Abstract
Verticillium dahliae is one of the most important soil pathogens, causing verticillium wilt. It is well known that the use of chemical products against this pathogen is not without side effects on the environment. In this regard, the present study was aimed to search for antagonistic rhizobacteria as an alternative of biological control against this causal agent. A total of 162 isolates were screened for their antagonistic activity according to the “double layer” and the “well diffusion” methods. Three of them (RS11, SF82 and ZO4), were subsequently selected as biological control agent (BCAs) according to their efficiency and were identified by 16S rRNA sequencing and Biolog microplate GEN III as Bacillus spp. Using 10 different lipopeptide gene primers, PCR reactions only revealed the involvement of genes responsible for iturins (ituA, ituD, ituC), bacillomycin (bmyA) and Bacilysin (bacA / B-F, bacA / B-R) biosynthesis. The Plant Growth Promoting Rhizobacteria traits [enzymatic activities, phytohormones production] of the three BCAs were also studied in vitro then on pepper plant (Capsicum annuum L.), indicating that Bacillus subtilis ZO4 was the most effective, enhancing leaf, stem and root growth comparing to the control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Verticillium dahliae is a soil borne plant pathogen that causes vascular wilt in over 160 agronomically important plant species worldwide. Verticillium wilt causes severe economic losses in many crops, including vegetables, fruits, flowers, oilseed crops, fiber crops and woody perennials (Usami et al. 2002; Wei et al. 2015; Markakis et al. 2016). The extraordinary impact of this fungus is largely due to the production of survival structures called microsclerotia, which can persist under field conditions for long periods even in the absence of a host. When the microsclerotia are close to the host plant root and the environmental conditions are favorable, they can easily germinate causing infection (Novo et al. 2017). In Mediterranean countries like Spain (Goicoechea et al. 2001) and Marocco (Douira et al. 2008) wilt is considered one of the most common diseases affecting pepper (Capsicum annuum L.).
Control management strategies of Verticillium dahliae have been mainly relied on chemical use, such as the antifungal antibiotic aureofungin, the fungicide benomyl, and the plant defense activator acibenzolar-S-methyl (Goicoechea 2009). However, studies have reported ineffectiveness as well as an increasing evidence of fungal resistance to these plant antibiotics (Calderon et al. 2015a, b). On the other hand, promising experimental data have shown that biological control, using selected biological control agents (BCA) could be an alternative approach (Angelopoulou et al. 2014) due to their ability to colonize the rhizosphere and to produce various inhibitory substances (Bhattacharyya and Jha 2012). For instance, rhizospheric bacteria, such as Pseudomonas fluorescens have been reported to be effective against the verticillium wilt (López-Escudero and Mercado 2011).
Bacillus species are well known for their ability to control plant diseases through various mechanisms, including the production of several lipopeptides such as bacillomycin, mycosubtilin, surfactin, iturin, and fengycin (Fernandes et al. 2007; Snook et al. 2009; Sriram et al. 2011). Among the species, Bacilus subtilis, Bacillus cereus and Bacillus amyloliquefaciens have been particularly reported to be effective for the control of plant diseases caused by soil borne, foliar, and postharvest fungal pathogens (Abeysinghe 2009) in laboratory, greenhouse, and field studies (Soares et al. 2016). Moreover, Bacillus spp. are also considered as plant growth promoting bacteria (PGPB), which colonize the root systems of plants and can stimulate plant growth through direct ways including biofertilization, phosphorus solubilization, production of plant hormones (Bent et al. 2001; Reyes et al. 2002), and excretion of diverse compounds like hydrolytic enzymes (Shakeel et al. 2015). Mechanisms of PGPR agents also include reducing the level of disease, induction of systemic resistance, and competition for nutrients and niches (Lugtenberg and Kamilova 2009), without conferring pathogenicity (Van Loon and Bakker 2005; Adesemoye and Kloepper 2009).
The objectives of the present study were first to isolate bacterial strains from a Tunisian Oasis soil and to characterize them based on their morphological, biochemical and molecular attributes; then to study their ability to suppress verticillium wilt disease in pepper plants and finally to assess their plant growth promoting effects.
Materials and methods
Bacterial isolation and culture conditions
For bacterial isolation, rhizosperic soil of the halophytic plant Zygophyllum album from an Oasis located in Zarzis, Tunisia was considered. 50 g of soil was sampled, transported and handled on the same day to the laboratory. Suspensions were made by adding 5 g of soil to 50 ml of distilled water and shaking at 200 rpm for 30 min at laboratory temperature. Ten-fold serial dilutions were prepared and one hundred microliters of the 10−3 dilution were plated on 25 ml of both Luria-Bertani (LB) medium (Sigma Aldrich). All plates were then incubated for 7 days at 28 °C (Park et al. 2005). A total of 162 isolates were then selected, purified and maintained for long-term storage at −80 °C using glycerol 30%.
Antifungal activity screening and BCAs selection
Bacterial isolates were screened for their ability to suppress the mycelial growth of six (6) fungal pathogens: Verticillium dahliae (V4) and Neofusicoccum mediterraneum (B0071), both kindly provided to us by the Plant Pathology Laboratory of the University of Rabanales, Cordoba, Spain; and Fusarium pseudograminearum (FO1), Phytophthora sp. and Botryosphaeria dothidea (IOT B002), which were provided by the Labaratory of Improvement and protection of olive genetic resources, Olive Tree Institute of Tunisia.
Dual culture assay
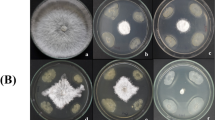
A loopful of each of the bacterial isolates was streaked on a fresh Potato Dextrose Agar (PDA) (Sigma Aldrich) plate at approximately 2 cm far from pathogen mycelium plugs. Petri dishes were incubated for 7 days at 24 ± 2 °C. Antagonistic activity was then evaluated by measuring the percentage of growth inhibition (Lahlali and Hijri 2010) according to the formula: \( GI\left(\%\right)=\frac{R1-R2}{R1}\ast 100 \), where R1 is the distance of fungal growth from the point of inoculation to the colony on control plates (bacteria free), and R2, the distance of fungal growth from the point of inoculation to the direction of the antagonist.
Antifungal activity of cell-free supernatant
The three most effective isolates were selected and antifungal activity of their cell-free supernatant (CFS) was evaluated by the well diffusion method. Five ml of soft agar (0.7%) containing a suspension (105 conidia ml−1) of each pathogen were poured into PDA plates. After cooling, wells of 6 mm diameter were cut. BCAs suspensions (108CFU ml−1) were centrifuged for 15 min, at 8000×g, then neutralized to pH 6.5 and filtered through a 0.22 μm filter. One hundred microlitres of each of the resulting CFS were then placed in the prepared wells. Petri dishes were incubated for 48 h at 24 ± 2 °C and the diameters of inhibition zone were measured according to Ouzari et al. (2008).
Phenotypic and biochemical characterization of BCAs
Phenotypic analyses of selected BCAs was performed using 96-well BIOLOG GENIII Microplates ™ using Omnilog Data collection software (Biolog, Inc., Hayward, CA) (Bochner et al. 2001; Bochner 2003). The test panel was comprised of sources of carbon, nitrogen, amino acids and organics acids; in addition to identifying other important physiological properties such as pH, NaCl and lactic acid tolerance, and susceptibility to antibiotics. Testing for Gram negative staining, oxidase and catalase activities were also performed.
Molecular identification of BCAs
Genomic DNA was extracted from selected BCAs according to Chen et al. (2016), and quantified by spectrophotometry (NanoDrop, Wilmington, USA). The Molecular identification was based on the 16S rDNA gene amplification using the universal primer pairs Fd1 and Rd1 (Issar et al. 2012).
The PCR mix consisted of a final volume of 25 μl containing 100 ng of genomic DNA, 1X My Taq Reaction buffer, 10 μM of each primer, 10 mM dNTP, and 1 U of My Taq DNA polymerase. Amplification was performed with initial denaturation at 94 °C for 3 min, 35 cycles at 94 °C for 1 min, 57 °C for 1 min, and 72 °C for 1 min 30 s, and a final elongation step of 5 min at 72 °C. PCR products were separated using electrophoresis in 1% agarose gel in 1 mol 1−1 TBE buffer and purified using an Ultra Clean PCR Clean-Up Kit (Mobio Laboratories Carlsbad, CA). The obtained amplicons were sequenced in both forward and reverse directions using an automated sequencer ABI PRISM 3130xl (Applied Biosystems, Foster City, CA) by the division of sequencing of the center of Biotechnology of Borj Cedria, Tunis, Tunisia (CBBC). The nucleotide sequences were edited using BioEdit Sequence Alignment and compared with sequences deposited in GenBank (http://www.ncbi.nlm.nih.gov/).
Screening for growth promoting traits and hydrolytic enzymes
Production of indole acetic acid (IAA), ammonia (NH3) and hydrogen cyanide (HCN)
IAA production was evaluated according to a modified method described by Upadhyay et al. (2009). Bacterial cultures were grown for 72 h in 5 ml of sterile peptone yeast extract broth (peptone – 10 g, beef extract – 3 g, NaCl – 5 g, l-tryptophan – 0.204 g, distilled water– 1 l; pH – 7) and incubated for 96 h in the dark at 28 °C. A volume of 1.5 ml of this broth was then centrifuged for 10 min at 12,850 x g, and 1 ml of Salkawaski reagent (50 ml, 35% of perchloric acid, 1 ml 0.5 M FeCl3 solution) was added to 1 ml of the supernatant. The tubes were subsequently incubated for 1 h at 30 °C in the dark and IAA production was indicated by the formation of red color in the medium. Three replicates were considered.
Quantitative analysis was evaluated at 535 nm absorbance using a pf UV/Visible spectrophotometer and concentration was measured according to a standard graph of IAA (HiMedia) obtained in the range of 10–100 μg/ml.
The production of NH3 was evaluated according to Dweipayan et al. (2014). Briefly, 50 μl of bacterial cell suspension was inoculated in 30 ml of peptone broth (4%), then incubated at 28 °C for 72 h, and amended with 1 ml Nessler’s reagent. The formation of yellow to brown precipitate indicated a positive reaction. Three replicates were considered.
BCAs were also screened for the production of hydrogen cyanide (HCN) using the method described by Dweipayan et al. (2014). Isolates were streaked on LB medium amended with 4.4 g glycine/l. A Whatman filter paper no.1 soaked in 0.5% picric acid solution (in 2% sodium carbonate) was placed at the top of each plate. Petri dishes (three replicates per BCA) were then incubated at 28 °C for 4 days. The development of orange to red color indicated HCN production.
Phosphate solubilization was evaluated according to (Thabti et al. 2016). A loopful of each BCA was spot-inoculated on Pikovskaya’s agar medium (Dinesh et al. 2013). All plates were then incubated at 28 °C for 5 days. The observation of clear zone around the bacterial colony indicated a positive reaction.
Hydrolytic enzymes production
-
(i).
Amylase activity was performed according to Teodoro and Martins (2000). BCAs were cultured on agar starch medium containing 1% soluble starch, 0.2% yeast extract, 0.5% peptone, 0.05% MgSO4, 0.05% NaCl, 0.015% CaCl2 and 2% agar at pH 7.0. All petri dishes were incubated at 28 °C for 48 h. Amylase activity was confirmed by the appearance of a clear halo after staining with Lugol.
-
(ii).
Protease activity was evaluated according to Benkiara et al. (2013). BCAs were spot-cultured on milk agar medium (0.5% tryptone, 0.3% yeast extract, 1.5% agar and 25% skimmed milk), and incubated at 28 °C for 48 h. Protease activity was confirmed by the appearance of a clear zone around the colony indicating the degradation of milk casein.
-
(iii).
Cellulase activity was performed according to Kasana et al. (2008), on CMC agar containing 0.2% carboxymethyl cellulose, 0.2% NaNO3, 0.1% K2HPO4, 0.05% MgSO4, 0.05% KCl, 0.02% peptone, and 2% agar. Cellulase activity was confirmed by the appearance of a clear halo around the tested BCA after treatment with Gram’s iodine. Three plates were used as replicates for each isolate.
-
(iv).
Mannanase activity was evaluated on the LBG medium. After 24 h incubation at 28 °C, all plates were treated with Congo-Red; mannanase activity was identified by the appearance of a clear halo around the tested isolate (Yin et al. 2012).
-
(v).
Urease activity was performed according to Singh et al. (2017). BCAs were cultured on urea indole medium and incubated at 28 °C for 24 h. A color change from yellow to bright pinkish-red indicated a positive reaction.
Identification of antifungal metabolites (AFS)
Physical and chemical properties of antifungal substances
The stability of the antifungal substances was tested against 7 different enzymes: trypsin,α-chymotrypsin, pepsin, peptidase, lysozyme, and proteinase K. Samples of aliquots of CFS were treated with each enzyme at a final concentration of 1 mg ml−1. The test tubes with and without the enzyme (control) were then incubated at 37 °C for 2 h and heated at 100 °C for 3 min in order to denature the enzyme (Compaoré et al. 2013). Thermal stability of the antifungal substances was also analyzed by exposing the CFS to temperatures of 50, 60, 70, 80, 90, and 100 °C for 30 min, as well as an autoclaving at 121 °C for 15 min. Using 1 mol l−1 NaOH or HCl, the effect of pH on the antifungal activity was tested by adjusting CFS to pH’s of 4, 6, 8, 9 and 10. CFS with adjusted pH, were then incubated for 2 h at 37 °C before being neutralized to pH 7. After each treatment, residual activity was determined using the agar well diffusion method according to the formula: RA (%) \( =\Big(\frac{D}{d\ } \)) * 100; where D: Diameter of maximum activity and d: diameter of inhibition growth.. An untreated CFS was considered as controls.
Detection of lipopeptide synthesis genes by PCR
Polymerase chain reactions were carried out to determine the presence of the iturin, iturin A, mycosubtilin, surfactin, fengycin, bacilysin and bacillomycin biosynthesis genes in the DNA of ZO4, RS11, and SF82 isolates (Table 1). The PCR amplifications were carried out in a 25 μl reaction mixture containing 2 × PCR Master Mix / Dream Taq Green (12.5 μl) (Fermentas GmbH, St Leon-Rot, Germany), high purity sterile water (9.5 μl), 1 μl of each Forward and Reverse primer (10 μmol μl-1) and 1 μl of template DNA. The amplifications were performed in a thermocycler (Applied Biosystems, 2720, Singapore) using the following PCR conditions: 94 °C for 4 min, 30 cycles of 94 °C for 30 s, 57 °C for 30 s, 72 °C for 75 s. The final elongation was at 72 °C for 7 min. The amplified products were detected by agarose gel electrophoresis. PCR was positive when a band of the appropriate size was visualized (Compaoré et al. 2013).
In planta experiments
-
(i)
Pathogenicity test
Pathogenicity of an isolate of V. dahliae was tested on 4 month years old pepper plants. Inoculum was prepared from 1-week old PDB (Potato Dextrose Broth) (Sigma Aldrich) cultures, which was filtrated then diluted at a concentration of 106 conidia ml−1 (Rekanovic et al. 2007). Inoculation was performed using the root-dip method by soaking for one hour in the prepared suspensions or in sterile distilled water (negative control). Inoculated and control plants were then repotted and incubated for two months at 23 ± 2 °C and 95% humidity, and visible changes were recorded daily.
-
(ii)
Suppression of Verticillium wilt by BCAs and disease assessment
Young pepper plants at four months years old were first inoculated as described previously, then treated by watering with either BCA’s suspensions (108CFU/g of soil) or sterile distilled water (negative control). All plants were subsequently placed at 23 ± 2 °C and 95% humidity for 8 weeks. Three replicates and 15 plants per replicate were considered for each treatment. Disease severity ratings were recorded each two weeks, according to a 0–4 rating scale: (0 for a healthy plant, 1 = 1 to 33% of a defoliated plant, 2 = 34 to 66% of a defoliated plant, 3 = 67 to 99% of a defoliated plant and 4 = a dead plant) (Trapero et al. 2013). For each replication a disease index (DI) was first calculated according to the formula: DI = (∑ni ∗ i)/N: where i is the severity (0 to 4), ni is the number of plants with the severity of i, and N is the total number of plants. The AUDPCs was then calculated as the area under the curve of DI over time.
Evaluation of plant growth promotion of BCAs
The experiment was carried out on young pepper plants (2 months) using the dipping method. Roots were dipped in BCAs suspensions (108 CFU) for 30 min prior to planting. Control plants were dipped in sterile distilled water (Karlidag et al. 2007). Growth promoting effects were evaluated by measuring the length (cm) of stem and roots, and the weight (fresh and dry) of leaves, stem and roots (g).
All the experiments above were performed in three replicates.
Statistical analysis
Statistical analysis was performed using SPSS software (version 20). A completely randomized design was used for all experiments with 3 replications for each treatment. The data presented are from representative experiments that were repeated twice with similar results. Treatments were compared via ANOVA using the Duncan’s test at 5% (P = 0.05) probability level.
Results
Biochemical and molecular identification of the BCA’s
A collection of one hundred and sixty two isolates obtained from the rhizosphere of South Tunisian Oasis was conserved in the Microorganisms and active biomolecules lab. According to dual culture assay and well diffusion method, only thirty five isolates showed an antifungal activity against the 5 tested pathogens (data not shown). Among these, the three selected BCAs: ZO4, RS11 and SF82 were the most effective, particularly against V. dahliae, which growth has been inhibited at 72.82 ± 0.76%, 43.80 ± 0.20% and 47.87% ± 0.31%, respectively (Table 2). The three BCA isolates were first identified as B. subtilis by the Biolog system with a similarity index ranged between 0.514 and 0.646. Characteristics of biochemical reactions obtained from GENIII are described in Table 3. However, 16S rDNA analysis revealed 100% similarity of both RS11 (KX179571) and SF82 (KX179571) with B. amyloliquefaciens isolate (KX179573) and 100% similarity of ZO4 (KX179573) with B. subtilis isolate (MF581450).
Growth promoting traits and hydrolytic enzymes
Potential PGPR mechanisms of the selected BCAs were evaluated in vitro based on IAA production in chemically defined medium, phosphate solubilization in agar plate, and HCN, ammonia and hydrolytic enzymes (α amylase, protease, cellulose, mannanase) production. Results summarized in Table 4, revealed that the best activity was recorded for ZO4 isolate, which exhibited the highest amount of produced IAA (28.84 ± 0.27 μg ml1) as well as a positive reaction for the other traits.
Pathogenicity test
One month after inoculating pepper plants with V. dahliae suspension, symptoms of fungal attack started to be gradually observed until becoming very clear by the end of the fifth week; a brownish color was observed on almost all leaves of affected branches; which became curly with weakened bases, leading to their easy fall to the simple touch. All inoculated plants died two months after inoculation whereas controls remained totally healthy (Fig. 1). Koch’s postulate was then verified.
“In planta” antagonism against Verticillium disease
After treatment by BCA’s cultures, the AUDPCs and the analysis of variance showed statistically significant differences in disease severity resulting especially in a total inhibition by ZO4 isolate (Fig. 2).
Growth promoting effect in pepper plants
As presented in Table 5, all isolates significantly increased the length and the weight (fresh and dry) of leaves, stem and roots compared to non-inoculated controls. The analysis of variance showed significant differences between the BCAs (p = 0.05) as B. subtilis ZO4 has been proved to be the most efficient (Fig. 3).
Effect of enzymes, heat and pH on antimicrobial activity
Results showed that the incubation of CFS at different pH ranges reduced the antifungal activity of ZO4, RS11 and SF82 from 87% to 73%, 78% to 82%, and 67% to 72%; respectively at pH 4 and 10 (data not shown). In addition, they also revealed that the substance(s) produced by the BCAs showed sensitivity toward all the used proteolytic enzymes (Table 6). Furthermore, following 30 min exposure to wide range temperatures (50–120 °C), the activity of BCAs decreased then was completely lost at 120 °C.
PCR detection of lipopeptide biosynthesis genes
All the isolates ZO4, RS11 and SF82 were found to be negative for the genes involved in the biosynthesis of the lipopeptides surfactin (srf), fengycin (fen) and mycosubtilin (myc/itu). However, they contained genes involved in the biosynthesis of the lipopeptides iturins (ituA, ituD, ituC), bacilysin (bacA / B-F, bacA / B-R) and bacillomycin (bmyA) (Fig. 4).
Discussion
The best sources of antagonistic microorganisms are their natural environments, where they compete with naturally colonized microbiota that includes plant pathogens or spoilage microorganisms. This study was conducted to screen bacterial strains isolated from an oasis soil against verticillium wilt of pepper caused by V. dahliae. Of one hundred and sixty-two isolated bacteria, only three showed remarkable activity against the pathogen. All Three BCAs were identified by BIOLOG test as B.subtilis. Several studies have reported the use of BIOLOG micro plate assay for bacterial biocontrol agents, particularly in defining their specific carbon sources (Altinok et al. 2013; Ahmad et al. 2017). However, based on their 16S rRNA sequences, they were identified as B. subtilis: ZO4 and B. amyloliquefaciens: RS11 and SF82. These results are consistent with other studies that confirm a clear uncertainty provided by the GENIII software for genus and species level identifications (Wragga et al. 2014).
Antagonistic activity of the bacterial isolates was first evaluated in vitro, based on their ability to inhibit mycelia growth. According to the diffusion well assay against Neofusicoccum mediterraneum, Phytophthora sp., Fusarium pseudograminearum, Botryosphaeria dothidea and Verticillium dahliae, results revealed that bacterial culture filtrates were found to be highly active against all tested fungi (> 42%) with a significant difference between bacteria isolate for the same pathogen as well as between pathogens for the same bacterial isolate. B. subtilis ZO4, in particular, recorded the highest growth inhibition against V. dahliae (72.8%). These findings are consistent with several studies indicating that rhizosphere may be a common source for the selection of Bacillus species with important potentials that are useful for the biocontrol of both soil-borne and foliar pathogenic fungi (Govindasamy et al. 2010; Hinarejos et al. 2016). Moreover, on the basis of these results, the antibiotic activities exhibited by our BCAs appeared to be extracellular and easily recovered in their supernatant, which could be explained by the production of antifungal metabolites and lytic enzymes that are able to penetrate the cells of the pathogen and chemically inhibit its growth. Several authors have reported the large spectrum of antifungal activity of Bacillus spp. (Yang et al. 2014; Han et al. 2015a, b; Jahanshir et al. 2016) and have suggested that antibiosis could be the most common mode of antagonism observed among these species (Edwards and Seddon 2008). Other studies have also reported that Bacillus spp. protect plants through a number of mechanisms, particularly through the synthesis of different lipopeptides with inhibitory activity against phytopathogens (Falardeau et al. 2013; Cawoy et al. 2015; Torres et al. 2016)
The beneficial plant–microbe interactions in the rhizosphere are known to be important determinants of plant health and soil fertility (Pii et al. 2015). For this matter, our isolates were then tested for their different PGPR traits. Results showed that all BCAs were IAA producers. Several studies reported the production of IAA by rhizobacteria isolated from corn, wheat and rice cultures (Cakmakci et al. 2007; Mehnaz et al. 2010); others have demonstrated that IAA enhances plant cell elongation and cell division which stimulates better the root growth (Dey et al. 2004; Gray and Smith 2005; Li et al. 2018). Moreover, results revealed that all isolates were able to solubilize phosphate and to produce HCN similarly to the findings of Dinesh et al. (2013). The production of HCN plays an important role in the biological control of plant pathogens. In fact, Blumer and Hass (2000) suggested that HCN could be proposed as a defense regulator against pathogenic diseases, such as wheat planting (Rana et al. 2011), since it acts as an indicator of the strain resistance. Furthermore, experiments showed that BCAs were able to produce ammonia, which has been reported in many studies (Singh et al. 2017; Vimal et al. 2018). Many researchers have also reported the involvement of such attributes in root and stem length (Beneduzi et al. 2012), which is consistent with our findings. In fact, the inoculation of pepper plants with BCAs revealed an important increase in their root and stem length compared to the control. A significant increase was also recorded for both fresh and dry leaf, stem and root weight. The Analysis of variance showed a significant difference between the bacterial isolates. Overall, the highest values were recorded by B. subtilis ZO4 isolate. These results are consistent with several studies that reported the importance of Bacillus spp. and especially B. subtilis as PGPR bacteria (Govindasamy et al. 2010; Yu et al. 2011; Xu et al. 2016).
Another mechanism interfering with fungal inhibition is the degradation of their cell wall by the action of lytic enzymes. Results revealed that BCAs degraded cellulose, mannanose, protease and amylase. Such findings are consistent with other studies which reported that these enzymes play an important role in fungal inhibition and in disease prevention through their capacity to degrade the fungal cell membrane, (Thabti et al. 2016).
Selected proteolytic enzymes, including Proteinase K, α–chymotrypsin and pepsin showed strong effect on the three CFS. This was highlighted by the significant loss of antifungal activity against V. dahliae compared to the control. For instance, antifungal activity of SF82 was completely lost after treatment with Lysozyme. Such results suggest that BCAs antifungal activities seem to be probably attributed to some protease sensitive compounds such as iturin group of antibiotics (Torres et al. 2016). Several studies have reported the proteinaceous characteristics of antimicrobial substances produced by Bacillus species (Compaoré et al. 2013; Ceresa et al. 2016; Caulier et al. 2018). On the other hand, inhibitory substances were found to be active in a wide pH range (4 to 10), and highly stable after heat treatment (resistance up to 100 °C). Numerous studies have reported the importance of such properties in biological control of soil-borne pathogens, particularly under field conditions (Han et al. 2015a, b; Nawaz et al. 2018)
The detection of lipopetide synthesis genes has shown that our isolates are capable of producing iturin A, bacilysin and bacillomycine. These results are consistent with those of Compaoré et al. (2013). Bacillomycin, belong to the Iturin family, known for its antifungal properties, particularly against filamentous fungi which allow their application as bio-control agents (Liu et al. 2011; Zeriouh et al. 2011). Several studies have also shown the ability of these lipopeptides to inhibit fungi such as V. dahliae (Zhao et al. 2017).
It is well known that, unlike in vitro tests which only involve the pathogen and the antagonist under natural conditions, the role played by the host plant is crucial (Anith et al. 2003). Therefore, a “screening” system involving the infectious agent, the antagonist and the host, is necessary in order to have better information regarding the true inhibitory power of the selected BCAs. The in planta biological control experiment undertaken in this study, revealed variable antagonistic activities of the three BCAs. In fact, wilt severity in the form of AUDPC was significantly affected by treatments. Both B. amyloliquefaciens SF82 and RS11 have significantly reduced the appearance of wilt symptoms. However, especially plants treated with B. subtilis ZO4 strain sustained significantly the lowest AUDPC value (AUDPC = 1.8) compared to the control (AUDPC = 98). Such disease suppression requires further research, mainly focused on ZO4 mechanism and plant roots interaction. Previous studies have proposed that root colonization by biological agents plays an important role in the suppression of soil pathogens (Compant et al. 2005; Cao et al. 2011). In fact, as reported by Haggag and Timmusk (2008), the colonization of roots by BCAs before pathogen establishment, enhance biological control by preventing pathogen penetration; moreover, fast colonization of roots could be an important factor for the establishment and the introduction of BCAs in the rhizosphere and thus for biocontrol efficacy (Gamalero et al. 2003). Several studies have confirmed the antifungal activity of Bacillus spp. and their role in increasing plant yield. For instance, Tjamos et al. (2004) reported the isolation of a B. amyloliquefaciens strain that significantly reduced verticillium disease of apple trees and increased the yields up to 25%.
In conclusion, this study has shown the potential of ZO4, RS11 and SF82 rhizobacterial strains as important biological control and PGPR agents capable of reducing or eliminating symptoms of verticillium wilt in pepper plants. Especially, B. subtilis ZO4 isolate seems to be a promising candidate. However, its application under field conditions needs more investigations regarding its concentration, formulation and delivery method.
References
Abeysinghe S (2009) Effect of combined use of Bacillus subtilis CA32 and Trichoderm harzianum RUOI on biological control of Rhizoctonia satani on Solanum melongena and Capsicum annuum. Plant Pathol J 8:9–16. https://doi.org/10.3923/ppj.2009.9.16
Adesemoye AO, Kloepper JW (2009) Plant-microbes interactions in enhanced fertilizer use efficiency. Appl Microbiol Biotechnol 85:1–12. https://doi.org/10.1007/s00253-009-2196-0
Ahmad Z, Wu J, Chen L, Dong W (2017) Isolated Bacillus subtilis strain 330-2 and its antagonistic genes identified by the removing PCR. Sci Rep 7:1777. https://doi.org/10.1038/s41598-017-01940-9
Altinok HH, Dikilitas M, Yildiz HN (2013) Potential of Pseudomonas and Bacillus isolates as biocontrol agents against fusarium wilt of eggplant. Biotechnol Biotechnol Equip 27:3952–3958. https://doi.org/10.5504/BBEQ.2013.0047
Angelopoulou DJ, Naska EJ, Paplomatas EJ, Tjamos SE (2014) Biological control agents (BCAs) of verticillium wilt: influence of application rates and delivery method on plant protection, triggering of host defence mechanisms and rhizosphere populations of BCAs. Plant Pathol 63:1062–1069. https://doi.org/10.1111/ppa.12198
Anith KN, Radhakrishan NV, Manpmohandas TP (2003) Screening of antagonistic bacteria for biological control of nursery wilt of black pepper (Piper nigrum). Microbiol Res 158:91–97. https://doi.org/10.1078/0944-5013-00179
Beneduzi A, Ambrosini A, Passaglia LMP (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 35:1044–1051. https://doi.org/10.1590/S1415-47572012000600020
Benkiara A, Zaraî JN, Badis A, Rebzani F, Boulkour TS, Rekik H, Naili B, Ferradji FZ, Bejar S, Jaouadi B (2013) Biochemical and molecular characterization of a thermo-and detergent-stable alkaline serine keratinolytic protease from Bacillus circulans strain DZ100 for detergent formulations and feather-biodegradation process. Int Biodeterior Biodegrad 83:129–138. https://doi.org/10.1016/j.ibiod.2013.05.014
Bent E, Tuzun S, Chanway CP, Eneback S (2001) Alterations in plant growth and in root hormone levels of lodgepole pines inoculated with rhizobacteria. Can J Microbiol 47:793–800. https://doi.org/10.1139/w01-080
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28:1327–1350. https://doi.org/10.1007/s11274-011-0979-9
Blumer C, Hass D (2000) Mechanism, regulation, and ecological role of bacterial cyanide biosynthesis. Arch Microbiol 173:170–177. https://doi.org/10.1007/s002039900127
Bochner BR (2003) New technologies to assess genotype–phenotype relationships. Nat Rev Genet 4:309–314. https://doi.org/10.1038/nrg1046
Bochner BR, Gadzinski P, Panomitros E (2001) Phenotype microarrays for high-throughput phenotypic testing and assay of gene function. Genome Res 11:1246–1255. https://doi.org/10.1101/gr.186501
Cakmakci R, Erat M, Erdogan U, Dönmez MF (2007) The influence of plant growth-promoting rhizobateria on growth and enzyme activities in wheat and spinach plants. J Plant Nutr Soil 170:288–295. https://doi.org/10.1002/jpln.200625105
Calderon AR, Dolores JR, Alcazar BJ, Belaj A, De la Rosa R, Leon L (2015a) Evaluation of verticillium wilt resistance in selections from olive breeding crosses. Euphytica 206:619–629. https://doi.org/10.1007/s10681-015-1463-7
Calderon AR, Rodríguez-Jurado RD, Leon L, Alcazar BJ, De la Rosa R, Belaj A (2015b) Pre-breeding for resistance to verticillium wilt in olive: fishing in the wild relative gene pool. Crop Prot 75:25–33. https://doi.org/10.1016/j.cropro.2015.05.006
Cao Y, Ling N, Yang XM, Chen LH, Shen QR (2011) Bacillus subtilis SQR 9 can control fusarium wilt in cucumber by colonizing plant roots. Biol Fertil Soils 47:495–506. https://doi.org/10.1007/s00374-011-0556-2
Caulier S, Gillis A, Colau G, Licciardi F, Liépin M, Desoignies N, Modrie P, Legrève A, Mahillon J, Bragard C (2018) Versatile antagonistic activities of soil-borne Bacillus spp. and Pseudomonas spp. against Phytophthora infestans and other potato pathogens. Front Microbiol 9(143). https://doi.org/10.3389/fmicb.2018.00143
Cawoy H, Debois D, Franzil L, De Pauw E, Thnart P, Ongena M (2015) Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/ amyloliquefaciens. Microb Biotechnol 8:281–295. https://doi.org/10.1111/1751-7915.12238
Ceresa C, Rinaldi M, Chiono V, Carmagnola I, Allegrone G, Fracchia L (2016) Lipopeptides from Bacillus subtilis AC7 inhibit adhesion and biofilm formation of Candida albicans on silicone. Antonie Van Leeuwenhoek 109:1375–1388. https://doi.org/10.1007/s10482-016-0736-z
Chen X, Zhang Y, Fu X, Li Y, Wang Q (2016) Isolation and characterization of Bacillus amyloliquefaciens PG12 for the biological control of apple ring rot. Postharvest Biol Technol 115:113–121. https://doi.org/10.1016/j.postharvbio.2015.12.021
Chung S, Kong H, Buyer JS, Lakshman DK, Lydon J, Kim SD, Roberts DP (2008) Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soilborne pathogens of cucumber and pepper. Appl Microbiol Biotechnol 80:115–123. https://doi.org/10.1007/s00253-008-1520-4
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases principles, mechanisms of action, future prospects. Appl Environ Microbiol 71:4951–4959. https://doi.org/10.1128/AEM.71.9.4951-4959.2005
Compaoré CS, Nielsen DS, Sawadogo-Lingani H, Berner TS, Nielsen KF, Adimpong DB, Diawara B, Ouedraogo GA, Jakobsen M, Thorsen L (2013) Bacillus amyloliquefaciens ssp. plantarum strains as potential protective starter cultures for the production of bikalga, an alkaline fermented food. J Appl Microbiol 115:133–146. https://doi.org/10.1111/jam.12214
Dey R, Pal KK, Bhatt DM, Chauhan SM (2004) Growth promotion and yield enhancement of peanut (Arachishypogaea L.) by application of plant growth promoting rhizobacteria. Microbiol Res 159:371–394. https://doi.org/10.1016/j.micres.2004.08.004
Dinesh R, Anandaraj M, Kumar A, Srinivasan V, Bini YK, Subila KP, Aravind R, Hamza S (2013) Effects of plant growth promoting rhizobacteria and NPK fertilizers on biochemical and microbial properties of soils under ginger (Zingiber officinale Rosc.) cultivation. Agric Res 2:346–353. https://doi.org/10.1007/s40003-013-0080-8
Douira A, Benkirane R, Touhami AO, Elhaloui NE (2008) Verticillium wilt of pepper (Capsicum annuum) in Morocco. J Phytopathol 143:467–470. https://doi.org/10.1111/j.1439-0434.1995.tb04556.x
Dweipayan G, Pinakin D, Pranav P, Janki NT (2014) Screening of PGPR from saline desert of Kutch: growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol Res 169:66–75. https://doi.org/10.1016/j.micres.2013.07.004
Edwards SG, Seddon B (2008) Mode of antagonism of Brevibacillus brevis against Botrytis cinerea in vitro (2008). J Appl Microbiol 91:652–659. https://doi.org/10.1046/j.1365-2672.2001.01430.x
Falardeau J, Wise C, Novitsky L, Avis TJ (2013) Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J Chem Ecol 39:869–878. https://doi.org/10.1007/s10886-013-0319-7
Fernandes PAV, De Arruda IR, Dos Santos AFAB, De Araujo AA, Souto Maior AM, Ximenes EZ (2007) Antimicrobial activity of surfactants produced by R14 against Bacillus subtilis multidrug resistant bacteria. Braz J Microbiol 38:704–709. https://doi.org/10.1590/S1517-83822007000400022
Gamalero E, Lingua G, Berta G, Lemanceau P (2003) Methods for studying root colonization by introduced beneficial bacteria. Agronomie 23:407–418. https://doi.org/10.1007/978-90-481-2666-8_37
Goicoechea N (2009) To what extent are soil amendments useful to control Verticillium wilt? Pest Manag Sci 65:831–839. https://doi.org/10.1002/ps.1774
Goicoechea A, Aguirreolea J, Cenoz S, García-Mina JM (2001) Gas exchange and flowering in Verticillium-wilted pepper plants. J Phytopathol 149:281–286. https://doi.org/10.1046/j.1439-0434.2001.00622.x
Govindasamy V, Senthilkumar M, Magheshwaran V, Kumar U, Bose P, Sharma V, Annapurna K (2010) Bacillus and Paenibacillus spp.: potential PGPR for sustainable agriculture. In: Plant Growth and Health Promoting Bacteria, vol 18. Part of the Microbiology Monographs book series (MICROMONO), pp 333–364. https://doi.org/10.1007/978-3-642-13612-2_15
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant bacterium signaling processes. Soil Biol Biochem 37:395–412. https://doi.org/10.1016/j.soilbio.2004.08.030
Haggag WM, Timmusk S (2008) Colonization of peanut roots by biofilm-forming Paenibacillus polymyxa initiates biocontrol against crown rot disease. J Appl Microbiol 104:961–969. https://doi.org/10.1111/j.1365-2672.2007.03611.x
Han JH, Shim H, Shin JH, Kim KS (2015a) Antagonistic activities of Bacillus spp. strains isolated from tidal flat sediment towards anthracnose pathogens Colletotrichum acutatum and C.gloeosporioides in South Korea. Plant Pathol J 31:165–175. https://doi.org/10.5423/PPJ.OA.03.2015.0036
Han Y, Zhang B, Shen Q, You C, Yu Y, Li P, Shang Q (2015b) Purification and identification of two antifungal cyclic peptides produced by Bacillus amyloliquefaciens L-H15. Appl Biochem Biotechnol 176:2202–2212. https://doi.org/10.1007/s12010-015-1708-x
Hinarejos E, Castellano M, Rodrigo I, Bellés JM, Conejero V, López-Gresa MP, Lisón P (2016) Bacillus subtilis IAB/BS03 as a potential biological control agent. Eur J Plant Pathol 146:597–608. https://doi.org/10.1007/s10658-016-0945-3
Issar S, Sharma S, Choudhary DK, Gautam HK, Gaur RK (2012) Molecular characterization of Pseudomonas spp. isolated from root nodules of various leguminous plants of Shekhawti Rgion, Rajasthan, India. Am J Plant Sci 3:60–63. https://doi.org/10.4236/ajps.2012.31005
Jahanshir A, Vahid F, Javadi T, Javad N (2016) Antifungal effect of plant essential oils on controlling Phytophthora species. Plant Pathol J 32:16–24. https://doi.org/10.5423/PPJ.OA.05.2015.0091
Karlidag H, Esitken A, Turan M, Sahin F (2007) Effects of root inoculation of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient element contents of apple. Sci Hortic 114:16–20. https://doi.org/10.1016/j.scienta.2007.04.013
Kasana RC, Salwan R, Dhar H, Dutt S, Gulati A (2008) A rapid and easy method for the detection of microbial cellulases on agar plates using gram’s iodine. Curr Microbiol 57:503–507. https://doi.org/10.1007/s00284-008-9276-8
Lahlali R, Hijri M (2010) Screening, identification and evaluation of potential biocontrol fungal endophytes against Rhizoctonia solani AG3 on potato plants. FEMS Microbiol Lett 311:152–159. https://doi.org/10.1111/j.1574-6968.2010.02084.x
Li Z, Zhang X, Zhao Y, Li Y, Zhang G, Peng Z, Zhang J (2018) Enhancing auxin accumulation in maize root tips improves root growth and dwarfs plant height. Plant Biotechnol J 16:86–99. https://doi.org/10.1111/pbi.12751
Liu J, Zhou T, He D, Li XZ, Wu H, Liu W, Gao X (2011) Functions of lipopeptides bacillomycin D and fengycin in antagonism of Bacillus amyloliquefaciens C06 towards Monilinia fructicola. J Mol Microbiol Biotechnol 20:43–52. https://doi.org/10.1159/000323501
López-Escudero FJ, Mercado-Blanco J (2011) Verticillium wilt of olive: a case study to implement an integrated strategy to control a soil-borne pathogen. J Plant Soil 344:1–50. https://doi.org/10.1007/s11104-010-0629-2
Lugtenberg B, Kamilova F (2009) Plant growth promoting rhizobacteria. Annu Rev Microbiol 63:541–556. https://doi.org/10.1146/annurev.micro.62.081307.162918
Markakis EA, Tjamos SE, Antoniou PP, Paplomatas EJ, Tjamos EC (2016) Biological control of Verticillium wilt of olive by Paenibacillus alvei, strain K165. BioControl 61:293–303. https://doi.org/10.1007/s10526-015-9669-0
Mehnaz S, Kowalik T, Reynolds B, Lazarovits G (2010) Growth promoting effects of corn (Zea mays) bacterial isolates under greenhouse and field conditions. Soil Biol Biochem 42:1848–1856. https://doi.org/10.1016/j.soilbio.2010.07.003
Nawaz HH, Rajaofera MJN, He Q, Anam U, Lin C, Miao W (2018) Evaluation of antifungal metabolites activity from bacillus licheniformis OE-04 against Colletotrichum gossypii. Pestic Biochem Physiol 146:33–42. https://doi.org/10.1016/j.pestbp.2018.02.007
Novo M, Silvar C, Merino F, Martínez-Cortés T, Lu F, Ralph J, Pomar F (2017) Deciphering the role of the phenylpropanoid metabolism in the tolerance of Capsicum annuum L. to Verticillium dahliae Kleb. Plant Sci 258:12–20. https://doi.org/10.1016/j.micres.2013.07.004
Ouzari H, Khsairi A, Raddadi N, Jaoua L, Hassen A, Zarrouk M, Daffonchio D, Boudabous A (2008) Diversity of auxin producing bacteria associated to Pseudomonas savastanoiinduced olive knots. J Basic Microbiol 48:370–377. https://doi.org/10.1002/jobm.200800036
Park M, Kim C, Yang J, Lee H, Shin W, Kim S, Sa T (2005) Isolation and characterization of diazotrophic growth promoting bacteria from rhizosphere of agricultural crops of Korea. Microbiol Res 160:127–133. https://doi.org/10.1016/j.micres.2004.10.003
Pii Y, Mimmo T, Tomasi N, Terzano R, Cesco S, Crecchio C (2015) Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol Fertil Soils 51:403–415. https://doi.org/10.1007/s00374-015-0996-1
Rana A, Saharan B, Joshi M, Prasanna R, Kumar K, Nain L (2011) Identification of multi-trait PGPR isolates and evaluating their potential as inoculants for wheat. Ann Microbiol 61:893–900. https://doi.org/10.1007/s13213-011-0211-z
Rekanovic E, Milijasevic S, Todorovic B, Potocnik I (2007) Possibilities of biological and chemical control of Verticillium wilt in pepper. Phytoparasitica 35:436–441. https://doi.org/10.1007/BF03020601
Reyes I, Bernier L, Antoun H (2002) Rock phosphate solubilization and colonization of maize rhizosphere by wild and genetically modified strains of Penicillium rugulosum. Microb Ecol 44:39–48. https://doi.org/10.1007/s00248-002-1001-8
Sarrangi NP, Athukorala WG, Dilantha F, Khalid YR (2009) Identification of antifungal antibiotics of Bacillus species isolates from different microhabitats using polymerase chain reaction and MALDI-TOF mass spectrometry. Can J Microbiol 55:1021–1032. https://doi.org/10.1139/W09-067
Shakeel M, Rais A, Hassan MN, Hafees YF (2015) Root associated Bacillus sp. improves growth, yield and zinc translocation for basmati Rice (Oryza sativa) varieties. Front Microbiol 6(1286). https://doi.org/10.3389/fmicb.2015.01286
Singh R, Kapil DP, Kumar A, Singh M (2017) PGPR isolates from the rhizosphere of vegetable crop Momordica charantia: characterization and application as biofertilizer. Int J Curr Microbiol App Sci 6:1789–1802. https://doi.org/10.20546/ijcmas.2017.603.205
Snook ME, Mitchell T, Hinton DM, Bacon CW (2009) Isolation and characterization of leu 7- surfactin from the endophytic bacterium Bacillus mojavensis RRC 101, a biocontrol agent for Fusarium verticillioides. J Agric Food Chem 57:4287–4292. https://doi.org/10.1021/jf900164h
Soares MA, Li HY, Bergen M, da Silva JM, Kowalski KP, White JF (2016) Functional role of an endophytic Bacillus amyloliquefaciens in enhancing growth and disease protection of invasive English ivy (Hedera helix L.). Plant Soil 405:107–123. https://doi.org/10.1007/s11104-015-2638-7
Sriram MI, Kalishwaralal K, Deepak V, Gracerosepat R, Srisakthi K, Gurunathan S (2011) Biofilm inhibition and antimicrobial action of lipopeptide biosurfactant produced by heavy metal tolerant strain Bacillus cereus NK1. Colloids Surf B Biointerfaces 85:174–178. https://doi.org/10.1016/j.colsurfb.2011.02.026
Stein T (2005) Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol 56:854–857. https://doi.org/10.1111/j.1365-2958.2005.04587.x
Teodoro CEDS, Martins MLL (2000) Culture conditions for the production of thermostable amylase by Bacillus sp. Braz J Microbiol 31:298–302. https://doi.org/10.1590/S1517-83822000000400011
Thabti W, Riahi Y, Gharsalli R, Belhaj O (2016) Screening and characterization of thermo-active enzymes of biotechnological interest produced by thermophilic Bacillus isolated from hot springs in Tunisia. Acta Biochim Pol 63:581–587. https://doi.org/10.18388/abp.2016_1271
Tjamos EC, Tsitsigiannis DI, Tjamos SE, Antoniou PP, Katinakis P (2004) Selection and screening of Endorhizosphere bacteria from solarized soils as Biocontrol agents against Verticillium dahliae of Solanaceous hosts. Eur J Plant Pathol 110:35–44. https://doi.org/10.1023/B:EJPP.0000010132.91241.cb
Torres MJ, Brandan P, Petroselli G, Erra- Balsells R, Audisio MC (2016) Antagonistic effects of Bacillus subtilis subsp. subtilis and B. amyloliquefaciens against Macrophomina phaseolina: SEM study of fungal changes and UV-MALDI-TOF MS analysis of their bioactive compounds. Microbiol Res 182:31–39. https://doi.org/10.1016/j.micres.2015.09.005
Trapero C, Diez CM, Rallo L, Barranco D, Lopez-Escudero FJ (2013) Effective inoculation methods to screen for resistance to Verticillium wilt in olive. Sci Hortic 162:252–259. https://doi.org/10.1016/j.scienta.2013.08.036
Upadhyay SK, Singh DP, Saikia R (2009) Genetic diversity of plant growth promoting Rhizobacteria isolated from Rhizospheric soil of wheat under saline condition. Curr Microbiol 59:489–496. https://doi.org/10.1007/s00284-009-9464-1
Usami T, Abiko M, Shishido M, Amemiya Y (2002) Specific detection of tomato pathotype of Verticillium dahliae by PCR assays. J Gen Plant Pathol 68:134–140. https://doi.org/10.1007/PL00013066
Van Loon L, Bakker P (2005) Induced systemic resistance as a mechanism of disease suppression by Rhizobacteria. In: Siddiqui ZA (ed) PGPR: Biocontrol and biofertilization. Springer, Dordrecht, pp 39–36
Vimal SR, Gupta J, Singh J (2018) Effect of salt tolerant Bacillus sp. and Pseudomonas sp. on wheat (Triticum aestivum L.) growth under soil salinity: a comparative study. Microbiol Res 9(7462). https://doi.org/10.4081/mr.2018.7462
Wei F, Fan R, Dong H, Shang W, Xu X, Zhu H, Yang J, Hu X (2015) Threshold microsclerotial inoculum for cotton Verticillium wilt determined through wet-sieving and real time quantitative PCR. Phytopathol 105:220–229. https://doi.org/10.1094/PHYTO-05-14-0139-R
Wragga P, Randall L, Whatmore AM (2014) Comparison of Biolog GEN III MicroStation semi-automated bacterial identification system with matrix-assisted laser desorption ionization-time of flight mass spectrometry and 16S ribosomal RNA gene sequencing for the identification of bacteria of veterinary interest. J Microbiol Methods 105:16–21. https://doi.org/10.1016/j.mimet.2014.07.003
Xu SJ, Hwan PD, Kim JY, Kim BS (2016) Biological control of gray mold and growth promotion of tomato using Bacillus spp. isolated from soil. Trop Plant Pathol 41:169–176. https://doi.org/10.1007/s40858-016-0082-8
Yang R, Fan X, Cai X, Hu F (2014) The inhibitory mechanisms by mixtures of two endophytic bacterial strains isolated from Ginkgo biloba against pepper phytophthora blight. Biol Control 85:59–67. https://doi.org/10.1016/j.biocontrol.2014.09.013
Yin LJ, Tai HM, Jiang ST (2012) Characterization of mannanase from a novel mannanase-producing bacterium. J Agric Food Chem 60:6425–6431. https://doi.org/10.1021/jf301944e
Yu X, Ai C, Xin L, Zhou G (2011) The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on fusarium wilt and promotes the growth of pepper. Eur J Soil Biol 47:138–145. https://doi.org/10.1016/j.ejsobi.2010.11.001
Zeriouh H, Romero D, Gutierrez GL, Cazorla FM, De Vicente A, Perez-García A (2011) The iturin like lipopeptides are essential components in the biological control arsenal of Bacillus subtilis against bacterial diseases of cucurbits. Mol Plant-Microbe Interact 24:1540–1552. https://doi.org/10.1094/MPMI-06-11-0162
Zhao H, Shao D, Jiang C, Shi J, Li Q, Huang Q, Rajoka MSR, Yang H, Jin M (2017) Biological activity of lipopeptides from Bacillus. Appl Microbiol Biotechnol 101:5951–5960. https://doi.org/10.1007/s00253-017-8396-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Guenoun, K., Chattaoui, M., Bouri, M. et al. Biological control of growth promoting rhizobacteria against verticillium wilt of pepper plant. Biologia 74, 237–250 (2019). https://doi.org/10.2478/s11756-018-00169-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-018-00169-9