Abstract
Polyphosphoric acid functionalized silica-coated magnetic nanoparticles with core–shell structure (NiFe2O4@SiO2–PPA) has been used as a magnetically recyclable green catalyst for the one-pot three-component synthesis of 2-thioxoquinazolinones by the reaction of isatoic anhydride, primary amines and thiourea under neat conditions. The catalyst is readily recovered by simple magnetic decantation and can be recycled five times with no significant loss of catalytic activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, much attention has been directed toward the production of magnetic nanoparticles (MNPs) [1–3], because these nanoparticles can be well dispersed in the reaction mixtures without a magnetic field providing large surfaces for ready access of substrate molecules. More importantly, after completing the reactions, the MNP catalysts can be isolated efficiently from the product solution through a simple magnetic separation process. The strategy of magnetic separation, taking advantage of MNPs, is typically more effective than filtration or centrifugation as it prevents loss of the catalyst [4, 5]. Magnetic separation of super MNPs is simple, economical, and promising for industrial applications. From both economic and environmental viewpoints, organic reactions under solvent-free conditions using recoverable catalysts have gained significant attention [5–8]. Rostamnia et al. reported the synthesis of MNPs for immobilizing sulfuric acid as a heterogeneous catalyst, and showed that their synthesized catalyst can act as a magnetic separable catalyst and after completing the reaction can be easily isolated by using an external magnet [9]. In another study, Mamani and et al. reported supporting sulfonic acid on silica-coated MNPs, and obtained good results in the catalytic activity of these catalysts [10]. Polyphosphoric acid (PPA) is an inorganic acid which is used as a catalyst in organic synthesis [11, 12], but because it is homogeneous, its separation is difficult after completing the reaction. Thus, we decided to support this acid on silica-coated MNPs.
Thioxoquinazolinones have been the object of intensive investigation due to their different biological properties such as antimycobacterial [13], antifungal [14], antihypertensive [15], antihistaminic [16], enzyme inhibitor [17], anticancer [18] and antiviral activities [19]. Although various procedures have been reported for the synthesis of thioxoquinazolinone derivatives [20–22], most of the methods have encountered some drawbacks, such as low yields, prolonged reaction time, expensive catalysts and use of toxic organic solvents. Thus, the development of an environmentally benign and high yielding methodology for the synthesis of thioxoquinazolinones is in great demand.
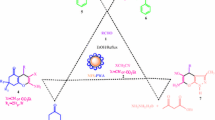
Recently, we developed approaches for the synthesis of biologically interesting products via multicomponent reactions [23–26]. The challenge in this field was developing efficient and rapid green methods. Based on our earlier success in the preparation of magnetic nanoparticles as catalysts [27–30] combined with our achievements in this area, we present here the results of an extended investigation into the activity of the PPA functionalized magnetic nanocatalyst [27] NiFe2O4@SiO2–PPA, as an efficient catalyst for the synthesis of 2-thioxoquinazolinones. Facile separation of the catalyst by using an external magnet and the recyclability of the catalyst (up to five times) are other important benefits of this system. To the best of our knowledge, there are no examples of the use of NiFe2O4@SiO2–PPA as a catalyst for the synthesis of thioxoquinazolinones from the condensation of isatoic anhydride, primary amines and thiourea under solvent-free conditions. Therefore, we wish to report a simple, rapid and an efficient synthetic method for the synthesis of thioxoquinazolinones using NiFe2O4@SiO2–PPA as a heterogeneous and reusable catalyst without the requirement for any solvent, salt, or additive (Scheme 1).
Experimental
Melting points were determined on an Electrothermal type 9100 melting point apparatus. IR spectra were recorded using a 4300 Shimadzu spectrophotometer with KBr plates. 1H NMR spectra were recorded on a Bruker DRX500 spectrometer. The products (4a–o) were isolated and characterized by comparison of physical and spectral data with those of known samples.
Preparation of NiFe2O4@SiO2–PPA
The magnetic nanoparticles (NiFe2O4; Fig. 1) were synthesized by a chemical co precipitation technique using ferric and nickel ions [31]. Based on our previous report [27], coating a layer of silica on the surface of the NiFe2O4 nanoparticles and then functionalization of the iron oxide with polyphosphoric acid were achieved by treatment with tetraethyl orthosilicate (TEOS) followed by polyphosphoric acid. As shown in Fig. 1, the SEM image of NiFe2O4 nanoparticles demonstrates that these MNPs are almost spherical and regular in shape. However, aggregation of the nanoparticles was found and this aggregation might occur during the coating and supporting process [27–30].
General procedure for the preparation of 3-(aryl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-ones (4a–i)
A mixture of isatoic anhydride (2.0 mmol), primary amines (2.0 mmol) with thiourea (2.0 mmol) under solvent-free condition at 120 °C in the presence of (0.02 g) of NiFe2O4@SiO2–PPA was heated in an oil bath for 18–30 min. Upon completion, cold ethanol was added to the reaction mixture (the product is soluble in ethanol) and the NiFe2O4@SiO2–PPA was placed on the side wall of the reaction vessel with the aid of an external magnet, then the catalyst was washed and dried to be reused in the next run. The solvent was evaporated and the resulting crude product was collected and gives compounds (4a–o) in high yields.
The spectral data of some representative 3-(aryl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-ones are the following:
3-phenyl-2-thioxo-2,3-dihydroquinazolin-4(1H)-one (4a):
mp 307–309 °C,1H NMR (500 MHz, DMSO-d 6): δ 7.2–8 (m, 9H, Ar–H), 13 (br, 1H, N–H exchanges with D2O), IR (KBr disc): 1,662 (C=O), 1,229 (C=S), 3,243 cm−1 (N–H).
3-(4-Methylphenyl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one (4d):
mp 305–307 °C, 1H NMR (500 MHz, DMSO-d 6): δ 2.38 (s, 3H, CH3), 7.1–8 (m, 8H, Ar–H), 13 (br, 1H, N–H exchanges with D2O), IR (KBr disc): 1,662 (C=O), 1,199 (C=S), 3,243 cm−1 (N–H).
3-Benzyl-2-thioxo-2,3-dihydroquinazolin-4(1H)-one (4e):
mp 254–255 °C, 1H NMR (500 MHz, DMSO-d 6): δ 5.6 (s, 2H, CH2), 7.2–8 (m, 9H, Ar–H), 13 (br, 1H, N–H exchanges with D2O), IR (KBr disc): 1,688 (C=O), 1,179 (C=S), 3,200 cm−1 (N–H).
3-(4-Chlorophenyl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one (4f):
mp 318–320 °C, 1H NMR (500 MHz, DMSO-d 6): δ 7.2–8 (m, 8H, Ar–H), 13 (br, 1H, N–H exchanges with D2O), IR (KBr disc): 1,661 (C=O), 1,233 (C=S), 3,244 cm−1 (N–H).
3-(2-Methylphenyl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one (4 h):
mp 265–267 °C, 1H NMR (500 MHz, DMSO-d 6): δ 2.94 (s, 3H, CH3), 7.1–8 (m, 8H, Ar–H), 13 (br, 1H, N–H exchanges with D2O), IR (KBr disc): 1,662 (C=O), 1,199 (C=S), 3,286 cm−1 (N–H).
Results and discussion
In order to determine the optimum conditions, the reaction between isatoic anhydride (2.0 mmol), aniline (2.0 mmol) with thiourea (2.0 mmol) at 120 °C were chosen as a model reaction. The best result has been obtained at 0.02 g of catalyst (Table 1) and under solvent-free conditions (Table 2). For comparison, and to show the effect of NiFe2O4@SiO2–PPA as a solid acid catalyst, synthesis of the model compound 4a, was also investigated using SiO2–PPA, PPA, and SiO2 under solvent free conditions separately. The results are given in Table 3 and, as shown, NiFe2O4@SiO2–PPA gave the best result (entry 1). Using SiO2–PPA as the catalyst gave the product 4a in good yield (84 %) (entry 2), but separation and recovery of SiO2–PPA were not easy, because we needed filtration or centrifugation, which are not eco-friendly processes. Also, we used PPA as a catalyst and the yield of the reaction was relatively good (68 %), but it was not recyclable (entry 3). Therefore, NiFe2O4@SiO2–PPA can act as a solid acid catalyst in the synthesis of 2-thioxoquinazolinones derivatives. The generality of this process was demonstrated by the wide range of substituted primary amines to synthesize the corresponding products in high yields (Table 4). Unlike some previously reported methods, the present method does not require toxic organic solvents to produce the 2-thioxoquinazolinones derivatives (4a–o). All the products were characterized by NMR, IR and melting point and also by comparison with the data reported in literature.
A probable mechanism for condensation of isatoic anhydride, aniline, thiourea and NiFe2O4@SiO2–PPA (as an acidic catalyst) for the synthesis of 2-thioxoquinazolinones is proposed as shown in Scheme 2.
It is of great importance that the core/shell material should possess sufficient magnetic and superparamagnetic properties for its practical applications. Magnetic hysteresis measurements for the NiFe2O4 were done in an applied magnetic field at r.t., with the field sweeping from −10,000 to +10,000 Oersted. As shown in Fig. 2, the M (H) hysteresis loop for the samples was completely reversible, showing that the nanoparticles exhibit superparamagnetic characteristics. The hysteresis loops of them reached saturation up to the maximum applied magnetic field. The magnetic saturation value of the NiFe2O4 is 16.71 emu g−1 at r.t. These particles showed high permeability in magnetization, and their magnetization was sufficient for magnetic separation with a conventional magnet (Fig. 2).
At the end of the reaction, to determine the applicability of catalyst recovery, ethanol was added to the reaction mixture to dissolve the product. With the aid of an external magnet, the catalyst was held on the side wall of the reaction vessel, while the solution was decanted. The catalyst was washed with diethyl ether to remove residual product, dried at 100 °C under vacuum, and reused in subsequent reactions in excellent yields (91, 91, 91, 90 and 89 %). It showed the same activity as the fresh catalyst without any significant loss of its activity (Fig. 3).
Conclusion
We have reported a simple new catalytic method for the synthesis of 2-thioxoquinazolinones by one-pot three-component condensation reaction of isatoic anhydride, primary amines and thiourea using NiFe2O4@SiO2–PPA as an efficient, reusable, and green heterogeneous catalyst under solvent-free conditions. Moreover, the catalyst could be readily separated by use of a magnetic force and reused without any significant loss of catalytic activity after five runs.
References
V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara, J.M. Basset, Chem. Rev. 111, 3036 (2011)
M.M. Lin, H.H. Kim, H. Kim, M. Muhammed, D.K. Kim, Nano Rev. 1, 4883 (2010)
D. Astruc, F. Lu, J.R. Aranzaes, Angew. Chem. Int. Ed. 44, 7852 (2005)
A. Hu, G.T. Yee, W. Lin, J. Am. Chem. Soc. 127, 12486 (2005)
M. Mojtahedi, M.S. Abaee, T. Alishiri, Tetrahedron Lett. 50, 2322 (2009)
L. Mamani, A. Heydari, M. Sheykhan, Appl. Catal. A 384, 122 (2010)
B. Karimi, E. Farhangi, Adv. Synth. Catal. 355, 508 (2013)
F. Shahbazi, K. Amani, Catal. Commun. 55, 57 (2014)
S. Rostamnia, K. Lamei, M. Mohammadquli, M. Sheykhan, A. Heydari, Tetrahedron Lett. 53, 5257 (2012)
L. Mamani, M. Sheykhan, A. Heydari, M. Faraji, Y. Yamini, Appl. Catal. A 377, 64 (2010)
D.W. Hein, R.J. Alheim, J.J. Leavitt, J. Am. Chem. Soc. 79, 427 (1957)
E. Alcalde, I. Dinarés, L. Pérez-García, T. Roca, Synthesis 1992, 395 (1992)
V. Aagarsamy, R. Giridhar, M.R. Yadav, R. Revathi, K. Ruckmani, Ind. J. Pharm. Sci. 68, 532 (2006)
A. Rajasekaran, V. Rajamanickam, S. Darlinquine, Eur. Rev. Med. Pharmacol. Sci. 17, 95 (2013)
R. Cortez, I.A. Rivero, R. Somanathan, G. Aguirre, F. Ramirez, E. Hong, Synth. Commun. 21, 285 (1991)
V. Alagarsamy, R. Giridhar, M.R. Yadav, J. Pharm. Pharmacol. 58, 1249 (2006)
M. Redondo, J.G. Zarruk, P. Ceballos, D.I. Perez, C. Perez, A.P. Castillo, M.A. Moro, J. Brea, C. Val, M.I. Cadavid, M.I. Loza, N.E. Campillo, A. Martinez, C. Gil, Eur. J. Med. Chem. 47, 175 (2012)
H.M. Jen, H.L. Jiau, K.S. Chu, X.Y. Bastow, B. Kenneth, N.K. Hamel, E. Lee, K. Hsiung, J. Med. Chem. 43, 4479 (2000)
S.K. Krishnan, S. Ganguly, R. Veerasamy, B. Jan, Eur. Rev. Med. Pharmacol. Sci. 15, 673 (2011)
F. Wanga, P. Zhaoa, C. Xi, Tetrahedron Lett. 52, 231 (2011)
S. Allameh, M.M. Heravi, M.M. Hashemi, F.F. Bamoharram, Chin. Chem. Lett. 22, 131 (2011)
A. Saeed, U. Shaheena, M. Bolteb, J. Chin. Chem. Soc. 57, 82 (2010)
A. Khojastehnezhad, F. Moeinpour, A. Davoodnia, Chin. Chem. Lett. 22, 807 (2011)
A. Khojastehnezhad, F. Moeinpour, A.R. Shams, Synth. React. Inorg. Met. Org. Chem. 42, 273 (2012)
F. Moeinpour, N. Dorostkar-Ahmadi, A. Sardashti-Birjandi, A. Khojastehnezhad, M. Vafaei, Res. Chem. Intermed. 40, 3145 (2014)
F. Moeinpour, N. Dorostkar, M. Vafaei, Synth. Commun. 42, 2367 (2012)
A. Khojastehnezhad, M. Rahimizadeh, F. Moeinpour, H. Eshghi, M. Bakavoli, C. R. Chim. 17, 459 (2014)
A. Khojastehnezhad, M. Rahimizadeh, H. Eshghi, F. Moeinpour, M. Bakavoli, Chin. J. Cat. 35, 376 (2014)
H. Eshghi, A. Khojastehnezhad, S.M. Seyedi, F. Moeinpour, M. Abbasi, M. Bakavoli, RSC Adv. 4, 39782 (2014)
M. Rahimizadeh, S.M. Seyedi, M. Abbasi, H. Eshghi, A. Khojastehnezhad, F. Moeinpour, M. Bakavoli, J. Iranian, Chem. Soc. (2014). doi:10.1007/s13738-014-0546-z
D. Zins, V. Cabuil, R. Massart, J. Mol. Liq. 83, 217 (1999)
R. Lakhan, M. Srivastava, Proc. Indian. Acad. Sci. Chem. Sci. 105, 11 (1993)
J. Azizian, A.A. Mohammadi, A.R. Karimi, Synth. Commun. 33, 415 (2003)
D. Guo-lan, W. Man-Man, H. Zhi-Bin, S. Da-Qing, J. Het, Chem. 46, 645 (2009)
V.K. Tiwari, D.D. Singh, H.A. Hussain, B.B. Mishra, A. Singh, Monatsh. Chem. 139, 43 (2008)
I. Yavari, S. Beheshti, Helv. Chim. Acta 94, 1825 (2011)
T. Kappe, W. Steiger, E. Ziegler, Monatsh. Chem. 98, 214 (1967)
Acknowledgment
The authors are grateful to Islamic Azad University, Bandar Abbas and Mashhad Branches for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eshghi, H., Khojastehnezhad, A., Moeinpour, F. et al. Nanomagnetically modified polyphosphoric acid (NiFe2O4@SiO2–PPA): an efficient, fast, and reusable catalyst for the synthesis of 2-thioxoquinazolinones under solvent-free conditions. Res Chem Intermed 41, 7915–7924 (2015). https://doi.org/10.1007/s11164-014-1866-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1866-4