF) m/z calculated for C26H19N5NaO2: 456.1436, found [M + Na]+: 456.1451.
4-(2-Methoxyphenyl)-5-methyl-2,7-diphenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4t)
Yellow solid, yield: 90%, m.p. 263-265°C; IR (KBr) (ν, cm−1): 3447 (NH), 1659 (C=O), 1592, 1497, 1377, 1225, 1114, 1026, 906, 854, 807, 752, 689, 651, 609; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 11.62 (s, 1H, NH), 8.25 (d, J = 8.0 Hz, 2H, ArH), 7.86 (d, J = 8.4 Hz, 2H, ArH), 7.58-7.51 (m, 3H, ArH), 7.46 (t, J = 7.6 Hz, 2H, ArH), 7.38 (d, J = 7.6 Hz, 1H, ArH), 7.32 (t, J = 7.6 Hz, 1H, ArH), 7.23-7.19 (m, 2H, ArH), 7.10 (t, J = 7.6 Hz, 1H, ArH), 3.72 (s, 3H, OCH3), 2.00 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 159.0 (C=O), 158.0, 156.8, 152.4, 145.3, 144.4, 139.3, 137.8, 131.2, 130.8, 129.5, 129.4, 126.2, 125.2, 121.6, 121.0, 120.4, 119.5, 113.9, 111.5, 105.8, 55.9 (OCH3), 14.2 (CH3); HRMS (ESI-TOF) m/z calculated for C27H21N5NaO2: 470.1593, found [M + Na]+: 470.1580.
4-(5-Bromo-2-methoxyphenyl)-5-methyl-2,7-diphenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4u)
Yellow solid, yield: 93%, m.p. > 280°C; IR (KBr) (ν, cm−1): 3439 (NH), 1661 (C=O), 1593, 1498, 1375, 1293, 1242, 1220, 1187, 1125, 1087, 1026, 866, 807, 756, 691, 619, 557; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 11.67 (s, 1H, NH), 8.24 (d, J = 8.0 Hz, 2H, ArH), 7.87 (d, J = 2.0 Hz, 2H, ArH), 7.70 (dd, J1 = 8.8 Hz, J2 = 2.4 Hz, 1H, ArH), 7.61 (d, J = 2.0 Hz, 1H, ArH), 7.54 (t, J = 7.6 Hz, 2H, ArH), 7.47 (t, J = 7.6 Hz, 2H, ArH), 7.30 (t, J = 7.6 Hz, 1H, ArH), 7.22 (t, J = 7.6 Hz, 1H, ArH), 7.17 (d, J = 9.2 Hz, 1H, ArH), 3.72 (s, 3H, OCH3), 2.04 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 158.8 (C=O), 157.8, 156.2, 152.4, 145.1, 142.2, 139.2, 137.7, 133.7, 133.0, 129.5, 129.4, 126.2, 125.3, 123.8, 121.0, 119.6, 113.8, 113.7, 111.9, 105.8, 56.4 (OCH3), 14.2 (CH3); HRMS (ESI-TOF) m/z calculated for C27H20BrN5NaO2: 548.0698, found [M + Na]+: 548.0715.
4-(3-Methoxyphenyl)-5-methyl-2,7-diphenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4v)
Yellow solid, yield: 94%, m.p. 233–234°C; IR (KBr) (ν, cm−1): 3424 (NH), 1662 (C=O), 1593, 1498, 1401, 1378, 1347, 1301, 1214, 1157, 1047, 812, 787, 754, 729, 690, 649; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 11.66 (s, 1H, NH), 8.23 (d, J = 8.0 Hz, 2H, ArH), 7.83 (d, J = 8.0 Hz, 2H, ArH), 7.57 (t, J = 7.6 Hz, 2H, ArH), 7.48–7.43 (m, 3H, ArH), 7.34 (t, J = 7.6 Hz, 1H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.16 (s, 1H, ArH), 7.12 (d, J = 6.8 Hz, 2H, ArH), 3.82 (s, 3H, OCH3), 2.04 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 158.9 (C=O), 157.8, 152.5, 147.3, 145.2, 139.2, 137.8, 133.8, 129.6, 129.4, 129.2, 126.3, 125.3, 122.1, 121.1, 119.6, 115.4, 115.2, 113.3, 105.0, 55.7 (OCH3), 15.3 (CH3); HRMS (ESI-TOF) m/z calculated for C27H21N5NaO2: 470.1593, found [M + Na]+: 470.1595.
4-(2,5-Dimethoxyphenyl)-5-methyl-2,7-diphenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4w)
Yellow solid, yield: 95%, m.p. > 280°C; IR (KBr) (ν, cm−1): 3438 (NH), 1659 (C=O), 1498, 1460, 1413, 1376, 1349, 1296, 1226, 1042, 810, 755, 690, 649; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 11.62 (s, 1H, NH), 8.23 (d, J = 8.0 Hz, 2H, ArH), 7.84 (d, J = 8.0 Hz, 2H, ArH), 7.59 (t, J = 7.6 Hz, 2H, ArH), 7.47 (t, J = 7.6 Hz, 2H, ArH), 7.35 (t, J = 7.6 Hz, 1H, ArH), 7.22 (t, J = 7.6 Hz, 1H, ArH), 7.13 (d, J = 9.2 Hz, 1H, ArH), 7.09 (dd, J1 = 9.2 Hz, J2 = 2.8 Hz, 1H, ArH), 7.00 (d, J = 2.8 Hz, 1H, ArH), 3.75 (s, 3H, OCH3), 3.66 (s, 3H, OCH3), 2.04 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 158.9 (C=O), 158.0, 153.1, 152.4, 150.8, 145.4, 144.2, 139.2, 137.8, 129.6, 129.4, 126.3, 125.3, 122.3, 121.1, 119.5, 116.5, 115.8, 113.9, 112.6, 105.8, 56.4 (OCH3), 56.0 (OCH3), 14.1 (CH3); HRMS (ESI-TOF) m/z calculated for C28H23N5NaO3: 500.1699, found [M + Na]+: 500.1683.
4-(3,4-Dimethoxyphenyl)-5-methyl-2,7-diphenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one (4x)
Yellow solid, yield: 92%, m.p. 253–254°C; IR (KBr) (ν, cm−1): 3439 (NH), 1685 (C=O), 1593, 1499, 1442, 1345, 1253, 1233, 1142, 1017, 867, 795, 760, 690, 669; 1H NMR (400 MHz, DMSO-d6) (δ, ppm): 11.58 (s, 1H, NH), 8.23 (d, J = 8.0 Hz, 2H, ArH), 7.83 (d, J = 8.0 Hz, 2H, ArH), 7.56 (t, J = 7.6 Hz, 2H, ArH), 7.46 (t, J = 7.6 Hz, 2H, ArH), 7.32 (t, J = 7.6 Hz, 1H, ArH), 7.23-7.19 (m, 2H, ArH), 7.12-7.07 (m, 2H, ArH), 3.86 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 2.10 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm): 159.0 (C=O), 158.0, 152.6, 150.1, 148.2, 147.8, 145.2, 139.2, 137.8, 129.5, 129.4, 126.2, 125.2, 124.4, 123.0, 121.1, 119.6, 114.2, 113.4, 111.1, 104.9, 56.1 (OCH3), 56.0 (OCH3), 15.7 (CH3); HRMS (ESI-TOF) m/z calculated for C28H23N5NaO3: 500.1699, found [M + Na]+: 500.1709.
Results and discussion
In our reported work, we have found that 3-amino-1-phenyl-1H-pyrazol-5(4H)-one was an important reagent for the synthesis of pyrazolo[3,4-b]pyridine derivatives [33]. In this research, we hoped it could also be used to synthesize pyrazolo[3,4-b]pyridine derivatives. Therefore, the reaction of 4-chlorobenzaldehyde, 3-amino-1-phenyl-1H-pyrazol-5(4H)-one, and 3-aminopyrazole was operated in MeCN using PTSA•H2O as catalyst, and, to our delight, a new compound was obtained with 56% yield. With the help of IR, NMR, and HRMS, we can confirm that it was the fused pyrazolo[3,4-b]pyridine, i.e. pyrazolo[3,4-b:4′,3′-e]pyridine derivative, because it achieved the product by removing a molecule of ammonia from the starting reagents. This is a new process for the synthesis of pyrazolo[3,4-b]pyridine derivatives.
Encouraged by this discovery, we undertook the synthesis of more pyrazolo[3,4-b:4′,3′-e]pyridine derivatives. So, 4-chlorobenzaldehyde 1a, 3-amino-1-phenyl-1H-pyrazol-5(4H)-one 2, and 3-aminopyrazole 3 were again chosen to screen the reaction conditions (Scheme 1). First, we commenced the optimization studies using PTSA•H2O as the catalyst, employing different solvents (MeCN, MeOH, EtOH, THF, CH2Cl2, toluene, and H2O) as reaction media (Table 1, entries 1–7). Unfortunately, the results of the reactions were not satisfactory. Considering our reported reaction [34], we decided to test a model reaction in the mixed solvents (H2O and HOAc). Gratifyingly, the model reaction readily occurred to afford pyrazolo[3,4-b:4′,3′-e]pyridine with different yields under the different ratios of mixed solvents (H2O:HOAc) (Table 1, entries 8–11). An excellent yield was obtained when the volume ratio of H2O and HOAc was 3:1 (Table 1, entry 10). Further study found that the best results could be gained at 80 °C for 6 h (Table 1, entry 12). We also found that the reaction temperature had a great influence on the yield, and that lower temperatures were unfavorable to the reaction (Table 1, entries 13–16). The reaction did not occur in pure water, while 77% yield was gained in acetic acid medium (Table 1, entries 17–18). The results are listed in Table 1.
With the optimal conditions in hand, the reactions of different aromatic aldehydes 1, 3-amino-1-phenyl-1H-pyrazol-5(4H)-one 2, and 3-aminopyrazole 3 were conducted in mixed solvents to test the validity of the screening conditions (Scheme 2); as expected, the corresponding compounds 4-aryl-2-phenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one derivatives were obtained with high yields (4a–4h). Next, 5-methyl-3-aminopyrazole was used in this process, and pyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one derivatives (4i–4l) were gained smoothly. In order to gain more pyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one derivatives, 3-methyl-1-phenyl-1H-pyrazol-5-amine was also used in this process, and the products 4m–4x were successfully produced with excellent yields. The aromatic aldehydes bearing either electron-withdrawing groups (F–, Cl–, Br–), or electron-donating groups (OH–, Me–, MeO–) have no obvious effect on the yields. The results are summarized in Table 2.
All the products were confirmed by IR, NMR, and HRMS spectra. 4d was chosen as an example to analyze the structures. In 4d 1H NMR, its spectrum recorded a singlet signal at δ = 13.60 (s, 1H) and 11.22 (s, 1H) ppm due to two NH protons. The chemical shift at 7.90 (s, 1H) ppm is a hydrogen (CH) on the pyrazole ring. A singlet signal at 3.74 ppm (s, 3H) is the CH3O group. The chemical shifts of the aromatic hydrogen (ArH) appear between 7.09 and 7.86 ppm. In its 13C NMR, the chemical shifts of the carbon atoms are at 159.4, 158.6, 157.2, 154.0, 142.4, 138.1, 135.5, 131.6, 131.4, 129.4, 125.1, 122.0, 120.5, 119.5, 113.4, 112.0, 105.1, and 55.9, respectively.
According to the obtained products, a possible mechanism is provided in Fig. 1. Firstly, the aromatic aldehyde reacted with 3-amino-1-phenyl-1H-pyrazol-5(4H)-one to give Knoevenagel product A. The addition reaction took place from A and the substituted 3-aminopyrazoles and intermediate B was obtained. The intermediate C was produced from the tautomerism of B. And then the cyclization reaction took place releasing a molecule of ammonia and the critical intermediate D was obtained. Finally, the tautomerism and oxidizing reaction operated and the products 4 were successfully formed.
Conclusions
In conclusion, we have reported efficient and green reactions of different aromatic aldehydes, 3-amino-1-phenyl-1H-pyrazol-5(4H)-one, and substituted 3-aminopyrazoles for the synthesis of pyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one derivatives with high yields. Differing from the reported methods, this is a new route for the generation of pyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one derivatives. According to the reported literature, these kinds of compounds have important optical properties, and have potential application prospects. Other advantages of this process are simple operation, easy separation, and a wide range of substrates. Best of all, this method possesses an environmentally benign procedure.
References
P.K. Chinthakindi, H.G. Kruger, T. Govender, T. Naicker, P.I. Arvidsson, J. Org. Chem. 81, 2618 (2016)
L. Yang, T. Dong, H.M. Revankar, C.P. Zhang, Green Chem. 19, 3951 (2017)
P. Norcott, S.P. McErlean, Org. Biomol. Chem. 14, 1288 (2016)
B. Li, P.H. Dixneuf, Chem. Soc. Rev. 42, 5744 (2013)
P. Czerwiński, M. Michalak, J. Org. Chem. 82, 7980 (2017)
J. Xu, L. Qiao, J. Shen, K. Chai, P. Zhang, Org. Lett. 19, 5661 (2017)
H. de Mello, A. Echevarria, A.M. Bernardino, M. Canto-Cavalheiro, L.L. Leon, J. Med. Chem. 47, 5427 (2004)
T.J. Tucker, J.T. Sisko, R.M. Tynebor, T.M. Williams, P.J. Felock, J.A. Flynn, M.T. Lai, Y. Liang, G. McGaughey, M. Liu, M. Miller, G. Moyer, V. Munshi, R. Perlow-Poehnelt, S. Prasad, J.C. Reid, R. Sanchez, M. Torrent, J.P. Vacca, B.L. Wan, Y. Yan, J. Med. Chem. 51, 6503 (2008)
H. Ochiai, A. Ishida, T. Ohtani, K. Kusumi, K. Kishikawa, S. Yamamoto, H. Takeda, T. Obata, H. Makai, M. Toda, Bioorg. Med. Chem. 12, 4089 (2004)
A. Cappelli, C. Nannicini, A. Gallelli, G. Giuliani, S. Valenti, G.P. Mohr, M. Anzini, L. Mennuni, F. Ferrari, G. Caselli, A. Giordani, W. Pereis, F. Makovec, G. Giorgi, S. Vomero, J. Med. Chem. 51, 2137 (2008)
L. Revesz, E. Blum, F.E. Di Padova, T. Buhl, R. Feifel, H. Gram, P. Hiestand, U. Manning, U. Neumann, G. Rucklin, Bioorg. Med. Chem. Lett. 16, 262 (2006)
F. Manetti, S. Schenone, F. Bondavalli, C. Brullo, O. Bruno, A. Ranise, L. Mosti, G. Menozzi, P. Fossa, M.L. Trincavelli, C. Martini, A. Martinelli, C. Tintori, M. Botta, J. Med. Chem. 48, 7172 (2005)
T. Tuccinardi, S. Schenone, F. Bondavalli, C. Brullo, O. Bruno, L. Mosti, A.T. Zizzari, C. Tintori, F. Manetti, C. Oiampi, M.L. Trincavelli, C. Martini, A. Martinelli, M. Botta, ChemMedChem 3, 898 (2008)
G.M. Shutske, J.E. Roehr, J. Heterocyclic Chem. 34, 789 (1997)
B.R. Henke, C.J. Aquino, L.S. Birkemo, D.K. Croom, R.W. Dougherty, G.N. Ervin, M.K. Grizzle, G.C. Hirst, M.K. James, M.F. Johnson, K.L. Queen, R.G. Sherrill, E.E. Sugg, E.M. Suh, J.W. Szewczyk, R.J. Unwalla, J. Yingling, T.M. Willson, J. Med. Chem. 40, 2706 (1997)
A. Straub, J. Benet-Buckholtz, R. Frode, A. Kern, C. Kohlsdorfer, P. Schmitt, T. Schwarz, H.M. Siefert, J.P. Stasch, Bioorg. Med. Chem. 10, 1711 (2002)
Y.S. Sanghvi, S.B. Larson, R.C. Willis, R.K. Robins, G.R. Revankar, J. Med. Chem. 32, 945 (1989)
C.H. Chuen, Y.T. Tao, Appl. Phys. Lett. 81, 4409 (2002)
K. Rechthaler, R. Schamschule, A.B.J. Parusel, K. Rotkiewicz, D. Piorun, G. Kohler, Acta Phys. Pol., A 95, 321 (1999)
Z. He, G. Milburn, A. Danel, A. Puchala, P. Tomasik, D. Rasala. J. Mater. Chem. 7, 2323 (1997)
A.B.J. Parusel, R. Schamschule, D. Piorun, K. Rechthaler, A. Puchała, D. Rasała, K. Rotkiewicz, G. Kohler, J. Mol. Struct. (TEOCHEM) 419, 63 (1997)
K.C. Joshi, K. Dubey, A. Dandia, Pharmazie 36, 336 (1981)
J. Quiroga, J. Portilla, B. Insuasty, R. Abonia, M. Nogueras, M. Sortino, S. Zacchino, J. Heterocycl. Chem. 42, 61 (2005)
N.J. Green, J. Xiang, J. Chen, L. Chen, A.M. Davies, D. Erbe, S. Tam, J.F. Tobin, Bioorg. Med. Chem. 11, 2991 (2003)
P. Acosta, B. Insuasty, A. Ortiz, R. Abonia, M. Sortino, S.A. Zacchino, J. Quiroga, Arab. J. Chem. 9, 481 (2016)
R. Ghahremanzadeh, M. Sayyafi, S. Ahadi, A. Bazgir, J. Comb. Chem. 11, 393 (2009)
B. Jiang, W. Fan, M.Y. Sun, Q. Ye, S.L. Wang, S.J. Tu, G. Li, J. Org. Chem. 79, 5258 (2014)
Z. Chen, Y. Shi, Q. Shen, H. Xu, F. Zhang, Tetrahedron Lett. 56, 4749 (2015)
K. Karnakar, S.N. Murthy, K. Ramesh, G. Satish, J.B. Nanubolu, Y.V.D. Nageswar, Tetrahedron Lett. 53, 2897 (2012)
X. Zhang, D. Li, X. Fan, X. Wang, X. Li, Mol. Divers. 14, 159 (2010)
A. Rahmati, T. Kenarkoohi, H.R. Khavasi, ACS Comb. Sci. 14, 657 (2012)
Z. Huang, Y. Hu, Y. Zhou, D.Q. Shi, ACS Comb. Sci. 13, 45 (2011)
S.S. Xu, Z.S. Wang, X.L. Li, L.C. Rong, S.J. Tu, Tetrahedron 72, 5754 (2016)
Z.S. Wang, L.L. Gao, Z.Y. Xu, Z. Ling, Y.Q. Qin, L.C. Rong, S.J. Tu, Tetrahedron 73, 385 (2017)
Acknowledgements
We are grateful to the National Natural Science Foundation of China (NSFC) (51174201, 21571087), the Open Foundation of Jiangsu Key Laboratory of Green Synthetic Chemistry for Functional Materials (K201312), the Major Projects of Natural Science Research in Jiangsu Province (15KJA150004), and the Priority Academic Program Development of Jiangsu Higher Education Institutions for financial support. This work was also sponsored by TAPP.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, H., Li, L., Wang, Z. et al. A green synthesis of 1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-3(2H)-one derivatives from deamination cyclization reactions in aqueous medium. Res Chem Intermed 44, 3211–3226 (2018). https://doi.org/10.1007/s11164-018-3302-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3302-7