Abstract
Global warming, fossil fuel depletion and fuel price increases have motivated scientists to search for methods for the storage and reduction of the amount of greenhouse gases, especially CO2. The hydrogenation process has been introduced as an emerging method of CO2 capture and convertion into value-added products. In this study, new types of catalysts are introduced for CO2 hydrogenation and are compared based on catalytic activity and product selectivity. The physical properties of the samples are specified using BET. Iron catalysts supported on γ-Al2O3 with different metal promoters (X = Ni, K, Mn, Cu) are prepared through the impregnation method. Moreover, Fe–Ni catalysts supported on HZSM5-Al2O3 and Ce–Al2O3 are synthesized. Samples are reduced by pure H2 and involved in hydrogenation reaction in a fixed bed reactor (H2/CO2 = 3, total pressure = 10 MPa, temperature = 523 K, GHSV = 2000, 1250 nml/min). All catalysts provide high conversion in hydrogenation reactions and the results illustrate that the selectivity of light hydrocarbons is higher than that of methane and CO. It is found that Ni has a promoting effect on the conversion fluctuations throughout the reaction with 66.13% conversion. Using combined supported catalysts leads to enhancing catalytic performance. When Fe–Ni/γ–Al2O3—HZSM5 is utilized, CO2 conversion is 81.66% and the stability of the Fe–Ni catalyst supported on Al2O3 and Ce–Al2O3 furthey improves.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global concerns such as earth temperature increase and climate change has put the world under pressure to limit the emissions of greenhouse gases and to find different routes of controlling the amount of them. CO2 is a greenhouse gas and its amount in the atmosphere has increased rapidly due to the industrial revolution. Therefore, attempts to decrease CO2 concentrations seem to be necessary. In addition, discharge of fossil fuels and increasing fuel prices have led scientists to study new economical and enironmentally frienly fuels [44,45,46]. Usage of CO2 is a strategy that either converts CO2 into the product or uses it directly in specific applications. The CO2 conversion process as a source of carbon for value-added products and fuels has been considered as a possible remedy for fossil fuel depletion and the global warming problem [6]. According to the free energy tables, CO2 is a thermodynamically stable component and it seems that hydrogen with a high energy level can be used as the reagent for CO2 transformation. It has been claimed that the hydrogenation reaction is one of the most important chemical reactions for converting carbon dioxide [7, 20, 36]. Carbon dioxide can be converted to hydrocarbons by a direct route shown in Eqs. (1)–(3). All heats of reaction are at 300 °C.
Generally, hydrogenation of CO2 can be categorized into two main groups according to the final product of the process resulting from various catalysts and operation conditions, such as fuels (hydrocarbons) and chemicals (DME and methanol). Both of these categories have advantages in transport and storage [8, 19, 31, 33]. A thermodynamic analysis has been performed to evaluate the optimal conditions and limitations of CO2 conversion to methane and methanol from a flue gas exhaust stream. It has been shown that the production of CH4 is more favorable than methanol from CO2 hydrogenation considering thermodynamical concepts [32].
Low-temperature catalytic conversion of carbon dioxide into methane has been carried out over Rh/γ–Al2O3 catalysts, and 100% selectivity for methane production was achieved [24]. The Co–Cu–ZrO2 nanomaterial catalyst was synthesized by the reverse co-precipitation method and used for CO2 hydrogenation. The result showed 58% methane selectivity, 1.27 times higher than the original Co–ZrO2 catalyst, and 42% CO2 conversion [43]. There is adequate information for CO2 hydrogenation to methane but the process is not recommended with respect to oxygenated (methanol, DME), because it includes more consumption of H2, more difficult storage, and lower energy production per volume [15]. Thus, synthesis of other kinds of fuels except methane especially as value-added products is indicated.
CO2 hydrogenation for hydrocarbons production goes through a two-step reaction: reverse water–gas shift (RWGS) and Fischer–Tropsch (FT) synthesis. Catalyst type, operating conditions and types of reactor are the factors that have effects on the process improvement [15]. According to Riedel et al. [38], the calculated equilibrium conversion of the CO2 hydrogenation reaction was about 72% and actual conversions were between 46 and 53%. Generally, CO2 conversion and selectivity for C2–C5+ have been reported in the range from 19% to 68% and 80%, respectively, in the literature [18, 35, 38, 47]. Fe/Co mixed catalysts have showed low selectivity for the favorable hydrocarbons, with 70 mol% methane production over a Co/Al2O3 catalyst in CO2 hydrogenation [26, 39, 48].
Synthesis of hydrocarbons through carbon dioxide hydrogenation was investigated by Nam et al. [34] using iron supported on alkali metal (Li, Na, K, Rb) ion-exchanged Y-zeolite catalysts in the hydrogenation reaction. The results illustrated that metal exchange in zeolite-Y affects the activity and selectivity of the catalysts by increasing the basicity of the catalyst surface.
CO2 was converted into iso-alkanes over Fe–Zn–M/Zeolite composite catalysts with different promoters (M=Cr, Mn, Zr, Al, La) in a study by Rongxian et al. [40]. It was found that Zr had a better promoting effect than the other promoters and that the acidity of the zeolite affected the hydrocarbon distribution and catalytic performance.
Satthawong et al. [42] reported that the addition of K promoter over Fe–Co bimetallic Al2O3-supported catalysts led to a significant increase in C2–C4 olefin formation in CO2 hydrogenation. They suggested that the result is due to the increasing effect of K on CO2 adsorption and its decreasing effect on H2 adsorption on the catalyst surface. Iron is recommended as an active site, and γ-Al2O3 and potassium are also suggested as the best support and promoter, respectively, for carbon dioxide hydrogenation via a two-step process [3, 18, 26, 35, 38, 39, 47, 48].
Lee et al. [28] examined the perfomance of CO2 hydrogenation using K promoter with Fe as an active site in different binders of alumina support. It was reported that alumina Fe-K/γ-Al2O3 (FA-A catalyst) was efficient for CO2 hydrogenation, while Fe-K/γ-Al2O3 (FS A catalyst) dramatically decreased the capability of the process.
Al-Dossary et al. [4] prepared a mesoporous structure catalyst, 0.05 Mn-Fe, using a one-step sol–gel method and used this high specific surface area catalyst in the CO2 hydrogenation process. The results showed that the catalyst was active in non-oxygenated hydrocarbon production (67.1% C2–C6+, C1 29.3%) and resulted in a small amount of CO production (7.7%).
Selective formation of light olefins of about 53.58% was observed in CO2 hydrogenation using Fe–Zn–K catalysts prepared via hydrothermal and impregnation procedures. Additionally, catalysts which had uniform particles showed high activity for the CO2 conversion [4].
Recently, Carlo Giorgio Visconti et al. [43] synthesized a K–Fe catalyst through thermal decomposition and impregnation processes. The catalyst provided high activation in CO2 hydrogenation into lower olefins at 5 bar. They proposed that hydrocarbons can be formed from CO produced as a primary component in the RWGS reaction.
Using N-functionalized carbon nanotubes as support in a K–Mn–Fe catalyst resulted in a suitable iron active phase for alkenes production in the CO2 hydrogenation process [23].
It has been reported that the addition of Mn to iron-based catalysts increases CO2 conversion and decreases methane formation via its structural and electrical properties [11, 18, 29, 47]. As a promoter, Cu acts naturally as Mn in iron-based CO2 hydrogenation and leads to increasing the dispersion of the catalyst components [30]. It has been demonstrated that K acts as an electrical promoter in FT and increases the CO2 canversion by donating electron density to the vacant d orbital of the iron [11,12,13, 18]. It has been reported that the addition of Ni to iron-based catalysts improves catalytic activity. It can be used as an active promoter in the C–C coupling process during CO hydrogenation under FTS reaction conditions so it can also be suggested for CO2 hydrogenation [17]. Using ZSM5 in the catalyst structure led to a higher conversion of carbon monoxide and enhanced selectivity shift from methane to higher hydrocarbons based on its ascidic-shape selective characteristic. The researches showed that CO2 conversion increased with adding Ce to the structure of catalyst. Ce is a WGS catalyst and could be considered as an ideal promoter for iron-based catalysts [16, 37].
In the current work, we have studied the effects of different promoters and combined supports on CO2 hydrogenation, CO2 conversion, hydrocarbon distribution, fluctuation of conversion throughout the reaction time and physical properties of samples. The synthesis of Iron catalysts with the promoters Ni, K, Cu and Mn on Al2O3 support and also a Fe–Ni catalyst on HZSM5-Al2O3 and Ce–Al2O3 combined supports were carried out through impregnation, while the combined supports were prepared via some specific techniques. The objective of this work is to recommend effective catalysts for CO2 hydrogenation.
Experimental
Catalyst preparation
All metals were purchased from Merck. Industrial gamma alumina has a surface area of 128 mm2/g which was used for the synthesized catalysts. All catalysts were prepared based on the conventional impregnation method by adding aqueous solutions of the metals into specific supports in the nominal composition of 0.35 promoter/1Fe/5 support. The aqueous solutions were composed of Fe(NO3)3·6H2O as active metal and one of the KMnO4, K2CO3, Cu(NO3)3·9H2O and Ni(NO3)2·6H2O compounds as promoter. In addition, Al2O3, Al2O3–HZSM5 and Ce-Al2O3 were used as different supports. As the first step, Al2O3 was entirely dried in a rotary drier for 1 h. The synthesis process of the two other supports and impregnation information is explained in the following sections.
Al2O3–Ce support preparation
Alumina and Ce(NO3)3·6H2O with 12 Ce/100Al weight ratio were mixing in a flask in the presence of deionized water for 6 h in order to prepare the Al2O3–Ce support. Then, the impregnated sample was dried at 100 °C for 12 h. Finally, the sample was calcined at 500 °C in ambient air for 6 h.
Al2O3-HZSM5 support preparation
In order to prepare the Al2O3–HZSM5 support, aqueous solutions of NaZSM5 and NH4NO3 (1 molar solution) were mixed at 40 °C for 4 h. Then, the solution was filtered and the precipitate was dried at 100 °C for 12 h. Subsequently, the dried precipitate was calcined at 500 °C for 6 h. This process was repeated three to five times and HZSM5 was obtained. Thereafter, the HZSM5 and Al2O3 in the same weight ratios were combined for 6 h and the impregnated sample dried at 100 °C for 12 h and calcined at 500 °C for 6 h.
Impregnation of the aqueous solution on supports
As the final step for the synthesis of CO2 hydrogenation catalysts, the aqueous solution of Fe, and one of the promoters were added to the support and were mixed homogeneously for 6 h. Then, the samples were dried at 120 °C for 12 h in ambient air and calcined at 500 °C for 6 h.
Process set up and operating condition
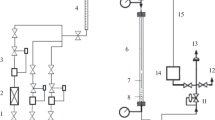
Process of CO2 hydrogenation was carried out in a catatest set up with a stainless steel fixed bed reactor in bench scale (1.6 cm inner diameter, 70 cm height) surrounded by an electric jacket. A schematic diagram of the process set up is shown in Fig. 1. Prior to the reaction, all catalyst samples were reduced in pure hydrogen for 10 h at the flow rate of 10 nml/min. Temperature and pressure of reduction were fixed at 400 °C and 1 bar, respectively. The reactant gases (H2 and CO2) were passed through an MFC (mass flow controller; Booker) at H2/CO2: 3/1 ratio. Reactions were carried out at 300 °C and the total flow rates of 1250 and 2000 ml/g-cat at STP condition. In addition, pressure was maintained at 10 bar using a back pressure regulator throughout the reaction. The outlet stream of the reaction was separated in a condenser and the gases were sent to a gas chromatography (Agilent 6890) equipped with HP-plotQ capillary column which uses H2 and He as internal standard gases. The consumption of the reactants and the production of different hydrocarbons were determined using thermal conductivity and flame ionization detectors (TCD and FID) results.
Characterization test using BET
The Brunauer–Emmett–Teller (BET) method was used to characterize the average pore volume, the mean pore diameter and the specific surface area of the catalyst samples. These physical properties were specified by a Quantachrome Autosorb 1 Automated Gas Sorption system (USA), using high-purity N2 adsorption–desorption isotherm at −196 °C (liquid nitrogen temperature) during 4 h.
Results and discussion
The effects of X promoter on the catalytic performance of Fe-Al2O3 supported catalyst
The CO2 conversion, CO selectivity and hydrocarbons distribution for different catalysts with X=K, Ni, Mn, Cu were calculated at GHSV = 2000 nml/min GHSV = 2000 nml/min and T = 300 °C and P = 10 bar. The results are illustrated in Table 1. The results showed that CO2 conversion and hydrocarbons distribution changes by using different promoters, according to their rules [11] The Mn promoter converted CO2 to products with the conversion of 71.70% and achieved the maximum conversion among other catalyst samples. Then, Ni, K and Cu had the conversions of 66.13, 65.35 and 63.17%, respectively. The samples generally showed a high tendency to form light hydrocarbons. For example, light hydrocarbons selectivity in the distribution of productions is 80.828% when Cu was applied. Furthermore, catatestMn promoter produced the highest CO mole percentage among all samples which is 8.732%. K–Fe/Al2O3 offered the methane selectivity of 13.014% which is the maximum value for methane production of all samples.
The low methane selectivity is related to the low concentration of the active carbon sites on the catalysts surface due to the CO2 low dissociation for acidic catalysts [43]. The increment of iron oxide concentration decreases the acidity of the catalysts surface leading to the reduction in methane selectivity.
The fluctuations of CO2 conversion throughout the reaction for different samples are shown in Fig. 2. When the promoter is Cu, the range of conversion fluctuations is the widest among all samples and there is a huge reduction in the amount of conversion during the reaction time. According to the results, Mn and Ni presented more stability in conversion throughout the reaction.
It has been reported that Mn acts as both a structural and electronic promoter. Applying the Mn promoter on the iron catalyst causes methane formation suppressing [1, 11, 18]. It is proposed that the Mn promoter raises the basicity of the catalyst’s surface. Using Mn has been reported to augment the reduction of the catalyst as well as the carburization and dispersion of Fe2O3-state being advanced by using Mn. The basicity of the catalyst surface is suppressed possibly by incorporating of the promoter into the iron lattice [29, 41]. Although Mn promotes catalyst activity in some aspects like increasing conversion, it leads to blocking the active sites of catalyst and decreasing CO conversion to hydrocarbons, which is the second step in CO2 hydrogenation [12, 29]. So, promoters other than Mn like Cu, Ni, K are preferred for the hydrogenation process.
It has been reported that the addition of Cu as a reducing metal raises the activity of the Fe catalyst and enhances the dispersion of the catalyst particles [5, 30]. It has been suggested that Cu promotes the reduction of the hematite particles during carburization. Active sites for the dissociability of the adsorbing hydrogen are created with reducing of the copper to its metallic form [18]. The metallic form of Cu supplies active sites for adsorbing dissociated hydrogen [41]. The copper performance is comparable with the Mn promoting performance in nature. According to the results, Cu showed more CO2 conversion and less fluctuations than the other promoters, so Ni and K are preferred as promoters.
It has been demonstrated that the electronic role of potassium in the FT process is more than its structural role, but generally the role of K promoter in CO2 hydrogenation and FT is not obviously specified [14, 41]. Adding the K promoter significantly affects the overall performance and also increases the CO2 conversion [9, 12, 25, 27]. It has been observed that the addition of K enhances the alkene/alkane ratio and shifts the products distribution to longer-chain hydrocarbons [12]. It has been demonstrated that K lowers the work function of metal via donating electron density to the iron d orbital, decreasing the H2 adsorption ability and increasing the adsorption of CO which results in the reduction in the hydrogenation of alkenes [11, 14]. Using K has been reported as a factor for increasing CO2 conversion [9, 27].
Nickel-based catalyst performance exhibits high CO2 conversion and offers more CH4 selectivity for the hydrogenation process [2, 21].
The effects of support on the performance of Fe–Ni catalyst
Table 2 shows the comparison between Fe–Ni catalysts on HAZM5-Al2O3 and Al2O3 supports in terms of CO2 conversion, CO selectivity and hydrocarbon distribution at GHSV = 2000 nml/min. Table 3 also illustrates the comparison between Fe–Ni catalysts on Ce–Al2O3 and Al2O3 supports based on the aforementioned parameters at GHSV = 1250 nml/min. According to the results, adding HZSM5 to Al2O3 increases CO2 conversion by 15.53% and enhances the composition of methane in the product. The conversion difference between Ce-Al2O3 and Al2O3 supports at GHSV = 1250 nml/min is not significant. However, methane selectivity by using Ce was three times less than a simple support. It has been demonstrated that decreasing GHSV from 2000 to 1250 nml/min results in the increment of conversion and methane selectivity. Figure 3 shows the fluctuations of CO2 conversion throughout the reaction for combined supports and Al2O3. As the figure indicates, the conversion changes are diminished by using combined supports. It is suggested that HZSM5 can have both a support role and a catalyitic role due to its high surface area and well-defined structure. Therefore, the conversion and the stability of catalyst increases by adding HZSM5 to the support [10, 22].
Ceria can be utilized on iron-based catalysts for WGS and RWGS reactions. It has beenis reported that using ceria and iron leads to large amounts of CO production from CO2. However, it has been shown that it prevents chain growth due to the active sites blocking of iron and deactivates the catalyst in the hydrogenation process [13, 16, 37]. According to these studies, HZSM5 is suggested as an added support to Al2O3 because it improves the conversion and stability of the catalyst throughout the reaction time.
Characterization of iron based catalysts
According to N2 adsorption–desorption isothermal diagrams, it can be concluded that the mesoporous structure of alumina is retained in all samples and that the impregnation process has no specific effect on the structure. Pore size of samples ranges from 57 to 60 nm and pore size distribution diagrams are similar. Specific surface area, average pore diameter and average pore volume for samples are summarized in Table 4. It is proposed that specific surface area for the metals on Al2O3 is directly related to the conversion and the values of specific surface area were obtained as 206.8, 205.8, 173.4 and 165.3 m2/g respectively in the presence of Mn, Ni, K and Cu promoters. Although catalysts with combined support Ce–Al2O3 offered maximum specific surface of about 347.2 m2/g, its catalytic performance was not the highest. The specific surface area of Fe–Ni/HZSM5–Al2O3 was low compared to other samples (166.5 m2/g); however, it provided high conversion and stability. Therefore, it is suggested that the catalytic activity for Fe–Ni/HZSM5–Al2O3 was useful.
Conclusion
An iron catalyst promoted by Ni, K, Mn, Cu on \(\gamma\)-alumina support was prepared and the catalytic activity of these samples were compared in the operating conditions. It was found that Ni showed a promoting effect on the conversion fluctuations throughout the reaction having the conversion of 66.13%. The catalytic performance of the Fe–Ni catalyst on HZSM5–Al2O3 and Ce–Al2O3 combined supports was studied. The results illustrated that HZSM5-Al2O3 increases CO2 conversion up to 81.66%. In addition, light hydrocarbons were the main product in the CO2 hydrogenation of all the catalyst samples. Moreover, methane production and CO2 conversion were increased with decreasing GHSV from 2000 to 1250 nml/min.
References
J. Abbott, N.J. Clark, B.G. Baker, Effects of sodium, aluminium and manganese on the fischer–tropsch synthesis over alumina-supported iron catalysts. J. Appl. Catal. 26, 141–153 (1986)
S. Abelló, C. Berrueco, D. Montané, High-loaded nickel–alumina catalyst for direct CO2 hydrogenation into synthetic natural gas (SNG). J. Fuel 113, 598–609 (2013)
A.N. Akin, M. Ataman, A.E. Aksoylu, Z.I. O¨nsan, CO2 fixation by hydrogenation over co-precipitated Co/Al2O3. React. Kinet. Catal. Lett. 76, 265–270 (2002)
M. Al-Dossary, A.A. Ismail, J.L.G. Fierro, H. Bouzid, S.A. Al-Sayari, Effect of Mn loading onto MnFeO nanocomposites for the CO2 hydrogenation reaction. J. Appl. Catal. B. 165, 651–660 (2015)
H. Ando, Q. Xu, M. Fujiwara, Y. Matsumura, M. Tanaka, Y. Souma, Hydrocarbon synthesis from CO2 over Fe–Cu catalysts. J. Catal. Today 45, 229–234 (1998)
M. Aresta, A. Dibenedetto, A. Angelini, The changing paradigm in CO2 utilization. J. CO2 Util. 3–4, 65–73 (2013)
M. Aresta, The fixation of carbon dioxide in inorganic and organic chemicals. J. Energy Convers. Manag. 34, 745–752 (1993)
G. Centi, S. Perathoner, Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. J. Catal. Today 148, 191–205 (2009)
P.H. Choi, K.-W. Jun, S.-J. Lee, M.-J. Choi, K.-W. Lee, Hydrogenation of carbon dioxide over alumina supported Fe–K catalysts. J. Catal. Lett 40, 115–118 (1996)
M. Dalil, M. Sohrabi, S.J. Royaee, Application of nano-sized cobalt on ZSM-5 zeo catalyst in Fischer-Tropsch synthesis. J. Ind. Eng. Chem. 18, 690–696 (2012)
R.W. Dorner, D.R. Hardy, F.W. Williams, H.D. Willauer, Heterogeneous catalytic CO2 conversion to value-added hydrocarbons. J. Energy Environ. Sci. 3, 884–890 (2010)
R.W. Dorner, D.R. Hardy, F.W. Williams, H.D. Willauer, K and Mn doped iron-based CO2 hydrogenation catalysts: detection of KAlH4 as part of the catalyst’s active phase. J Appl. Catal. A. 373, 112–121 (2010)
R.W. Dorner, D.R. Hardy, F.W. Williams, H.D. Willauer, Effects of ceria-doping on a CO2 hydrogenation iron–manganese catalyst. J. Catal. Commun. 11, 816–819 (2010)
M.E. Dry, T. Shingles, L.J. Boshoff, G.J. Oosthuizen, Heats of chemisorption on promoted iron surfaces and the role of alkali in Fischer–Tropsch synthesis. J. Catal. 15, 190–199 (1969)
P. Dumrongbunditkul, T. Witoon, M. Chareonpanich, T. Mungcharoen, Preparation and characterization of Co–Cu–ZrO2 nano materials and their catalytic activity in CO2 methanation. J. Ceram. Int. 42, 10444–10451 (2016)
Q. Fu, H. Saltsburg, M. Flytzani-Stephanopoulos, Active Nonmetallic Au and Pt Species on Ceria-Based Water-Gas Shift Catalysts. J. Science. 301, 935–938 (2003)
S. Golestan, A.A. Mirzaei, H. Atashi, CO hydrogenation reaction over nano structured Fee–Nie–Mn catalyst: kinetic and mechanistic studies. J. Nat. Gas Sci. Eng. 37, 280–290 (2017)
T. Herranz, S. Rojas, F.J. Perez-Alonso, M. Ojeda, P. Terreros, J.L.G. Fierro, Hydrogenation of carbon oxides over promoted Fe–Mn catalysts prepared by the micro emulsion methodology. J. Appl. Catal. A. 311, 66–75 (2006)
P. Jessop, F. Joó, Ch-Ch. Tai, Recent advances in the homogeneous hydrogenation of carbon dioxide. J. Coord. Chem. Rev. 248, 2425–2442 (2004)
Z. Jiang, T. Xiao, V.L. Kuznetsov, P.P. Edwards, Turning carbon dioxide into fuel. Philos. Trans. R. Soc. Lond. 368, 3343–3364 (2010)
V. Jiménez, C. Jiménez-Borja, P. Sánchez, A. Romero, E.I. Papaioannou, D. Theleritis, S. Souentie, S. Brosda, J. Luis Valverde, Electrochemical promotion of the CO2 hydrogenation reaction on composite Ni or Ru impregnated carbon nanofiber catalyst-electrodes deposited on YSZ. J. Appl. Catal. B. 107, 210–220 (2011)
S.-H. Kang, J. Wook Bae, K.-J. Woo, P.S. Sai Prasad, K.-W. Jun, ZSM-5 supported iron catalysts for Fischer–Tropsch production of light olefin. J. Fuel Process. Technol. 91, 399–403 (2010)
P. Kangvansura, L.M. Chew, W. Saengsui, P. Santawaja, Y. Poo-arporn, M. Muhler, H. Schulz, A. Worayingyong, Product distribution of CO2 hydrogenation by K- and Mn-promoted Fe catalysts supported on N-functionalized carbon nanotubes. J. Catal. Today 27, 59–65 (2016)
A. Karelovic, P. Ruiz, CO2 hydrogenation at low temperature over Rh/γ-Al2O3 catalysts: effect of the metal particle size on catalytic performances and reaction mechanism. J. Appl. Catal. B. 113–114, 237–249 (2012)
M. Khobragade, S. Majhi, K.K. Pant, Effect of K and CeO2 promoters on the activity of Co/SiO2 catalyst for liquid fuel production from syngas. J. Appl. Energy 94, 385–394 (2012)
J.-S. Kim, S. Lee, S.-B. Lee, M.-J. Choi, K.-W. Lee, Electrochemical and XAFS studies of effects of carbonate on the oxidation of arsenite. J. Catal. Today 115, 228–234 (2006)
D.L. King, J.B. Peri, An infrared study of nitric oxide chemisorption on alumina-supported iron and alkalized iron Fischer–Tropsch catalyst. J. Catal. 79, 164–175 (1983)
S.-C. Lee, J.-H. Jang, B.-Y. Lee, M.-C. Kang, M. Kang, S.-J. Choung, The effect of binders on structure and chemical properties of Fe-K/γ-Al2O3 catalysts for CO2 hydrogenation. J. Appl. Catal. A. 253, 293–304 (2003)
T. Li, Y. Yang, C. Zhang, X. An, H. Wan, Z. Tao, H. Xiang, Y. Li, F. Yi, B. Xu, Effect of manganese on an iron-based Fischer–Tropsch synthesis catalyst prepared from ferrous sulfate. J. Fuel. 86, 921–928 (2007)
S. Li, S. Krishnamoorthy, A. Li, G.D. Meitzner, E. Iglesia, Promoted iron-based catalysts for the fischer–tropsch synthesis: design, synthesis, site densities, and catalytic properties. J. Catal. 206, 202–217 (2002)
J. Ma, N. Sun, X. Zhang, N. Zhao, F. Xiao, W. Wei, Y. Sun, A short review of catalysis for CO2 conversion. J. Catal. Today 148, 221–231 (2009)
C.V. Miguel, M.A. Soria, A. Mendes, L.M. Madeira, Direct CO2 hydrogenation to methane or methanol from post combustion exhaust streams—a thermodynamic study. J. Nat. Gas Sci. Eng. 22, 1–8 (2015)
M. Mikkelsen, M. Jørgensen, F.C. Krebs, The teraton challenge. A review of fixation and transformation of carbon Dioxide. J. Energy Environ. Sci. 3, 43–81 (2010)
S.-S. Nam, H. Kim, G. Kishan, M.-J. Choi, K.-W. Lee, Catalytic conversion of carbon dioxide into hydrocarbons over iron supported on alkali ion-exchanged Y-zeolite catalysts. J. Appl. Catal. A. 179, 155–163 (1999)
S.-S. Nam, S.-J. Lee, H. Kim, K.-W. Jun, M.-J. Choi, K.-W. Lee, Catalytic conversion of carbon dioxide into hydrocarbons over zinc promoted iron catalysts. J. Energy Convers. Manag. 38((Suppl.)), S397–S402 (1997)
S.N. Riduan, Y. Zhang, Recent developments in carbon dioxide utilization under mild conditions. J. RSC 39, 3347–3357 (2010)
F.J. Pe´ rez-Alonso, M. Ojeda, T. Herranz, S. Rojas, J.M. Gonza´ lez-Carballo, P. Terreros, J.L.G. Fierro, Carbon dioxide hydrogenation over Fe–Ce catalysts. J. Catal. Commun. 9, 1945–1948 (2008)
T. Riedel, G. Schaub, K.-W. Jun, K.-W. Lee, Kinetics of CO2 hydrogenation on a K-promoted Fe catalyst. J. Ind. Eng. Chem. Res. 40, 1355–1363 (2001)
T. Riedel, M. Claeys, H. Schulz, G. Schaub, S.-S. Nam, K.-W. Jun, M.-J. Choi, G. Kishan, K.-W. Lee, Comparative study of Fischer-Tropsch synthesis with H2/CO and H2/CO2 syngas using Fe-and Co-based catalysts. Appl. Catal. A. 186, 201–213 (1999)
B. Rongxian, T. Yisheng, H. Yizhuo, Study on the carbon dioxide hydrogenation to iso-alkanes over Fe–Zn–M/zeolite composite catalysts. J. Fuel Process. Technol. 86, 293–301 (2004)
S. Saeidi, N. SaidinaAmin, M. Rahimpour, Hydrogenation of CO2 to value-added products—a review and potential future developments. J. CO2 Util. 5, 66–81 (2014)
R. Satthawong, N. Koizumi, Ch. Song, P. Prasassarakich, Light olefin synthesis from CO2 hydrogenation over K-promoted Fe–Co bimetallic catalysts. J. Catal. Today 251, 34–40 (2015)
C.G. Visconti, M. Martinelli, L. Falbo, A. Infantes-Molina, L. Lietti, P. Forzatti, G. Iaquaniello, E. Paloc, B. Picutti, F. Brignoli, CO2 hydrogenation to lower olefins on a high surface area K-promoted bulk Fe-catalyst. J Appl Catal. B 200, 530–542 (2017)
W. Wang, S. Wang, X. Ma, J. Gong, Recent advances in catalytic hydrogenation of carbon dioxide. J. Chem. Soc. Rev. 40, 3703–3727 (2011)
T. Witoon, Characterization of calcium oxide derived from waste eggshell and its application as CO2 sorbent. J. Ceram. Int. 37, 3291–3298 (2011)
D.A. Wood, Carbon dioxide (CO2) handling and carbon capture utilization and sequestration (CCUS) research relevant to natural gas: a collection of published research (2009–2015). J. Nat. Gas Sci. Eng. 25, A1–A9 (2015)
L. Xu, Q. Wang, D. Liang, X. Wang, L. Lin, W. Cui, Y. Xu, The promotions of MnO and K2O to Fe/silicalite-2 catalyst for the production of light alkenes from CO2 hydrogenation. J Appl. Catal. A. 173, 19–25 (1998)
Y. Zhang, G. Jacobs, D.E. Sparks, M.E. Dry, B.H. Davis, CO and CO2 hydrogenation study on supported cobalt Fischer–Tropsch synthesis catalysts. J. Catal. Today 71, 411–418 (2002)
Acknowledgements
The authors would like to honor the memory of Prof. Morteza Sohrabi, who passed away unexpectedly during this research project. We also acknowledge the Amirkabir University of Technology for its support and help in this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bashiri, N., Royaee, S.J. & Sohrabi, M. The catalytic performance of different promoted iron catalysts on combined supports Al2O3 for carbon dioxide hydrogenation. Res Chem Intermed 44, 217–229 (2018). https://doi.org/10.1007/s11164-017-3099-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3099-9