Abstract
The configurations, stabilities, electronic, and magnetic attributes of the first row transition metal (TM)-substituted TMC6N7 clusters have been investigated at the PBE level. The results display that the first row TM atoms are inclined to replace the N atom which approaches the N atom out of the C–N rings except for Cu and Zn. As for the CuC6N7 and ZnC6N7 clusters, the N atom out of the C-N rings is inclined to be substituted. The ScC6N7(GS), TiC6N7(GS), VC6N7(GS), CoC6N7(GS), TiC6N7(a), and CoC6N7(a) clusters display more structurally stabilities than the pristine C6N8 clusters. The ground-state 3d TMC6N7 clusters exhibit more dynamic stabilities than the pristine C6N8 clusters except for Zn. Partial of 4s orbital electrons of TM atoms is transferred to the neighbor C atoms. The V, Cr, Mn, and Fe atoms of the ground-state TMC6N7 clusters display the opposite spin to Co and Ni atoms of the ground-state TMC6N7 clusters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Graphite carbon nitride (g-C3N4) has been regarded as an excellent carbon carrier of catalyst [1]. It has the advantages of low cost, simple synthesis, thermal and chemical stabilities, biocompatibility, and non-toxicity, etc. [2,3,4,5]. However, the photocatalytic efficiency of pristine g-C3N4 is limited by the intrinsic lack of light-absorbance, charge mobility, and recombination rate of electron–hole pairs [6]. To solve these problems, doping has been widely adopted to modulate the electronic configurations of g-C3N4 to improve conductivity, optics, magnetism, etc. [7]. That is, doping reduces electronic transition energy and provides a ‘bridge’ for lower energy photo-generated electrons [3]. Various non-metals and metals have been considered to modify the intrinsic characteristics and obtain novel properties of layered g-C3N4 [8]. g-C3N4, with rich pyridine-like nitrogen, prefers to trap TM atoms to the hexagonal rings of g-C3N4 [1, 3]. At present, a large number of studies have been performed on first row TM (i.e., Ti [9], V [10], Cr [4], Mn [1, 8, 11], Fe [1, 7, 11,12,13,14,15,16,17,18], Co [1, 11, 14, 16, 19], Ni [20], Cu [11, 12, 16, 21, 22], and Zn [11, 17, 23]) doping on the g-C3N4 sheet. The strong hybridization between 3d orbitals of TM atoms and the pπ orbitals of g-C3N4 not only can improve the photocatalytic properties but also can induce spin polarization [1, 3, 8]. Given the complexity of the reaction process, we have calculated the 3d TM-substituted TMC5N8 clusters [24]. However, the difference in the radius of C (0.86 nm) and N (0.80 nm) atoms is so small [3]. The precise recognition of the effective active sites has not been explored [25]. gh-C3N4 is more stable than gt-C3N4 [8], and the structural unit of g-C3N4 is ordered tri-s-triazine (C6N7) which is planar and aromatic [26]. C6N7 can provide many binding sites that interact with metals or other pollutants [18].
C3N4 exhibits versatile electronic and magnetic properties which originate from the holey geometrical character [27, 28]. In this study, the configurations, stabilities, electronic, and magnetic properties of the TMC6N7 clusters are investigated by using density functional theory (DFT). It is very important to understand the evolution mechanisms of gh-C3N4-based materials. It is also helpful to control defects and design novel properties of C3N4-based materials [28].
Computational details
The pristine C6N8 clusters are extracted by gh-C3N4 in Ref. [8]. A TM atom is adopted to substitute a N atom of the pristine C6N8 clusters in order to design the TMC6N7 clusters, respectively. The optimization and property calculations have been performed by DFT which are implemented in the DMol3 package. The Perdew–Burke–Ernzerhof (PBE) functional (including a semi-empirical van der Waals (vdW) correction) within the generalized gradient approximation (GGA) is adopted for the exchange–correlation [26, 29]. Because of strong electronic coupling of TM atoms of the TMC6N7 clusters [30], the approximate semi-classical dispersion correction scheme DFT + D must be selected [31, 32]. In order to avoid omitting certain configurations of the TMC6N7 clusters, symmetry unconstraints is adopted [33, 34]. Considering the electron relativity effects of the TM atoms, all electron relativistic treatment is selected [35]. Spin polarized is selected as a result of certain TM atoms exhibit high spins [34]. Double numerical plus polarization (DNP) is used [32, 36]. Furthermore, the Mülliken population analysis is executed to obtain the net charges and spin properties of the TMC6N7 clusters [34].
The average binding energy (Eb) of the TMC6N7 clusters was calculated to determine the structural stability [34]:
where E(TM), E(C), and E(N) present the total energies of isolated TM, C, and N atoms, respectively. E(TMC6N7) is the total energy of the TMC6N7 clusters.
In order to determine the accuracy of the PBE functional selected, the calculated distance (6.840 Å) between two nitride pores is compared with the corresponding experimental value (6.81 Å) [37]. And the calculated lattice constant (7.138 Å) of gh-C3N4 agree well with the other computational results (7.13 Å [38] and 7.14 Å [39]) and the experimental value (7.13 Å [40]). As for the TM atom doping, it has been confirmed in our previous work [35]. Consequently, the PBE functional is selected to investigate the TMC6N7 clusters.
Results and discussion
Configurations
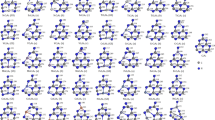
The optimized configurations of the TMC6N7 clusters have been displayed in Fig. 1. The gray balls present C atoms, the blue balls present N atoms, and other balls present TM atoms, respectively. Owing to the radius of TM atoms is larger generally than the substituted N atoms. It leads to the symmetry of the pristine C6N8 clusters is degraded to C1. The unplanar structures of the TMC6N7 clusters in Fig. 1 are labeled a star (*). As for these unplanar TMC6N7 clusters, the pseudo-Jahn–Teller distortion due to the electronic coupling of TM atoms leads to the planar C6N8 clusters is transformed into the characteristic buckling [41]. And TM atoms will advance the transformation from the quasi-planar configurations to three-dimensional structures [42]. As for the planar TMC6N7 clusters which have not yield any significant distortion, it suggests that the interaction between them is weaker [43]. 3d atoms are inclined to replace the N atom which approaches the N atom out of the C–N rings except for Cu and Zn. As for the CuC6N7 and ZnC6N7 clusters, the N atom out of the C–N rings is inclined to be substituted. It derives from the polarization of Cu+ ion and Zn2+ ion is stronger than that of other 3d TM atoms according to electron configurations, which leads to the prominent polarization between them and the C6N7 fragments, and the corresponding chemical bonds transition from ionic bonds to covalent bonds. The isomers (d) which the N atoms in the centre of C–N rings substituted except for ZnC6N7(d) are the most unstable structures. It originates from a larger effective decay radius of Zn which indicates a more intense localization of impurity states [43]. Wang et al. [2] have observed the pore size of the Zn-doped C3N4 sheet expands by the BET analysis. The ZnC6N7(b) clusters become a typical. Even it is more exaggerating than the ZnC5N8(c) clusters [24]. And Yue et al. [23] have confirmed that Zn doping partly damages the structure of g-C3N4. Nevertheless, cross-link effect of Zn between the interlayers of g-C3N4 sheet is helpful to obstruct the exfoliation of g-C3N4 [2]. However, Zn doping will create active sites on the surface of g-C3N4 for reducing protons into H2 [44].
Stabilities
The average binding energies Eb of the TMC6N7 clusters have been displayed in Fig. 2. The first row TM symbols are listed to expound the abscissa of Figs. 2, 3, 4, 5. The negative value of the binding energy presents a stable doping system [3]. ScC6N7(GS), TiC6N7(GS), VC6N7(GS), CoC6N7(GS), TiC6N7(a), and CoC6N7(a) actually improve the structural stabilities of the C6N8 clusters. As for other 3d TM atoms, they prefer to segregate and then aggregate the TM clusters [26]. In our previous work, only ScC5N8(GS), TiC5N8(GS), and VC5N8(GS) can increase the structural stabilities of the pristine C6N8 clusters [24]. Xiong et al. [45] have confirmed Ti doping can improve the thermodynamic stabilities of the CNx coatings.
The energy gaps between the highest occupied molecular orbital (HOMO) states and the lowest unoccupied molecular orbital (LUMO) states of the TMC6N7 clusters have been plotted in Fig. 3. Although the calculated HOMO–LUMO gap (0.694 eV) of the pristine C6N8 clusters is significantly narrower than the experimental value (2.7 eV) of C3N4 [4], it seems to be confirmed by that (1.24 eV) of monolayer CN [38] and that (1.22 eV) of two-dimensional C6N8 [28], and that (1.040 eV) of the sole g-C3N4 [29]. The HOMO–LUMO gaps of the ground-state TMC6N7 clusters as an example, the TM substituting can improve the dynamic stabilities of the pristine C6N8 clusters except for Zn. It is significantly different from those of the TMC5N8 clusters [24]. It originates from the hybridization between d orbitals of the TM atoms and p orbitals of the neighbor C atoms in the TMC6N7 clusters which is less than that between d-p orbitals of TM and N atoms of the TMC5N8 clusters [24, 26]. The metallic properties of Zn at the top site of the ZnC6N7 clusters are better kept because of Zn atoms exhibit the all paired 3d10 orbital systems [26]. The metal-like electronic density of states of Zn will increase the non-adiabatic instability of the pristine C6N8 clusters [46].
To expound the influence of TM atom substituted the pristine C6N8 clusters, the HOMO and LUMO states of the ground-state TMC6N7 clusters have been displayed in Table 1. The blue regions prefer to trap electrons, and the yellow regions prefer to release electrons [47]. The total charge density displays a high charge density around the TM atoms [47, 48], projecting to the C-TM bonds, indicating charge transfer from TM to C atoms. The degree of charge redistribution is mainly proportional to the interaction strength [48]. As for the ground-state TMC6N7 clusters except for the ZnC6N7 clusters, the HOMO and LUMO states display the hybridization between the 3d orbital electrons of the TM atoms and the 2p orbital electrons of the C atoms. While for the ground-state ZnC6N7 clusters, the hybridization effect is limited because of the Zn atoms exhibit the all paired 3d10 orbital systems [26].
Electronic properties
The net charges of the TM atoms of the TMC6N7 clusters have been plotted in Fig. 4. The positive charges on the TM atoms decrease generally as atomic number of the TM atoms increasing which is similar to that on TM embedded the sole g-C3N4 [29]. As for the ground-state TMC6N7 clusters, the TM atoms loss a few electrons within the scope of 0.113 |e| and 1.039 |e|. As for the transferred charge amounts of TM atoms of the TMC6N7 clusters are generally less than those (from 0.375|e| to 1.150|e|) of TM atoms of the TMC5N8 clusters [24]. It derives from the TM atoms and the neighbor C atoms of the TMC6N7 clusters prefer to the electropositive which is different from that of the TM atoms and the neighbor negative N atoms of the TMC5N8 clusters [48]. Z. Zhu et al. [38] have confirmed that the charge transfer of Co and g-C3N4 by Raman enhancement factors. The electron transfer follows the formation of chemical bonds [49]. The covalent bonding character of TM-C of TM-adsorbed BC6N have been confirmed [43]. The differences in the net charges of TM atoms of the TMC6N7 isomers are shown in Fig. 4 and the Mülliken charges of the C and N atoms of the TMC6N7 clusters are given in Table 2. (The Mülliken charges in Table 2 have been listed by the order from top to bottom and from left to right of atoms of the TMC6N7 isomers in Fig. 1.) It originates from the different atomic arrangements [50].
The natural electron configurations of the ground-state TMC6N7 clusters have been listed in Table 3. Compare the natural electron configurations of isolated TM atoms with those of the TM atoms of the TMC6N7 clusters in Table 3, the 4s orbital of the TM atoms of the TMC6N7 clusters lose more electrons than the 3d and 4p orbitals of the obtained. It confirms the hybridization mechanism of the sp orbital electrons of the TMC6N7 clusters.
Magnetic properties
The spins of TM atoms of the TMC6N7 clusters have been displayed in Fig. 5. The spins of the TM atoms of the TMC6N7 clusters are found to be lower than those of isolated TM atoms in Ref. [35], it originates from the certain d orbital electrons are transferred or magnetic couplings in the TMC6N7 clusters [26, 48]. As for the ground-state TMC6N7 clusters, the maximum spins of the TM atoms appear at Cr and Fe. It derives from the 3d orbitals of Cr which display strong spin exchange splitting εd [51]. The V, Cr, and Fe atoms doping the g-C3N4 display ferromagnetic states [26], while Mn exhibits an anti-ferromagnetic behavior [26, 52]. While for Cu and Zn atoms of the TMC6N7 clusters display the nonmagnetic states because of the all paired d orbital structures [26]. The Cu and Zn atoms of TM-g-C3N4 sheets display nonmagnetic which has been confirmed [26]. As for the discrete spins of the TMC6N7 isomers, it derives from the spatial arrangements of interacting magnetic orbitals [26]. The neighboring Cedge atoms exhibit oppositely spin polarized with respect to the TM atoms [8].
Conclusions
In summary, the configurations, stabilities, electronic, and magnetic attributes of the first row transition metal (TM)-substituted TMC6N7 clusters have been investigated at the PBE level. The results display that the 3d TM atoms are inclined to replace the N atom which approaches the N atom out of the C-N rings except for Cu and Zn. As for the CuC6N7 and ZnC6N7 clusters, the N atom out of the C-N rings prefers to be substituted. The ScC6N7(GS), TiC6N7(GS), VC6N7(GS), CoC6N7(GS), TiC6N7(a), and CoC6N7(a) clusters display more structurally stabilities than the C6N8 clusters. The ground-state 3d TMC6N7 clusters exhibit more dynamic stabilities than the C6N8 clusters except for Zn. A small amount of 4s orbital electrons of the TM atoms is transferred to the neighbor C atoms by the net charge distribution of the TMC6N7 clusters. The V, Cr, Mn, and Fe atoms of the ground-state TMC6N7 clusters display the opposite spin to Co and Ni atoms of the ground-state TMC6N7 clusters.
References
Q. Liu, J.Y. Zhang, Langmuir 29, 3821 (2013)
Z.T. Wang, J.L. Xu, H. Zhou, X. Zhang, Rare Met. 38, 459 (2019)
R. Zhang, S. Niu, X. Zhang, Z. Jiang, J. Zheng, C. Guo, Appl. Surf. Sci. 489, 427 (2019)
Y. Zhang, Q. Zhang, Q. Shi, Z. Cai, Z. Yang, Sep. Purif. Technol. 142, 251 (2015)
H.A. Bicalho, J.L. Lopez, I. Binatti, P.F.R. Batista, J.D. Ardisson, R.R. Resende, E. Lorencon, Mol. Catal. 435, 156 (2017)
C. Sun, H. Zhang, H. Liu, X. Zheng, W. Zou, L. Dong, L. Qi, Appl. Catal. B Environ. 235, 66 (2018)
J. Gao, Y. Wang, S. Zhou, W. Lin, Y. Kong, ChemCatChem. 9, 1708 (2017)
D. Ghosh, G. Periyasamy, B. Pandey, S.K. Pati, J. Mater. Chem. 2, 7943 (2014)
Y. Wang, Y. Wang, Y. Chen, C. Yin, Y. Zuo, L.-F. Cui, Mater. Lett. 139, 70 (2015)
G.D. Ding, W.T. Wang, T. Jiang, B.X. Han, H.L. Fan, G.Y. Yang, Chemcatchem. 5, 192 (2013)
Z. Ding, X. Chen, M. Antonietti, X. Wang, Chemsuschem 4, 274 (2011)
Z. Li, C. Kong, G. Lu, J. Phys. Chem. C. 120, 56 (2016)
J. Ma, Q. Yang, Y. Wen, W. Liu, Appl. Catal. B: Environ. 201, 232 (2017)
X. Chen, J. Zhang, X. Fu, M. Antonietti, X. Wang, J. Am. Chem. Soc. 131, 11658 (2009)
S. Tonda, S. Kumar, S. Kandula, V. Shanker, J. Mater. Chem. A. 2, 6772 (2014)
W. Oh, V.W.C. Chang, Z. Hu, R. Goei, T. Lim, Chem. Eng. J. 323, 260 (2017)
X.C. Wang, X.F. Chen, A. Thomas, X.Z. Fu, M. Antonietti, Adv. Mater. 21, 1609 (2009)
Q. Liao, D. Zou, W. Pan, W. Linghu, R. Shen, Y. Jin, G. Feng, X. Li, F. Ye, A.M. Asiri, H.M. Marwani, Y. Zhu, X. Wu, W. Dong, J. Mol. Liq. 258, 275 (2018)
L. Deng, M. Zhu, RSC Adv. 6, 25670 (2016)
L. Kong, Y. Dong, P. Jiang, G. Wang, H. Zhang, N. Zhao, J. Mater. Chem. A. 4, 9998 (2016)
B. Tahir, M. Tahir, N.A.S. Amin, Appl. Surf. Sci. 419, 875 (2017)
M. Ji, J. Huang, K. Zhang, D. He, S. Chang, D. Luo, E. Zhang, M. Xu, J. Liu, J. Zhang, J. Xu, J. Wang, C. Zhu, Inorg. Chem. Front. 5, 2420 (2018)
B. Yue, Q. Li, H. Iwai, T. Kako, J. Ye, Sci. Technol. Adv. Mater. 12, 034401 (2011)
Z. Li, Z. Zhao, Z. Liu, H. Wang, Q. Wang, Res. Chem. Intermediat. 46, 2099 (2020)
S. Sarkar, S.S. Sumukh, K. Roy, N. Kamboj, T. Purkait, M. Das, R. Sundar Dey, J. Colloid Interf. Sci. 558, 182 (2019)
E. Kroke, Angew. Chem. Int. Edit. 53, 11134 (2014)
A. Bafekry, C. Stampfl, S. Farjami Shayesteh, ChemPhysChem. 21, 164 (2020)
A. Bafekry, S. Farjami Shayesteh, F.M. Peeters, J. Appl. Phys. 126, 215104 (2019)
T. Wang, G. Yu, J. Liu, X. Huang, W. Chen, Phys. Chem. Chem. Phys. 21, 1773 (2019)
Y. Yang, C. Yin, K. Li, H. Tang, Y. Wang, Z. Wu, J. Electrochem. Soc. 166, F755 (2019)
S.A. Khandy, D.C. Gupta, RSC Adv. 6, 48009 (2016)
Y. Guo, C. Tang, X. Wang, C. Wang, L. Fu, Chin. Phys. B. 28, 048102 (2019)
Z. Zhao, Z. Li, Q. Wang, T. Shi, Mater. Chem. Phys. 240, 122220 (2020)
Z. Zhao, Z. Li, Mod. Phys. Lett. B. 33, 1950459 (2019)
Z. Zhao, Z. Li, Q. Wang, Chem. Phys. Lett. 739, 136922 (2020)
B. Delley, J. Chem. Phys. 113, 7756 (2000)
X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J.M. Carlsson, K. Domen, M. Antonietti, Nat. Mater. 8, 76 (2009)
Z. Zhu, X. Tang, T. Wang, W. Fan, Z. Liu, C. Li, P. Huo, Y. Yan, Appl. Catal. B Environ. 241, 319 (2018)
J. Cui, S. Liang, X. Wang, J. Zhang, J. Mater. Chem. Phys. 161, 194 (2015)
P. Niu, L. Zhang, G. Liu, Adv. Funct. Mater. 22, 4763 (2012)
D. Jose, A. Datta, J. Chem. Phys. C. 116, 24639 (2012)
T. Teshome, A. Datta, Acs Appl. Mater. Inter. 9, 34213 (2017)
A. Bafekry, Physica E. 118, 113850 (2020)
H. Sudrajat, S. Hartuti, Optik. 181, 1057 (2019)
Z.-W. Xiong, L.-H. Cao, J. Alloy. Compd. 775, 100 (2019)
S.M. Pratik, C. Chowdhury, R. Bhattacharjee, S. Jahiruddin, A. Datta, Chem. Phys. 460, 101 (2015)
A. Bafekry, C. Stampfl, M. Ghergherehchi, S.F. Shayesteh, Carbon 157, 371 (2020)
A. Bafekry, S. Farjami Shayesteh, M. Ghergherehchi, F.M. Peeters, J. Appl. Phys. 126, 144304 (2019)
A. Bafekry, C. Stampfl, S. Farjami Shayesteh, F.M. Peeters, Adv. Electron. Mater. 5, 1900459 (2019)
G. Ge, Q. Jing, Z. Yang, Y. Luo, Chin. Phys. Lett. 26, 083101 (2009)
L.J. Shi, Phys. Lett. A. 374, 1292 (2010)
W. Zhang, H.Y. Cho, Z. Zhang, W. Yang, J. Korean Phys. Soc. 69, 1445 (2016)
Acknowledgements
We gratefully acknowledge the financial support from the Key Fund Project of the National Science Foundation, People’s Republic of China (Grant No. 51634004), Key Laboratory of Chemical Metallurgy Engineering Liaoning Province, University of Science and Technology LiaoNing (Grant No.USTLKFSY201711) and the Fund Project of University of Science and Technology Liaoning (Grant No.2017YY02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Zhao, Z. & Shao, Tt. First-principles calculations on the first row transition metals-substituted TMC6N7 clusters. Res Chem Intermed 46, 3097–3107 (2020). https://doi.org/10.1007/s11164-020-04137-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04137-4