Abstract
Transition metals can enhance the electronic attributes of tungsten oxides. In this study, we focused on W2On (n = 1–6) clusters as a representative examples of tungsten oxide clusters with varying oxygen concentrations. The structures and electronic properties of the TMWOn (TM = Mn–Ni) clusters have been calculated using first-principles. The ground-state TMWOn clusters share some structural similarities with the ground-state W2On (n = 1–6) clusters. The W–O bonds of the TMWO2 (TM = Fe–Ni) clusters are significantly distorted into a triangular structure. The NiWOn (n = 1–2) and CoWOn (n = 3–5) clusters display greater thermodynamic stability than other TMWOn clusters. Among the TMWOn clusters, the W2O4, W2O6, MnWO, MnWO3, MnWO6, FeWO, FeWO4, FeWO6, CoWO, CoWO6, NiWO2, NiWO5 clusters are more kinetically stable. Furthermore, the amount of charge transfer between the TM atoms and W2On clusters increases from 0.050 |e| to 1.066 |e| as the number of oxygen atoms increases. The 4s orbital electrons of the TM atoms for the TMWOn clusters are partially transferred to the neighboring O atoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tungsten oxides (WO3) are important materials with a variety of industrial applications, including electrochromic devices, chemical sensors, dye-sensitized solar cells, and catalysts etc. [1, 2]. Due to the similarity of the chemical bond formed on the WO3 surface to a cluster-like bond, investigating WO3 clusters can provide insight into the properties of bulk surfaces [3]. In fact, the WO3 clusters have been observed experimentally on the surfaces of WO3 films [4, 5]. Theoretical investigations on the WO3 clusters have also been extensively conducted. For example, Li et al. [6] have investigated the ground-state structures of the (WO3)n (n = 1–6) clusters using the B3LYP gradient-corrected exchange–correlation functional. Sai et al. [1] have investigated the ground-state structures of the (WO3)n (2 ≤ n ≤ 12) clusters using first-principles. These studies have revealed that the WO3 clusters exhibit unique electronic, magnetic, and chemical properties compared to bulk materials [7]. It is important to note that the WO3 clusters contain both terminal and bridging O atoms, except for the smallest WO3 molecule [3]. On the other hand, to highlight the influence of oxygen concentrations on the electronic properties of tungsten oxide clusters, some studies have been performed. Such as, Zhai et al. [8] have investigated the electronic structures and chemical bonding of WOn and WOn− species (n = 3–5) using anion photoelectron spectroscopy (PES) and density functional theory (DFT) calculations. Huang et al. [9] have investigated the electronic structure and chemical bonding of the W3On− and W3On (n = 7–10) clusters. Zhai et al. [10] have investigated the electronic structures and chemical bonding of the W2On− and W2On (n = 1–6) using PES and DFT calculations. However, the larger band gap of the WO3 clusters restricts their potential applications [10, 11]. To address this issue, various methods have been considered to reduce the energy gap, including transition metal doping [12]. For example, Zhao et al. [13] have investigated the structures, electronic and magnetic properties of the TM@W6O18 clusters using DFT. Hameed et al [14] have investigated the influence of the TM (TM = Fe–Zn) concentrations on the catalytic properties of WO3 nanoparticles using ultraviolet laser irradiation. Mansouri et al. [15] have investigated the effect of TM substitution and vacancies the in WO3 crystal using Ab Initio method. However, no reports on the electronic properties of the TM substituted small tungsten oxide clusters with different oxygen concentrations.
In this study, to highlight the influence of oxygen concentrations on the electronic properties of magnetic TM-substituted tungsten oxide clusters, the structures, electronic properties and dipole magnitudes of the TMWOn (TM = Mn–Ni, n = 1–6) clusters have been investigated using DFT.
2 Computational details
The ground-state structures of the W2On (n = 1–6) clusters were obtained from Ref. [10]. Then a W atom of the W2On clusters was substituted by a TM (TM = Mn–Ni) atom to construct the hypothetical TMWOn clusters. The geometry optimization and property calculations were executed using the DMol3 software [16, 17]. The generalized gradient approximation (GGA) and Perdew-Burke-Ernzerhof (PBE) were adopted to consider exchange-correction interaction [1, 18]. The structures of the TMWOn clusters were optimized without any symmetry constraints [1, 7]. Spin unrestricted was chosen [1, 13, 19]. All electron relativistic calculations were adopted to account for electron–ion interactions, as W is a heavy element [1, 19]. Double numerical plus polarization (DNP) was considered for each atom [19]. Mülliken population analysis was performed to obtain the net charge of each atom [20]. Harmonic vibrational analysis of the frequencies of the TMWOn clusters was executed to ensure the presence of saddle points on the potential energy surfaces [1, 21, 22]. It has been confirmed that there are no imaginary frequencies of the TMWOn clusters. The convergence thresholds for self-consistent field calculations were set: 1 × 10−5 Hartree/Bohr for the energy gradient, 2 × 10−3 Hartree/Å for the maximum force and 5 × 10−3 Å for the atomic displacement, respectively. For total energy convergence, the threshold was set at 1 × 10−5 Hartree, and for charge density, it was set at 1 × 10−6 e/Å3. The width of smearing was selected as 0.005 eV and the project out zero frequency modes option was selected.
The binding energies per atom of the W2On (n = 1–6) and TMWOn (TM = Mn–Ni, n = 1–6) clusters were calculated to investigate their thermodynamic stability [13]
where E(W), E(O) and E(TM) represent the atomic energies of single W, O and TM, respectively. E(W2On) and E(TMWOn) represent the total energies of W2On and TMWOn clusters, respectively.
The temperature dependence of the free energy of the ground-state W2On (n = 1–6) and TMWOn (TM = Mn–Ni, n = 1–6) clusters [23]
where the second item is the zero point vibrational energy, k is Boltzmann constant, h is Planck constant and F(ω) is the phonon density of states. Due to the change of PΔV is rather small under constant pressure, F is approximately equal to the Gibbs free energy G.
To ensure the accuracy of the selected PBE functional, the calculated bond length (1.757 Å) and the binding energy (1.082 eV) of WO3 clusters were compared to the corresponding calculated values (1.752 Å and 1.10 eV) [7]. Similarity, the calculated bond length (1.619 Å) and binding energy (4.180 eV) of FeO molecules were compared to experimental results (1.62 Å and 4.17 eV) [24]. These comparisons demonstrate the suitability of the PBE functional for calculating TMWOn clusters.
3 Results and discussion
3.1 Structures
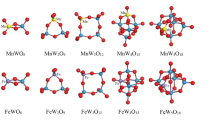
The binding energies per atom Eb have been used to determine the lowest-energy configurations of the TMWOn (TM = Mn–Ni, n = 1–6) clusters. Only the ground-state configurations of the calculated TMWOn (TM = Mn–Ni, n = 1–6) clusters have been shown in Fig. 1. It is worth noting that the lowest-energy structures of the TMWOn clusters are largely inherited from those of the W2On (n = 1–6) clusters. However, the point groups of the TMWOn clusters exhibit a degeneracy, resulting in an asymmetric structure C1. Compare these TMWOn clusters, it can be found that the sizes of TM atoms have less effect on the tungsten oxide structures [25]. For instance, in the case of the TMWO2 (TM = Fe–Ni) clusters, the W–O bonds are significantly distorted into a triangular structure due to the influence of TM-d electrons, leading to the Jahn–Teller distortion [8, 21]. Zhao et al. [13] have also found that the structural distortions of the Co@W6O18 clusters using DFT.
3.2 Stabilities
The calculated binding energies per atom Eb of W2On and TMWOn (TM = Mn–Ni, n = 1–6) clusters have been exhibited in Fig. 2. The more the negative binding energies, the more the stability will be [26]. The binding energies per atom of the W2On clusters decrease with the increase of O concentrates. Wang et al. [27] have revealed that more O atoms prefer to firm W atoms. When comparing the binding energy per atom of the W2On clusters to the TMWn-1O3n clusters, it is evident that the W2On clusters have lower binding energies per atom. This indicates that the W2On clusters are less thermodynamically stable. When comparing the different TMWOn clusters, it can be observed that the NiWOn (n = 1–2) clusters display greater thermodynamic stability than the other TMWOn (TM = Mn, Fe and Co, n = 1–2) clusters. This is likely due to the ability of these clusters to maximize the coordination number of the surface atom [28], as well as the strong binding of oxygen-2p electrons [8, 9]. Similarly, the CoWOn (n = 3–5) clusters exhibit more thermodynamic stability than the other TMWOn (TM = Mn, Fe and Ni, n = 3–5) clusters. On the other hand, the FeWO6 clusters show more thermodynamic stability than the other TMWO6 (TM = Mn, Co and Ni) clusters. This is mainly due to differences in the electron affinity of the TM atoms [29, 30].
The structure distortions should lead to the electron re-distributions [31]. The calculated gaps between the highest molecular occupied orbital (HOMO) and the lowest molecular unoccupied orbital (LUMO) states of the W2On and TMWOn (TM = Mn–Ni, n = 1–6) clusters have been plotted in Fig. 3. The calculated values of the HOMO–LUMO gaps are determined by the basis set [2], but it is still possible to investigate the relative electronic stability of the W2On and TMWOn clusters. According to previous research, the clusters with larger HOMO–LUMO gaps tend to be more stable and chemically inert [13]. Among the clusters studied, the W2O4, W2O6, MnWO, MnWO3, MnWO6, FeWO, FeWO4, FeWO6, CoWO, CoWO6, NiWO2, NiWO5 clusters display higher kinetic stability compared to their neighboring TMWOn clusters. On the other hand, the W2O, W2O5, MnWO2, MnWO4, FeWO2, FeWO5, CoWO5, NiWO, NiWO4, NiWO6 clusters exhibit higher kinetic activity. This can be attributed to the distortion of the HOMO mainly on the O sites and the LUMO mainly on the W atoms. These results are a result of the distortion of the atoms and charge redistribution near the atoms [18].
Clusters with both good structural and thermodynamic stability tend to be synthesized experimentally. The NiWOn (n = 1–2) and CoWOn (n = 3–5) clusters display greater thermodynamic stability than other TMWOn clusters. The MnWO, MnWO3, MnWO6, FeWO, FeWO4, FeWO6, CoWO, CoWO6, NiWO2, NiWO5 clusters display higher kinetic stability compared to their neighboring TMWOn clusters. It indicates that the NiWO2 and CoWO6 clusters prefer to synthesize.
The HOMO and LUMO orbitals of the W2On and TMWOn (TM = Mn–Ni, n = 1–6) clusters have been depicted in Figs. 4 and 5. In the HOMO states of the TMWOn clusters, there are more electrons surrounding the TM atoms, except for the NiWO, FeWO2, CoWO2 clusters. Similarly, in the LUMO states of the TMWn−1O3n clusters, there are more electrons surrounding the TM atoms, except for the NiWO, FeWO2, CoWO2, NiWO2, CoWO3, NiWO3, MnWO4, CoWO4 clusters [21, 32]. The HOMO and LUMO states of the TMWOn clusters, σ-type bonds and π-type bonds are coexist [32]. This can be attributed to the contributions of TM-d and O-p orbital electrons [1, 25, 33, 34].
The thermodynamical stability of the W2On and TMWOn (TM = Mn, Fe, Co and Ni, n = 1–6) clusters can be analyzed by Gibbs free energy (G). The Gibbs free energies of the ground-state W2On and TMWOn clusters have been plotted in Fig. 6. The Gibbs free energies of the W2On and TMWOn clusters gradually decreases with the increase of the cluster size. It demonstrates that the W2On and TMWOn clusters prefer to spontaneous grow with the increase of temperature. It demonstrates that the thermal stability of the W2On and TMWOn clusters gradually increase with the increase of the cluster size. It results from the shell closing effect which obvious affects the activity of clusters [25].
3.3 Electronic attributes
The calculated Mülliken-charges of TM atoms of TMWOn (TM = Mn–Ni, n = 1–6) clusters have been plotted in Fig. 7. The amount of charge transferred between the TM atoms and WOn clusters increases significantly as the number of oxygen atoms increases. This suggests that the W atoms become more positively charged with an increase in the number of O atoms. This indicates a conversion of the TM-O bond from a covalent bond to a partially ionic bond [1], which can help stabilize the remaining d electrons and increase their binding energies [10]. The amount of charge transferred of the MnWOn clusters is greater than in other TMWOn (TM = Fe, Co and Ni) clusters. While the amount in the CoWOn clusters is less than in other TMWOn clusters, except for the NiWO2 clusters. The difference can be attributed to discrepancies in the electron affinity of the TM atoms [29, 30].
The natural electron configurations of TM atoms of TMWOn (TM = Mn–Ni, n = 1–6) clusters have been displayed in Table 1. Upon comparison of the valence electrons (3d54s2, 3d64s2, 3d74s2 and 3d84s2) of a single Mn, Fe, Co and Ni atom with those of TMWOn clusters, it can be observed that there is internal charge transfer of TM atoms of the TMWOn clusters from the 4s orbital to the 3d and 4p orbitals. Thus is evident in the Mülliken charges of the TM atoms of TMWOn clusters (See Fig. 6). This indicates that the 4s orbital electrons of the TM atoms for the TMWOn clusters are also partially transferred to the neighboring O atoms [13]. Similarly, there is internal charge transfer in the O atoms of the TMWOn clusters, with electrons from the 2s orbital being transferred to the 2p orbital [13]. This suggests that the 2p and 3d orbital electrons of the O atoms are obtained from the TM atoms.
3.4 Dipole magnitudes
Considering the high dipole moment leads to higher reactivity but less stability. The dipole magnitudes of the TMWOn (TM = Mn–Ni, n = 1–6) clusters have been displayed in Fig. 8. The nonzero dipole moments of the TMWOn clusters emerge. This is due to the symmetry of the W2On clusters being degenerated by the TM substitution [35]. This is caused by insufficient hybridization between the TM-d electrons and O-p electrons of the TMWOn clusters. It causes the TMWOn (TM = Mn–Ni, n = 1–6) clusters display less structural stability. In general, the dipole moments of the TMWOn clusters decrease with the increase of the cluster sizes. It is due to the compensation effect of free electrons of O atoms on the dipole moments of TMWOn clusters. Similarly, the structural stability of the TMWOn clusters increases with the increase of the cluster sizes. The dipole magnitudes of the MnWO3, FeWO2, CoWO2, NiWO2 clusters are larger than those of neighboring TMWOn clusters.
4 Conclusions
The structures and electronic properties of the TMWOn (TM = Mn–Ni) clusters have been calculated using first-principles. The ground-state TMWOn clusters share some structural similarities with the ground-state W2On (n = 1–6) clusters. In the case of the TMWO2 (TM = Fe–Ni) clusters, the W–O bonds are significantly distorted into a triangular structure. However, the TMWOn clusters are less thermodynamically stable compared to their corresponding W2On clusters. The NiWOn (n = 1–2) and CoWOn (n = 3–5) clusters display greater thermodynamic stability than the other TMWOn clusters. Among the TMWOn clusters, the W2O4, W2O6, MnWO, MnWO3, MnWO6, FeWO, FeWO4, FeWO6, CoWO, CoWO6, NiWO2, NiWO5 clusters are more kinetically stable. As the number of O atoms increases, there is a significant increase in the amount of charge transferred from 0.050 |e| to 1.066 |e| between the TM atoms and W2On clusters. This charge transfer is highest in the MnWOn clusters compared to other TMWOn (TM = Fe, Co and Ni) clusters. The 4s orbital electrons of the TM atoms for the TMWOn clusters are also partially transferred to the neighboring O atoms. Additionally, the dipole magnitudes of the MnWO3, FeWO2, CoWO2, NiWO2 clusters are larger than those of neighboring TMWOn clusters.
References
Sai L, Tang L, Huang X, Chen G, Zhao J, Wang J (2011) Chem Phys Lett 544:7
Valentin CD, Wang F, Pacchioni G (2013) Top Catal 56:1404
Jin H, Zhu J, Hu J, Li Y, Zhang Y, Huang X, Ding K, Chen W (2011) Theor Chem Acc 130:103
Kim YK, Dohnalek Z, Kay BD, Rousseau R (2009) J Phys Chem C 113:9721
Santo N, Filipescu M, Ossi PM, Dinescu M (2010) Appl Phys A 101:325
Li S, Dixon DA (2006) J Phys Chem A 110:6231
Sun Q, Rao BK, Jena P, Stolcic D, Kim YD, Gantefor G, Castleman AWJ (2004) J Chem Phys 121:9417
Zhai H-J, Kiran B, Cui L-F, Li X, Dixon DA, Wang L-S (2004) J Am Chem Soc 126:16134
Huang X, Zhai H-J, Li J, Wang L-S (2006) J Phys Chem A 110:85
Zhai H-J, Huang X, Cui L-F, Li X, Li J, Wang L-S (2005) J Phys Chem A 109:6019
Li D, Huang W-Q, Xie Z, Xu L, Yang Y-C, Hu W, Huang G-F (2016) Mod Phys Lett B 30:1650340
Xu L, Yin M-L, Liu S (2014) Sci Rep-UK 4:6745
Zhao Z, Wu Z, Li Z (2023) Struct Chem 34:1395
Hameed A, Gondal MA, Yamani ZH (2004) Catal Commun 5:715
Mansouri M, Mahmoodi T (2016) Acta Phys Pol A 129:8
Delley B (1990) J Chem Phys 92:508
Delley B (2000) J Chem Phys 113:7756
Li W, Da P, Zhang Y, Wang Y, Lin X, Gong X, Zheng G (2014) ACS Nano 8:11770
Zhao Z, Li Z, Xue G, Shen X, Wu J (2021) Mater Chem Phys 262:124272
Mulliken RS (1955) J Chem Phys 23:1841
Cora F, Patel A, Harrison NM, Dovesi R, Catlow CRA (1996) J Am Chem Soc 118:12174
Li Z, Shen X, Zhao Z (2022) Res Chem Intermediat 48:339
Baroni S, de Gironcoli S, Corso AD, Giannozzi P (2001) Rev Mod Phys 73:515
Fan J, Wang L-S (1995) J Chem Phys 102:8714
Geusic EM, Morse MD, Smalley RE (1985) J Chem Phys 82:590
Li Z, Zhou Z, Zhao Z, Wang Q (2018) Int J Mod Phys B 32:1850187
Wang S, Zhan J, Chen K, Ali A, Zeng L, Zhao H, Hu W, Zhu L, Xu X (2020) ACS Sustain Chem Eng 8:8214
Zhang J-M, Duan Y-N, Xu K-W, Ji V, Ma Z-Y (2008) Phys B 403:3119
Zhao L, Qu X, Wang Y, Lv J, Zhang L, Hu Z, Gu G, Ma Y (2017) J Phys: Condens Matter 29:265401
Zheng H, Ou JZ, Strano MS, Kaner RB, Mitchell A, Kalantar-zadeh K (2011) Adv Funct Mater 21:2175
Pan H, Wu Y, Li C, Li H, Gong Y, Niu L, Liu X, Sun CQ, Xu S (2022) Appl Surf Sci 571:151230
Ingham B, Hendy SC, Chong SV, Tallon JL (2005) Phys Rev B 72:075109
Zhao YR, Xu YQ, Chen P, Yuan YQ, Qian Y, Li Q (2021) Results Phys 26:104341
Zhang XY, Zhao YR, Li HX, Cheng KG, Liu ZR, Liu ZP, He H (2023) Chinese Phys B 32:066102
Li Z, Wu Z-H (2024). Surf Rev Lett. https://doi.org/10.1142/S0218625X24500392
Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 51634004).
Author information
Authors and Affiliations
Contributions
ZL contributed to data curation, formal analysis, investigation, methodology, writing-original draft, writing-review and editing. ZHW contributed to investigation, writing-review and editing. ZZ contributed to funding acquisition, writing-review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z., Wu, Zh. & Zhao, Z. First-principles calculations on the structures and electronic properties of the TMW2On (TM = Mn–Ni, n = 1–6) clusters. Theor Chem Acc 143, 36 (2024). https://doi.org/10.1007/s00214-024-03113-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-024-03113-0