Abstract
A 2,2′-Bipyridine-based ionic compound named 2,2′-bipyridinium dihydrogen phosphate was synthesized by addition of phosphoric acid to a solution of 2,2′-Bipyridine in dichloromethane. After the characterization using FT-IR, mass, 1H, 13C and 31P NMR techniques, it was used as a Bronsted dicationic acidic catalyst for the promotion of the synthesis of 2-arylidene malononitrile and 5-arylidene barbituric acid derivatives via Knoevenagel condensation reaction in water. Some of the advantages of this method are the utilization of an easy preparable, cost-effective and eco-friendly organic salt as a catalyst within high rates and yields of the reactions, simple and quick work-up and acceptable reusability of the catalyst.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ionic liquids are known for their unique properties such as recyclability, low vapor pressure, tunable viscosity, good thermal and chemical stability and the ability to dissolve many organic and inorganic substances [1,2,3].
Brönsted acidic ionic liquids (BAILs) are a category of ionic liquids that contain protic acidic sites in their structures, so in addition to the properties of general ionic liquids, they also have acidic properties. Because that the number of possible combinations and nature of cations and anions in ionic liquids determines their physical and chemical properties [4, 5], halides, tetrafluoroborates, hexafluorophosphates, nitrates, phosphates, sulfates and sulfonates are common anions used in the preparation of acidic ionic liquids [6]. Due to the non-toxicity and less pollution of phosphoric acid in comparison with other conventional mineral acids, the use of phosphorous anion in the preparation of ionic liquids is more eco-friendly. In this way, some of the BAILs containing phosphate anion have been synthesized and their structures, properties and applications have been studied. Some of these compounds are choline dihydrogen phosphate (CDHP) as a buffer ionic liquid [7], l-butyl-3-methylimidazolium dihydrogen phosphate ([Bmim][H2PO4]) as a catalyst for the Mannich reaction [8] and Esterifications [9], diammonium hydrogen phosphate (DHP) as a catalyst for the synthesis of 2-aminothiazole and 2-iminothiazolidine derivatives [10], a series of alkylammonium salts ([Et3NH][H2PO4], [Et2NH2][H2PO4], [EtNH3][H2PO4]) as catalyst and medium for Fischer esterification reaction [11] and [Et3NH][H2PO4] and [Me3NH][H2PO4] in the cracking of dialkoxypropanes [12].
The Knoevenagel condensation is an important reaction that occurs between an aldehyde (or ketone) and a compound that consists of an active methylene moiety leading to a carbon–carbon double bond formation [13]. In this regard, the Knoevenagel condensation reaction between malononitrile and aldehydes is a suitable way for the preparation of alkenes containing electron-withdrawing groups caused them to be used as various chemical intermediates in a variety of organic reactions [14,15,16,17,18].
Barbituric acid contains an active methylene group and because of it can be entered in condensation reactions with aldehydes or ketones. This cyclic amide having a pKa = 4.01 in water. It is partially soluble in solvents such as water and methanol [19]. The reaction of barbituric acid with carbonyl compounds was studied since 1864 [20]. The products of this reaction are useful as intermediates in the synthesis of heterocyclic compounds [21], benzyl barbituric derivatives [22], oxadiazaflavines [23], and unsymmetrical disulfides [24].
Due to its importance and applications, different catalysts and conditions have been employed for the Knoevenagel reaction which of the [(DABCO)2C3H5OH].2Cl [25], Bis-Su [26], [C4(MIm)2].2Cl [27], [C4(MIm)2].2HSO4 [27], γ-Fe2O3@SiO2@[Bis-APTES]Cl2 NPs [28], Taurine [29, 30], [H2-pip][H2PO4]2 [31], H2-Bsim[ClO4]2 [32] and H2-Bim[HSO4]2 [32] are the most important ones.
Experimental
Materials
All chemicals were obtained from Fluka, Merck and Aldrich Chemical Companies. All yields refer to isolated pure products. Products were characterized by their physical constants, comparison with authentic samples and IR and NMR spectroscopy. The purity determination of the substrate and reaction monitoring was accompanied by thin-layer chromatography (TLC) on silica-gel polygram SILG/UV 254 plates.
Instrumentation
The FT-IR spectra were recorded on a Perkin- Elmer Spectrum BX series spectrophotometer as neat films on KBr pellets. The 1H NMR (500 MHz), 13C NMR (125 MHz) and 31P NMR (202 MHz) spectra were run on a Bruker AVANCE spectrometer in DMSO using TMS as an internal standard (δ in ppm). Melting points were recorded on a Büchi B-545 apparatus in open capillary tubes.
Preparation of 2,2′-bipyridinium dihydrogen phosphate ([H2-Bpy][H2PO4]2)
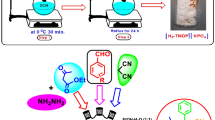
In a 100 mL round-bottomed flask in an ice bath, 2,2′-bipyridine (10.0 mmol, 1.562 g) was dissolved in 30.0 mL dichloromethane. Then an excess amount of phosphoric acid 85% (w/w) (2.0 mL ~ 28 mmol) was added drop-wise to it. During the addition of H3PO4, precipitation of the ionic product is observable. After completion of the addition, the mixture was stirred for an additional 24 h. Then, the solvent was decanted. The obtained white solid was washed with diethyl ether (3 × 30 mL) and ethanol (2 × 20 mL). Finally, the solid was completely dried using a rotary system and the desired [2,2′-bipyridine]-1, 1′-diium dihydrogen phosphate was obtained in 93% yield (Scheme 1).
Spectral and physical data of [H2-Bpy][H2PO4]2
M.P: 250 °C Dec; FT-IR (KBr, cm−1) υmax: 3103, 2707, 2330, 1708, 1604, 1583, 1528, 1455, 1435, 1356, 1224, 953, 807, 751, 608, 545; 1H NMR (500 MHz, DMSO) δ (ppm): 7.42 (m, 2H), 7.92 (t, J = 8.5 Hz, 2H), 8.38 (d, J = 7.5 Hz, 2H), 8.70 (s, 2H), 10.53 (S, 6H); 13C NMR (125 MHz, DMSO) δ (ppm): 121.1, 124.9, 138.1, 149.7, 155.6; 31P NMR (202 MHz, DMSO) δ (ppm): 2.26.
General procedure for the synthesis of 2-arylidene malononitriles in the presence of [H2-Bpy][H2PO4]2
In a 25 mL round-bottomed flask a mixture of an aromatic aldehyde (1.0 mmol), malononitrile (1.1 mmol) and [H2-Bpy][H2PO4]2 (10 mg) in water (5 mL) was stirred in an oil-bath at 70 °C. The progress and completion of the reaction were monitored by thin-layer chromatography (TLC) using n-hexane: ethyl acetate (7:3) as an eluent. After completion of the reaction, the obtained residue was separated by filtration and washed several times with water. Finally, the product was dried at room temperature and characterized by its spectral data and melting points while compared with the authentic samples.
General procedure for the synthesis of 5-arylidene barbituric acids in the presence of [H2-Bpy][H2PO4]2
In a 25 mL round-bottomed flask a mixture of aldehyde (1.0 mmol), barbituric acid (1.0 mmol) and [H2-Bpy][H2PO4]2 (10 mg) was stirred in water (5 mL) and heated in an oil-bath at 70 °C. The progress and completion of the reaction were monitored by TLC [n-hexane: ethyl acetate: EtOH (7:3:1)]. After completion, the product was separated by filtration and washed several times with water. Finally, the product was dried at 50 °C and characterized by its spectral data, melting point and comparison with authentic samples.
Results and discussion
Based on the obtained structural information and our previous work with bpy (SO3H)2(HSO4)2 [34], it is predicted that [H2-Bpy][H2PO4]2 can be used as an efficient catalyst to promote the reactions needing acidic catalysts to speed-up, so we were interested to investigate the performance of this catalyst in the acceleration of the Knoevenagel condensation reaction for the preparation of 2-arylidene malononitrile and 5-arylidene barbituric acid derivatives.
Characterization of the catalyst
After preparation of the [H2-Bpy][H2PO4]2, various spectroscopic techniques were used to characterize the structure of the compound. Here are some elucidations in detail:
Comparison of the solubility and melting points
Investigations on the solubility of 2,2′-bipyridine and [H2-Bpy][H2PO4]2 revealed that while 2,2′-bipyridine quickly dissolves in dichloromethane and ethanol the ionic product is completely insoluble in these solvents. Moreover, [H2-Bpy][H2PO4]2 dissolves in water very fast while 2,2′-bipyridine is slightly soluble in it.
A comparison between the melting points showed that [H2-Bpy][H2PO4]2 be decomposed at temperatures higher than 250 °C, while the precursor melts around 70–73 °C.
The above macroscopic differences prove that the nature of 2,2′-bipyridine is changed in the designed ionic structure.
FT-IR analysis
In the FT-IR spectrum of 2,2′-bipyridinium dihydrogen phosphate, the broadening in the area of 2100–3100 cm−1 is attributed to acidic N−H and O−H stretching vibrations. P=O stretching vibration is observed at 1224 cm−1 and the two peaks at 953, and 807 cm−1 are assigned to asymmetric and symmetric stretching vibrations of P−O.
NMR spectroscopy
In the 1H NMR spectrum of [H2-Bpy][H2PO4]2, four peaks related to eight hydrogens of the 2,2′-bipyridine ring are observed in the aromatic area. Single peak with the integral of six is related to six acidic hydrogens of the compound (two NH+ and two H2PO‾4) which are appeared in the same chemical shift.
The 13C NMR spectrum of [H2-Bpy][H2PO4]2 as a symmetrical compound, shows 5 peaks in the aromatic area related to five types of carbons.
In the 31P NMR spectra of [H2-Bpy][H2PO4]2 as a symmetrical compound, one peak at 2.26 ppm is observed which is related to the phosphorus of dihydrogen phosphate anion. This single peak proves the presence of H2PO4 anion in the structure of the catalyst.
Mass spectroscopy
In the mass spectrum of [H2-Bpy][H2PO4]2, the molecular ion peak (M+) appeared at m/e = 352.0, which is corresponding to the mass of the catalyst. Also, other peaks related to the other fragmentations such as bipyridine at m/e = 156.2 and pyridine m/e = 78.0 can be observed.
Optimizations and derivations
To evaluate the effectiveness of various factors in the Knoevenagel reactions, the influence of different solvents, temperatures and the amounts of the catalyst was investigated in the preparation of 4-chlorobenzaldehyde derivatives of both of the target reactions (Tables 1 and 2).
The obtained data showed that the best results can be achieved in the presence of 10 mg of [H2-Bpy][H2PO4]2 in the water at 70 °C (Scheme 2).
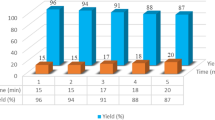
After the optimization studies, a variety of 2-arylidene malononitrile and 5-arylidene barbituric acid derivatives were prepared efficiently from the reaction of malononitrile or barbituric acid with different types of aldehydes in the presence of [H2-Bpy][H2PO4]2 (Tables 3 and 4).
The results showed that all the products can be obtained in high yields during short reaction times, without the considerable influence of the electron-donating or -withdrawing substituents located on the aromatic ring of the aldehyde.
Reusability of the catalyst
To show the reusability potential of the catalyst, the synthesis of compounds 1d and 2e was studied again. In these investigations and after the completion of the reactions, the products were isolated by filtration and the same reactions repeated in the filtrated solution without the addition of extra amounts of the catalyst. The results show that this catalyst can be reused at least four times in both of the mentioned reactions without significant loss of its catalytic activity.
Mechanistic study
The proposed mechanism for the synthesis of 2-arylidene malononitrile in the presence of [H2-Bpy][H2PO4]2 is shown in Scheme 3. Based on this mechanism, at first aldehyde and malononitrile are activated by the acidic protons of the catalyst. After that, the activated aldehyde is attacked by the activated nitrile compound to obtain intermediate (Ia). After the elimination of a molecule of water, the corresponding 2-arylidene malononitrile is achieved. This scheme also presents a suggested mechanism for the catalytic role of [H2-Bpy][H2PO4]2 in the 5-arylidene barbituric acid synthetic process. As can be seen, the catalyst enolized barbituric acid attacks the aldehyde which is also activated by the acidic proton of the catalyst. After the elimination of a water molecule from (Ib), the desired 5-arylidene barbituric acid derivative is achieved.
Comparison of the catalytic ability
To demonstrate the efficiency of the proposed method, and also carry out a correct comparison and investigation of the produced catalyst function, the results related to the preparation of the Knoevenagel condensation products have been compared with the same results obtained using other catalysts. In all cases, the introduced catalyst show advantages from the view-point of lesser amounts of the used catalyst, lower temperature and the lack of use of organic solvents (Table 5).
Conclusion
In this work, we described an efficient and clean synthetic protocol for the preparation of arylidene malononitrile and arylidene barbituric acid derivatives catalyzed by [H2-Bpy][H2PO4]2 as a new Brönsted acidic ionic liquid via Knoevenagel condensation reactions. This method offers important benefits such as the use of non-toxic catalyst and solvent, high rates and yields of the reactions, ease of work-up and separation of the products and high thermal stability and good reusability of the catalyst.
References
S. Keskin, D. Kayrak-Talay, U. Akman, O. Hortaçsu, J. Supercrit. Fluids. 43, 150 (2007)
P. Wasserscheid, T. Welton (2008) Ionic liquids in synthesis, John Wiley & Sons 1, p. 619
P. Wasserscheid, W. Keim, Angew. Chem. Int. Ed. 39, 3772 (2000)
C. Chiappe, D. Pieraccini, J. Phys. Org. Chem. 18, 275 (2005)
Z. Zamiraei, M. Golzar, H. Hamidi, Adv. J. Chem. A 1, 105 (2018)
S.T. Handy, Curr. Org. Chem. 9, 959 (2005)
D.R. MacFarlane, R. Vijayaraghavan, H.N. Ha, A. Izgorodin, Chem. Commun. 46, 7703 (2010)
G. Zhao, T. Jiang, H. Gao, B. Han, J. Huang, D. Sun, Green Chem. 6, 75 (2004)
J. Fraga-Dubreuil, K. Bourahla, M. Rahmouni, J.P. Bazureau, J. Hamelin, Catal. Commun. 3, 185 (2002)
S. Balalaie, S. Nikoo, S. Haddadi, Synth. Commun. 38, 2521 (2008)
P.A. Ganeshpure, G. George, J. Das, J. Mol. Catal. A Chem. 279, 182 (2008)
C. Wang, L. Guo, H. Li, Y. Wang, J. Weng, L. Wu, Green Chem. 8, 603 (2006)
L.F. Tietze, Pergamon. Oxford 32, 341 (1991)
F. Freeman, Chem. Rev. 69, 591 (1969)
R.H. Vekariya, H.D. Patel, Synth. Commun. 44, 2756 (2014)
E. Bombardelli, A. Cristoni, P. Morazzoni (1997) U.S. Patent 5,665,365
A. Corma, R.M. Martin-Aranda, Appl. Catal. A 105, 271 (1993)
L.F. Tietze, J. Heterocycl. Chem. 27, 47 (1990)
M. Windholtz, D. W. Green (1983), The merck index, 10th edn. by N. J. Rahway: Merck & Co (eds.) Inc.
A. Baeyer, Liebigs Ann. Chem. 130, 129 (1864)
J.T. Bojarski, J.L. Mokrosz, H.J. Bartoń, M.H. Paluchowska, Adv. Heterocycl. Chem. 38, 229 (1985)
Q. Yan, R. Cao, W. Yi, Z. Chen, H. Wen, L. Ma, H. Song, Eur. J. Med. Chem. 44, 4235 (2009)
J.D. Figueroa-Villar, E.R. Cruz, N. Lucia dos Santos, Synth. Commun. 22, 1159 (1992)
K. Tanaka, X. Chen, F. Yoneda, Tetrahedron 44, 3241 (1988)
M. Zabihzadeh, A. Omidi, F. Shirini, H. Tajik, M.S.N. Langarudi, J. Mol. Struct. 1206, 127730 (2020)
F. Hassanzadeh, N. Daneshvar, F. Shirini, M. Mamaghani, Res. Chem. Intermed. 46, 4971 (2020)
Z. Sharifi, N. Daneshvar, M.S.N. Langarudi, F. Shirini, Res. Chem. Intermed. 45, 4941 (2019)
R. Karimi-Chayjani, N. Daneshvar, F. Shirini, H. Tajik, Res. Chem. Intermed. 45, 2471 (2019)
F. Shirini, N. Daneshvar, RSC Adv. 6, 110190 (2016)
N. Daneshvar, F. Shirini, M.S.N. Langarudi, R. Karimi-Chayjani, Bioorg. Chem. 77, 68 (2018)
S. Darvishzad, N. Daneshvar, F. Shirini, H. Tajik, J. Mol. Struct. 1178, 420 (2019)
N. Daneshvar, M. Nasiri, M. Shirzad, M.S.N. Langarudi, F. Shirini, H. Tajik, New J. Chem. 42, 9744 (2018)
N. Safari, F. Shirini, H. Tajik, J. Iran. Chem. Soc. 16, 887 (2019)
F. Shirini, M. Abedini, N. Mahmoodi, M. Biglari, M.S.N. Langrudi, Phosphorus Sulfur Silicon Relat. Elem. 190, 1912 (2015)
Acknowledgements
We are thankful to the Research Council of the University of Guilan for the partial support of this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Darvishzad, S., Daneshvar, N., Shirini, F. et al. Knoevenagel condensation in aqueous media promoted by 2,2′-bipyridinium dihydrogen phosphate as a green efficient catalyst. Res Chem Intermed 47, 2973–2984 (2021). https://doi.org/10.1007/s11164-021-04445-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04445-3