Abstract
We report synthesis of triphenyl(propyl-3-hydrogen sulfate)phosphonium bromide ([TPPHSP]Br) as a reusable green Brønsted-acidic ionic liquid catalyst and its application for synthesis of 5-arylidene barbituric acids and pyrano[2,3-d]pyrimidine derivatives by condensation reaction between aromatic aldehydes and barbituric acid or aromatic aldehydes, malononitrile, and barbituric acid in EtOH–H2O in reflux condition with good to excellent yield. The [TPPHSP]Br IL catalyst was characterized by Fourier-transform infrared (FT-IR) spectroscopy, 1H and 13C nuclear magnetic resonance (NMR), and thermogravimetric (TG) analysis and showed good catalytic activity and reusability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, ionic liquids (ILs) have attracted considerable attention in organic synthesis as solvents, catalysts, or dual-purpose catalyst-solvents due to their special physical and chemical features, such as low volatility, good thermal stability, low melting point, insignificant vapor pressure, and recyclability [1,2,3,4,5,6,7,8,9]. Ionic liquids are generally constituted of large asymmetric organic cations of nitrogen or phosphorus and many different inorganic or organic anions [10]. Over the past few years, synthesis of certain ionic liquids known as task-specific ionic liquids (TSILs) with Brønsted-acidic functional groups in their structure has attracted significant interest because of their advantages over traditional mineral liquid and solid acids. Moreover, compared with conventional mineral acids, TSILs present various interesting properties including high catalytic efficiency, high polarity, easy product separation, and environmentally friendly nature [11,12,13,14,15,16].
Heterocycles are very important in synthesis of active biological compounds. These compounds are also the main structural component of most pharmaceutical molecules [17,18,19]. Among them, barbituric acid derivatives have attracted much attention in medicinal chemistry. The seductive and hypnotic properties of 5-arylidene barbituric acid derivatives have been known for years [20,21,22,23]. Moreover, pyrano[2,3-d]pyrimidines show numerous pharmacological activities, including antitumor [24], anticancer [25], antiviral [26], antimicrobial, and antifungal activities [27]. 5-Arylidene barbituric acid derivatives have been synthesized in presence of various catalysts such as 1,4-diazabicyclo[2.2.2]octane (DABCO) [28], l-tyrosine [29] Ni nanoparticles (NPs) [30], silicotungstic acid [31], NH2SO3H [32], [bmim]BF4 [33], ethylammonium nitrate [34], cetyltrimethylammonium bromide [35], and taurine [36]. Several procedures have also been reported for synthesis of pyrano[2,3-d]pyrimidines, e.g., using DABCO [37], basic ionic liquid [38], l-proline [39], urea-SO3H [40], Al-HMS-20 [41], nano-Al2O3 [42], alum [43], taurine [36], [H-Suc]HSO4 [44], CuO/ZnO nanocatalyst [45], B(OH)3 [46], Al-HMS-20 [41], and trichloroisocyanuric acid [47].
Multicomponent reactions (MCRs) are considered to be an important approach in organic synthesis [48], providing a powerful means to synthesize complex compounds in one step without separation of intermediates. Since three or more components react with together in a one-pot process in these reactions, they show high atom economy and high selectivity [49,50,51,52]. One type of MCR involves synthesis of pyrano[2,3-d]pyrimidines through one-pot, three-component condensation reaction between aldehydes, malononitrile, and barbituric acid. Although several catalysts and different procedures have been reported for synthesis of pyrano[2,3-d]pyrimidines, identification of an environmentally compatible approach using a recyclable catalyst is still a great demand in organic synthesis.
In continuation of our research on preparation and application of green catalysts in multicomponent reactions [53,54,55,56,57,58], we report herein preparation and use of triphenyl(propyl-3-hydrogen sulfate)phosphonium bromide ([TPPHSP]Br) as a green, efficient, and reusable ionic liquid catalyst (Scheme 1) for synthesis of 5-arylidene barbituric acid derivatives and pyrano[2,3-d]pyrimidines via one-pot condensation of aromatic aldehydes, malononitrile, and barbituric acid in EtOH–H2O in reflux condition (Scheme 1).
Experimental
All chemicals were purchased from Merck and Sigma-Aldrich companies. All reagents were applied without any further purification. All known compounds were identified by comparison of their melting points, IR, and NMR data with those reported in literature. Reaction progress was monitored by thin-layer chromatography (TLC) using silica gel SIL G/UV 254 plates. 1H and 13C NMR spectra were obtained using a Bruker Ultrashield spectrometer at 400 MHz and 100 MHz, respectively. Thermogravimetric analysis (TGA) was carried out using a METLLER apparatus. Melting points were measured with an Electrothermal 9100 apparatus and are uncorrected. FT-IR spectra were recorded with a JASCO 6300 spectrometer from KBr pellets in the range of 400–4000 cm−1.
Preparation of triphenyl(propyl-3-hydrogen sulfate)phosphonium bromide as ionic liquid
Hydroxyl-functionalized phosphonium IL was synthesized using the procedure described in literature with minor modifications [59]. Triphenylphosphine and 3-bromopropanol were combined in equimolar quantities in toluene and heated at reflux condition for 24 h under Ar atmosphere. The reaction mixture was then cooled to room temperature, and the toluene was decanted. The residue was washed several times (3 × 20 mL) with toluene and diethyl ether, then dried under vacuum to give the product as a yellow viscous oil. After this, hydroxyl-functionalized phosphonium IL (2.3 g, 6.0 mmol) in dry CH2Cl2 (30 mL) was charged into a 100-mL round-bottomed flask in an ice bath, then chlorosulfonic acid (0.7 g, 6.0 mmol) was added dropwise. After chlorosulfonic acid addition, the reaction mixture was stirred for 30 min, then allowed to stirred for 2 h at room temperature. The solvent was evaporated under reduced pressure to give the desired IL as a yellow viscous liquid. Spectroscopic data for the ionic liquid: IR (KBr, υ, cm−1) = 3302, 1595, 1499, 1474, 1231, 1119, 754, 692, 539. 1H NMR (400 MHz, CDCl3): δ 8.23 (s, 1H, OH), 7.32–7.42 (m, 15H), 4.04–4.07 (t, 2H), 3.26–3.33 (m, 2H), 1.86–1.91 (m, 2H) ppm. 13C NMR (100 MHz, CDCl3): δ 133.4, 133.3, 132.1, 132.0, 129.0, 128.9, 118.3, 117.4, 66.0, 21.1, 21.0, 19.4, 18.8 ppm.
General procedure for synthesis of 5-arylidene barbituric acid derivatives
Typically, a mixture of aromatic aldehyde (1.0 mmol), barbituric acid (1.0 mmol), and [TPPHSP]Br (0.01 g, 2 mol%) as catalyst was added to 5 mL EtOH–H2O (3:1) in a 25-mL round-bottomed flask. The reaction mixture was refluxed with stirring for appropriate time (Table 2). After reaction completion, as monitored by TLC (n-hexane:EtOAc), the precipitate was isolated by filtration and washed several times with cold ethanol for purification (Scheme 2). All of the desired product(s) were characterized by comparison of their physical properties and spectral data (IR and NMR) with those of known compounds.
General procedure for synthesis of pyrano[2,3-d]pyrimidine derivatives
Aromatic aldehydes (1.0 mmol), malononitrile (1.0 mmol), barbituric acid (1.0 mmol), and 5 mL EtOH–H2O as solvent were loaded into a 25-mL round-bottomed flask, then [TPPHSP]Br (0.01 g, 2 mol%) was added to the mixture. The resulting reaction mixture was stirred at reflux condition for appropriate time (Table 4). After reaction completion, monitored by TLC (n-hexane:EtOAc), the reaction mixture was cooled to room temperature and filtered. After filtration, the solid products were washed with cold water and recrystallized from ethanol to obtain pure products in good to excellent yield (Scheme 3, Table 4). The filtrate of ionic liquid [TPPHSP]Br was then recovered and reused for subsequent reactions. All desired products were characterized by comparison of their physical properties and spectral data (IR and NMR) with those of known compounds.
Results and discussion
In recent years, synthesis of task-specific ionic liquids with Brønsted-acidic functional groups, particularly SO3H and SO4H functional groups, has become increasingly popular [60, 61]. In this research, we synthesized triphenyl(propyl-3-hydrogen sulfate)phosphonium bromide as a new Brønsted-acidic ionic liquid. With the intention of investigating the applicability of [TPPHSP]Br in organic reactions, synthesis of barbiturate derivatives was selected as the model reaction.
Characterization of [TPPHSP]Br as Brønsted-acidic ionic liquid catalyst
The structure of the Brønsted-acidic ionic liquid [TPPHSP]Br was recognized by studying its FT-IR, 1H NMR, 13C NMR, TG, and differential thermogravimetric (DTG) spectra.
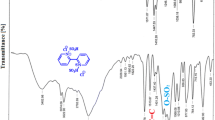
In the FT-IR spectrum of [TPPHSP]Br, the characteristic band at 3302 cm−1 corresponds to stretching vibration of O–H in SO4H group. The strong absorptions at 1231.33, 1185.38, 1119.48, and 539.97 cm−1 are related to asymmetric stretching and symmetric bending vibrations of S–O of SO4 group (Fig. 1).
Also, we studied the structure of [TPPHSP]Br by 1H and 13C NMR spectroscopy (Fig. 2). The acidic hydrogen of SO4H shows a significant peak in the 1H NMR spectrum of the ionic liquid, observed at 8.23 ppm as a broad singlet. Also, there are two multiplet peaks at 1.86–1.91 and 3.26–3.33 ppm and a triplet at 4.05 ppm, related to aliphatic hydrogens, and a multiplet peak at 7.32–7.48 ppm that can be assigned to aromatic ring protons of IL. The signals in the 13C NMR spectrum are in accordance with the structure of [TPPHSP]Br IL. The peaks at δ = 117.4–133.4 are related to aromatic ring carbons of PPh3. Furthermore, the peaks at 66.0 as a singlet and the two doublet peaks at 18.8–21.1 ppm are related to aliphatic carbons in the IL coupled with phosphorus atom (Fig. 3).
To investigate the thermal stability of the [TPPHSP]Br catalyst, TG and DTG analyses were performed. As depicted in Fig. 4, a first weight loss step was attributed to loss of adsorbed solvent used during preparation of the catalyst, taking place below 100 °C. The major weight loss and decomposition of the catalyst took place between 250 and 320 °C.
Application of [TPPHSP]Br catalyst in synthesis of barbiturate derivatives
After characterization of the new ionic liquid, we studied its catalytic activity as acidic catalyst in synthesis of 5-arylidene barbituric acids. To define effective reaction conditions, the reaction between 4-nitrobenzaldehyde and barbituric acid was chosen as a model and optimized in terms of various parameters such as solvent, amount of catalyst, and effect of temperature. The results are summarized in Table 1. The effect of different solvents and solvent-free condition was investigated using the model reaction. The results showed that the reaction progressed efficiently in a mixture of EtOH and H2O as solvent. The progress of the reaction increased considerably on increasing the temperature. Hence, reflux condition was chosen as the best temperature. The amount of catalyst in EtOH–H2O under reflux condition was optimized. As seen from Table 1, 2 mol% [TPPHSP]Br was the optimum amount of catalyst for this reaction (Table 1, entry 9). The results showed that increasing the amount of catalyst had no considerable effect on the product yield (Table 1, entry 10).
Then, synthesis of 5-arylidene barbituric acids was performed using various aromatic aldehydes and barbituric acid under the optimized conditions, viz. 2 mol% catalyst in EtOH–H2O under reflux. The results obtained are summarized in Table 2, revealing that all the aromatic aldehydes carrying either electron-donating or electron-withdrawing groups gave the desired products in high yield.
After successful use of [TPPHSP]Br in synthesis of 5-arylidene barbituric acids, we decided to investigate its ability to catalyze synthesis of pyrano[2,3-d]pyrimidines.
We used the condensation reaction of malononitrile, 4-nitrobenzaldehyde, and barbituric acid as a model to determined the best conditions. At first, the condensation reaction was investigated under solvent-free condition; as shown in Table 3, no desired product was formed. Therefore, the model reaction was performed in various solvents. The results showed that EtOH–H2O is a desirable solvent for this reaction. In n-hexane as solvent, no condensation product was observed (Table 3, entry 4). Also, the reaction was performed in chloroform as solvent, and the desired product was achieved in 50 % yield but the reaction did not reach completion (Table 3, entry 3).
The amount of catalyst was also investigated. When the reaction was performed without any catalyst, no desired product was formed. The amount of catalyst in the model reaction was then changed from 0.5 to 3 mol%. The results showed that 2 mol [TPPHSP]Br is the optimal amount of catalyst in terms of reaction time and yield, while increasing the amount of catalyst had no significant effect on the progress of the reaction (Table 3, entries 9, 10). To investigate the effect of temperature in improving the reaction, the model reaction was carried out at different temperatures. It was observed that the progress of the reaction was very slow at room temperature (Table 3, entry 11). Increasing the temperature from room temperature to reflux condition led to an increase in product yield from 40 to 90 % while the reaction time decreased from 150 to 50 min. Therefore, reflux was chosen as the best temperature. According to these results, the optimum condition for synthesis of pyrano[2,3-d]pyrimidines is 2 mol% [TPPHSP]Br as catalyst in EtOH–H2O under reflux condition.
After optimization of the reaction conditions (Table 3), the generality of the reaction was investigated in synthesis of pyrano[2,3-d]pyrimidines using different substituted aromatic aldehydes (Table 4). All reactions proceeded efficiently within 35–90 min to give the corresponding pyrano[2,3-d]pyrimidine derivatives in good to excellent yield (76–95 %). These results indicate that all the aromatic aldehydes with various substituents on the aromatic ring reacted efficiently with barbituric acid and malononitrile to afford the desired products in high yield.
To confirm the validity of the present approach versus literature, the results obtained in this work using triphenyl(propyl-3-hydrogen sulfate)phosphonium bromide are compared with those obtained using other reported catalysts for synthesis of 5-arylidene barbituric acid and pyrano[2,3-d]pyrimidine derivatives in Table 5, revealing that the performance of the ionic liquid is comparable to the others in terms of reaction time and product yield.
Scheme 4 illustrates a possible mechanism for preparation of above-mentioned barbiturate derivatives catalyzed by [TPPHSP]Br. Initially, arylaldehyde is activated by hydrogen bonding with IL catalyst. Then, malononitrile or barbituric acid intermediate adds to the activated aldehyde. Finally, through nucleophilic attack followed by cyclocondensation then tautomerization, the final product is formed.
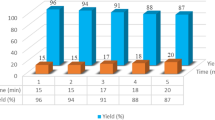
The reusability of the catalyst was also studied using the condensation reaction of 4-nitrobenzaldehyde, malononitrile, and barbituric acid. After reaction completion, the solid product was washed with water, then evaporated under reduced pressure to recover the catalyst, which was washed with acetone, dried, and reused for the same reaction. The recovered catalyst was reused five times with minimal change in activity (Table 6).
Conclusions
An efficient Brønsted-acidic ionic liquid catalyst triphenyl(propyl-3-hydrogen sulfate)phosphonium bromide was synthesized and characterized. Its catalytic activity was studied in synthesis of 5-arylidene barbituric acids and pyrano[2,3-d]pyrimidine derivatives via three-component, one-pot condensation reaction between several aromatic aldehydes, malononitrile, and barbituric acid. Short reaction time, high yield, and easy workup are some advantages of this catalyst. The [TPPHSP]Br catalyst is recyclable and can be reused in five consecutive cycles without significant loss of activity.
References
H.O. Bourbigou, L. Magna, D. Morvan, Appl. Catal. A Gen. 373, 1 (2010)
J. Pavlinac, M. Zupan, K.K. Laali, S. Stavber, Tetrahedron 65, 5625 (2009)
B.C. Ranu, S. Banerjee, Org. Lett. 7, 3049 (2005)
T. Joseph, S. Sahoo, S.B. Halligudi, J. Mol. Catal. A Chem. 234, 107 (2005)
D. Saha, A. Saha, B. Ranu, Tetrahedron Lett. 50, 6088 (2009)
M.A.P. Martins, C.P. Frizzo, D.N. Moreira, N. Zanatta, H.G. Bonacorso, Chem. Rev. 108, 2015 (2008)
A.K. Chakraborti, S.R. Roy, D. Kumar, P. Chopra, Green Chem. 10, 1111 (2008)
P. Wasserscheid, T. Welton, Ionic Liquids in Synthesis (Wiley-VCH, Weinheim, 2003)
J.D. Holbrey, R.D. Rogers, R.A. Mantz, P.C. Trulove, V.A. Cocalia, A.E. Visser, J.L. Anderson, J.L. Anthony, J.F. Brennecke, E.J. Maginn, T. Welton, Ionic Liquids in Synthesis (Wiley, Weinheim, 2008)
T. Welton, Coord. Chem. Rev. 248, 2459 (2004)
C. Yue, A. Mao, Y. Wei, M. Lü, Catal. Commun. 9, 1571 (2008)
S. Tang, G.A. Baker, H. Zhao, Chem. Soc. Rev. 41, 4030 (2012)
M.M.A. Pereira, Curr. Org. Chem. 16, 1680 (2012)
Q. Zhang, S. Zhang, Y. Deng, Green Chem. 13, 2619 (2011)
F. Shirini, N.G. Khaligh, S. Akbari-dadamahaleh, J. Mol. Catal. A 365, 15 (2012)
B.C. Ranu, L. Adak, S. Banerjee, Can. J. Chem. 85, 366 (2007)
E.C. Taylor, R.J. Knopf, R.F. Meyer, A. Holmes, M.L. Hoefle, J. Am. Chem. Soc. 82, 5711 (1960)
G. Heckmann, T. Bach, Angew. Chem. Int. Ed. 44, 1199 (2005)
H. Xu, H. Tang, H. Feng, Y. Li, Eur. J. Med. Chem. 73, 46 (2014)
F. Grams, H. Brandstetter, S. DAlò, D. Geppert, H.W. Krell, H. Lenert, V. Livi, E. Menta, A. Oliva, G. Zimmermann, Biol. Chem. 382, 1277 (2001)
F.N.M. Naguib, D.L. Levesque, E.-C. Wang, R.P. Panzica, M.H.E. Kouni, Biochem. Pharmacol. 46, 1273 (1993)
X. Chen, K. Tanaka, F. Yoneda, Chem. Pharm. 38, 307 (1990)
H. Wamhoff, Adv. Heterocycl. Chem. 38, 229 (1985)
J.A. Valderrama, P. Colonelli, D. Vásquez, M.F. González, J.A. Rodríguez, C. Theoduloz, Bioorgan. Med. Chem. 16, 10172 (2008)
M. Nogueras, J. Cobo, M.L. Quijano, M. Melguizo, A. Sánchez, Nucleosides Nucleotides 13, 447 (1994)
A.H. Shamroukh, M.E. Zaki, E.M. Morsy, F.M. Abdel-Motti, F.M. Abdel-Megeid, Arch. Pharm. (Weinh.) 340, 236 (2007)
B.D. Dhorajiya, B.Z. Dholakiya, R.M. Mohareb, Med. Chem. Res. 23, 3941 (2014)
A.S. Waghmare, S.S. Pandit, J. Saudi Chem. Soc. 21, 286 (2017)
G. Thirupathi, M. Venkatanarayana, P.K. Dubey, Y.B. Kumari, Chem. Sci. Trans. 2, 441 (2013)
J.M. Khurana, K. Vij, Catal. Lett. 138, 104 (2010)
J.T. Li, M.X. Sun, Aust. J. Chem. 62, 353 (2009)
J. Li, H. Dai, D. Liu, T. Li, Synth. Commun. 36, 789 (2006)
X.Z. Chun Wang, J.J. Ma, X. Zhou, P.C. Zhi Wang, Y.J. Gao, Synth. Commun. 35, 2759 (2005)
Y. Hu, Z.C. Chen, Z.G. Le, Synth. Commun. 34, 4521 (2004)
Z. Ren, W. Cao, W. Tong, X. Jing, Synth. Commun. 32, 1947 (2002)
N. Daneshvar, F. Shirini, M.S.N. Langarudi, R. Karimi-Chayjani, Bioorg. Chem. 77, 68 (2018)
F. Shirini, M.S.N. Langarudi, N. Daneshvar, J. Mol. Liq. 234, 268 (2017)
O.G. Jolodar, F. Shirini, M. Seddighi, Chin. J. Catal. 38, 1245 (2017)
M. Bararjanian, S. Balalaie, B. Movassag, A.M. Amani, J. Iran. Chem. Soc. 6, 436 (2009)
M.A. Zolfigol, R. Ayazi-Nasrabadi, S. Baghery, Appl. Organomet. Chem. 30, 273 (2016)
B. Sabour, M. Peyrovi, M. Hajimohammadi, Res. Chem. Intermed. 41, 1343 (2015)
N. Montazeri, Int. J. Nano Dimens. 6, 283 (2015)
A. Mobinikhaledi, N. Foroughifar, Inorg. Nano-Met. Chem. 40, 179 (2010)
O. Goli-Jolodar, F. Shirini, M. Seddighi, J. Iran. Chem. Soc. 13, 457 (2016)
J. Albadi, A. Mansournezhad, T. Sadeghi, Res. Chem. Intermed. 41, 8317 (2015)
A. Khazaei, H.A.A. Nik, A.R. Moosavi-Zare, J. Chin. Chem. Soc. 62, 675 (2015)
D.N. Chavan, D.R. Patil, D.R. Kumbhar, M.B. Deshmukh, Chem. Sci. Rev. Lett. 4, 1051 (2015)
J. Yang, F. Mei, S. Fu, Y. Gu, Green Chem. 20, 1367 (2018)
B. Jiang, T. Rajale, W. Wever, S.J. Tu, G. Li, Chem. Asian J. 5, 2318 (2010)
J.E. Biggs-Houck, A. Younai, J.T. Shaw, Curr. Opin. Chem. Biol. 14, 371 (2010)
E. Ruijter, R. Scheffelaar, R.V.A. Orru, Angew. Chem. Int. Ed. 50, 6234 (2011)
A. Hasaninejad, M. Shekouhy, N. Golzar, A. Zare, M.M. Doroodmand, Appl. Catal. A Gen. 402, 11 (2011)
J. Albadi, M. Jalali, A. Momeni, Res. Chem. Intermed. 44, 2395 (2018)
J. Albadi, A. Momeni, A. Mansournezhad, Jordan J. Chem. 12, 233 (2017)
J. Albadi, A. Alihoseinzadeh, A. Razeghi, Catal. Commun. 49, 1 (2014)
J. Albadi, J.A. Shrini, A. Mansournejhad, J. Chem. Sci. 126, 147 (2014)
H. Tajik, I. Mohammadpoor-Baltork, J. Albadi, Synth. Commun. 37, 323 (2007)
F. Shirini, M.A. Zolfigol, A.R. Aliakbar, J. Albadi, Synth. Commun. 40, 1022 (2010)
W. Dai, Y. Zhang, Y. Tan, X. Luo, X. Tu, Appl. Catal. A Gen. 514, 43 (2016)
P. Wasserscheid, M. Sesing, W. Korth, Green Chem. 4, 134 (2002)
K. Qiao, C. Yokoyama, Catal. Commun. 7, 450 (2006)
Acknowledgements
We are grateful to the Research Council of Shahrekord University for support of this research. The authors would like to thank Dr. Heshmat A. Samimi for reading this manuscript and giving helpful assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karami, S., Momeni, A.R. & Albadi, J. Preparation and application of triphenyl(propyl-3-hydrogen sulfate)phosphonium bromide as new efficient ionic liquid catalyst for synthesis of 5-arylidene barbituric acids and pyrano[2,3-d]pyrimidine derivatives. Res Chem Intermed 45, 3395–3408 (2019). https://doi.org/10.1007/s11164-019-03798-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03798-0