Abstract
Hydrogenation of CO2 to CO and hydrocarbons is carried out over a wide range of catalysts. Group of VIIIB transition metals have proved high conversion and selectively for CO and methane. Meanwhile, low cost and effective catalysts are preferable in an industrial scale. In this work, the synergistic effect of iron content on the catalytic performance were investigated in carbon dioxide hydrogenation reaction. Incipient wetness impregnation procedure was used for the preparation of four γ-Al2O3 supported iron-based catalysts. BET, XRD, H2-TPR and TEM techniques were employed for the catalyst characterization. The evaluation of catalysts were carried out in a fixed bed reactor at the process conditions of temperature of 300°C, pressure of 20 atm, H2 to CO2 ratio of 3 and GHSV of 3 NL/(h gCat). It was found that the promoter addition improves the activity of Fe catalyst for both Fischer-Tropsch synthesis (FTS) and Reverse Water Gas Shift (RWGS) reactions. The results showed that conversion of CO2 was from 15.6 to 35.6% with major products of methane, C2 to C4, C5+ and CO. It was also found that impact of K and Ce promoters into iron catalyst showed the highest conversion and hydrocarbon yield due to the synergistic effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 INTRODUCTION
The problem of global warming is related to increasing of CO2 emissions. CO2 is not only one of the main greenhouse gases that is caused global warming but it also is an inexpensive, abundant and readily available chemical [1–4]. Hydrogenation of carbon dioxide to methanol is a solution to solve two main problems needed to reduce CO2 emissions and the reduction in the usage of fossil fuels. As an ideal strategy for the use of CO2, hydrogenation of CO2 and the production of chemicals and fuels such as methane, methanol, syngas and dimethyl ether have been already considered [5–8]. One of the best ways to reduce environmental degradation and future shortages of fossil fuels production of hydrocarbons is through hydrogenation of CO2 within the modification of Fischer-Tropsch Synthesis (FTS), which CO2 is used instead of CO.
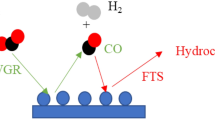
The structure of the catalyst for CO2 hydrogenation is similar to that of FTS. Hydrogenation of CO2 is a sequential reaction to reduce CO2 to CO and then CO hydrogenation for producing various hydrocarbons compounds and oxygenated compounds [9, 10]. The distribution of the hydrocarbon products due to the hydrogenation of carbon dioxide is similar to the hydrogenation of carbon monoxide at the same temperature and pressure conditions [11]. Investigation of this subject have been done, which can be divided into two categories: methanol-mediated and non-methanol-mediated reactions [12, 13]. In the methanol intermediate method, CO2 and H2 react in the presence of copper-zinc catalysts to produce methanol, which is subsequently converted to other hydrocarbons such as gasoline [14]. In the second one, CO2 hydrogenation occurs through two steps: Reverse Water Gas Shift (RWGS) reaction and FT synthesis. Iron oxides are used as catalysts in the FT process, which is active in both Water Gas Shift (WGS) and RWGS reactions [15, 16]. Iron catalysts are attractive for the synthesis of hydrocarbons due to the production of olefinic products [17]. One method of converting CO2 to liquid fuels is to hydrogenate CO2 through modified Fisher- Tropsch synthesis in the presence of catalysts. Riedel et al. investigated the catalytic performance of Fe and Co catalysts in the mixtures of CO, CO2 and H2. As CO2 increases and CO in the feed (syngas) decreases, the composition of the products obtained from the Co catalysts changes from heavy hydrocarbons to almost methane, while iron catalysts result in the same hydrocarbon products as CO2/H2 or CO/H2 [18]. It has been found that iron catalysts containing various promoters such as potassium, copper and manganese improved its performance [19]. In this paper, the hydrogenation of carbon dioxide were investigated in four iron-based catalysts. Moreover, the synergistic effects between the promoters were studied for this reaction. The catalysts were characterized using different techniques of BET, XRD, H2-TPR and TEM.
2 EXPERIMENTAL
2.1 Catalyst Preparation
The Fe catalysts were synthesized via wet impregnation method. In this procedure, an aqueous solution of Fe(NO3)3·9H2O (Aldrich, 99.99%), Cu(NO3)2·4H2O (Alfa Aesar, ≥98%) and Zn(NO3)2·4H2O (98.5%), KNO3 (Aldrich, 99.0%) or Ce(NO3)3·4H2O (Aldrich, ≥99.99%) or both of K/Ce salts [20] was prepared. γ-Al2O3 support (Condea Vista Catalox B γ-alumina) is used as a powder form. Typically, 7 g of γ-Al2O3 support was impregnated with 16 mL of the solution. The impregnated sample was dried at 120°C for 24 h in an electric oven, then samples were calcinated in a furnace at 400°C for 3 h. The catalyst compositions were designated in terms of the weight ratios as 15Fe/γ-Al2O3; 15Fe/3Cu/10Zn/2K/γ-Al2O3; 15Fe/3Cu/10Zn/2Ce/ γ-Al2O3, and 15Fe/3Cu/10Zn/1K/1Ce/ γ-Al2O3. All catalysts were pressed into pellets, crushed, and sieved to obtain particle sizes in the range of 20–30 mesh.
2.2 Catalyst Characterization
BET Surface area, pore volume and mean pore size of the catalysts were determined in a Micromeritics Tri star 3020 automated system. An XRD spectrum of fresh catalysts were conducted with a Philips PW1840 X-ray diffractometer with monochromatized CuKα radiation to determine the catalysts phases. Temperature—Programmed Reduction (TPR) profile of the calcined catalysts were recorded using a Micromeritics TPD-TPR 2900 system. The TPR of 50 mg of each sample was performed in 5% H2–95% Ar gas mixture. The catalysts were heated from 50 to 900°C at 10°C/min. Average particle size of the calcined catalysts were investigated by LEO 912AB Transmission Electron Microscopy (TEM).
2.3 Catalytic Activity Test
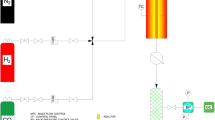
As shown in Fig. 1, the catalytic reaction was conducted in a fixed-bed stainless steel reactor. Electronic mass flow and pressure controllers are used to control the flow rate of feed and the pressure of reactor. The required amount of catalyst (2 g) was conducted into a stainless steel reactor with inner diameter of 0.95 cm and length of 70 cm. The length of catalyst bed in every loading is about 9 cm [21, 22]. The catalyst was reduced by a 20% H2–80% N2 flow gas at 400°C for 2 h. After the activation process, CO2 hydrogenation was carried out under the operational conditions of pressure of 20 atm, temperature of 300°C, H2/CO2 ratio of 3, GHSV of 3 NL/(h gCat). The products were analyzed online by a gas chromatography (GC) of Agilent 7890A [19, 20]. Using GC analysis, CO2 conversion and product selectivity were calculated and the following equations were used (Eqs. (1)–(5)):
The schematic diagram of fixed-bed reactor system: (1) valve; (2) carbonyl trap; (3) mass flow controller; (4) static mixer; (5) pressure gauge; (6) fixed-bed reactor; (7) catalyst; (8) quartz; (9) hot trap; (10) cold trap; (11) back pressure regulator; (12) wet gas flow meter; (13) vent; (14) gas chromatography; (15) by-pass line.
3 RESULTS AND DISCUSSION
3.1 Catalyst Characterization Results
Surface area, pore volume and mean pore size of the prepared catalysts were measured using a BET instrument. An appropriate amount of catalyst (0.3 g) was taken into the sample tube and slowly heated to 300°C for 2 h. The sample was then transferred to the adsorption unit, and the N2 adsorption/desorption was measured. The results obtained from BET for prepared catalysts are illustrated in Table 1. According to the BET results:
By adding Fe, Cu, Zn, K and Ce to the support, the BET surface area and pore volume were decreased. Furthermore, it might promote the aggregation of the catalyst crystallites and block up the pores. Figure 2 shows the XRD patterns for the three catalysts and γ‑Al2O3 support. The patterns of the catalysts show the peaks at 2θ = 37.6°, 39.5°, 45.8°, and 66.8° attributed to the primary support of γ-Al2O3 support. For the three calcined iron catalysts, in addition to the peaks attributed to the γ-Al2O3 support, the peaks of hematite, Fe2O3 at 24.1°, 33.3°, 35.7°, 41°, 49.5°, 54.2°, 62.3°, and 64.2° can be intuited. In the XRD pattern associated with the magnetite phase, exist two additional peaks located at 24° and 26.10°. On the other hand, the formation of FeAl2O4 spinel cannot be discarded. The diffraction peaks corresponding to the second metal oxide phases were not observed in the calcined catalysts [23].
TEM image revealed the morphology of the catalyst. TEM images of Fe–Zn–Cu (K–Ce)/γ-Al2O3 catalysts are presented in Fig. 3. Irregular shapes and agglomerated particles with the size of 20–80 nm are shown for the catalysts. The elements of Fe–Zn–Cu with combination of Ce and K are well dispersed over the surface of γ-Al2O3. Catalyst morphology was rough as all the particles were aggregated and agglomerated over the surface of the support [24, 25].
H2-TPR determines the reduction behavior of the catalysts. The first peak is the transformations of CuO to Cu
(1) \({\text{CuO}} \to {\text{Cu}}\).
The second peak is the transformation of Fe2O3 to Fe3O4, and the third peak represents the transformation of Fe3O4 to Fe [20, 23]
(2) Fe2O3 \( \to \) Fe3O4,
(3) Fe3O4 \( \to \) Fe.
Hydrogen consumption occurs mainly at 160–420°C due to the simultaneous reduction of CuO to Cu and Fe2O3 to Fe3O4. The reduction of iron catalysts by hydrogen is known to be a two or three-staged process [26–29]. In this process, Fe2O3 is first reduced at 397°C to Fe3O4, which is reduced to metallic iron at 690°C [28]. In all catalysts, iron oxides were reduced to Fe3O4 and they are probably converted partly to oxygen-deficient Fe3O4 species during the TPR to 400°C. H2-TPR profile of the catalysts are shown in Fig. 4.
According to Fig. 4, the un-promoted iron catalyst (F) exhibited two peaks attributed to the reduction by H2 at around 340 and 620°C, which corresponds to the two-step reduction of hematite (reactions (2) and (3)) [30–32]. Each catalysts of FCZ2K, FCZ2Ce and FCZ1K1Ce exhibited three peaks attributed to the reduction at around (220, 330 and 630°C), (230, 340 and 590°C) and (210, 315, 530°C), respectively, which correspond to the three-step reduction (reactions of 1, 2 and 3). The reduction of Fe species can be promoted by the addition of Cu species, leading to a shift of a peak attributed to the reduction of Fe2O3 to Fe3O4 to a lower temperature in H2-TPR profiles [30, 31], whereas K and Ce species suppresses the reduction of Fe species due to the interaction between K species and the metal species [33]. However, the second and third peaks of all the promoted iron-based catalysts was shifted to higher temperatures in comparison with that of the FCZ1K1Ce catalyst. Iglesia and coworkers exhibited the initial removal of only a very small amount of lattice oxygen from Fe2O3 which facilitated the formation of the active sites during the FTS reaction [34].
3.2 CO2 Hydrogenation Over Iron Supported γ-Al2O3 Catalysts
CO2 hydrogenation is performed in two steps with the CO intermediate being formed by the reverse water–gas shift (RWGS) reaction [26]:

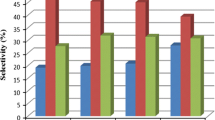
CO2 conversion and product selectivity were calculated using the data collected at 32 h time on stream. Products selectivity of the catalysts are indicated in Table 2. It shows that promoters affect on the selectivity of light hydrocarbons (methane and C2–C4), and heavy hydrocarbons (\({\text{C}}_{5}^{ + }\)). Based on the above discussion, the catalytic performances of CO2 hydrogenation over different catalysts are compared and listed in Table 2.
The product distribution (\({\text{C}}_{5}^{ + }\)) that is represented by carbon number and Productivity (gHydrocarbon/(h gCat)) are shown in Fig. 5. Hydrocarbon chain is formed by step-wise insertion or addition of C1 with constant growth probability (α), the chain length distribution can be modelled by Anderson–Schulz–Flory (ASF) distribution assuming a constant value for α:
here Wn is the mass fraction of the species with carbon number n and α is chain growth probability. By plotting of log (Wn/n) versus n, α can be obtained from the slope [43]. The calculated α of four types of catalysts including F/γ-Al2O3; FCZ/2K/γ-Al2O3; FCZ/2Ce/ γ-Al2O3, and FCZ/1K/1Ce/γ-Al2O3 are 0.58; 0.72; 0.64; 0.68, respectively.
CO2 hydrogenation over iron catalysts is occurred through a consecutive mechanism in which CO2 is first converted to CO by the RWGS reaction, and then CO is hydrogenated to produce hydrocarbons. Therefore, the mechanism of hydrocarbon chain growth from carbon dioxide is similar to that of CO hydrogenation (FT process). K has been extensively studied as a promoter of iron-based FT catalysts. It has been shown that it favors CO and CO2 adsorption and increases the production of hydrocarbons. The CO2 conversion of catalysts at identical operating conditions was recorded for about 48 h. In Fig. 6, the CO2 conversion of the catalysts decreases with time on stream. Promoters increase intrinsic activity of surface sites by lowering the activation energy or entropy for a chemical reaction [10].
For Ce-promoted Fe catalyst, the CO2 conversion is reduced in different time. Moreover, addition of the K2O has more destructive effect on CO2 conversion. It illustrates the catalyst FeCuZn-1Ce-1K/γ-Al2O3 with highest selectivity and almost relatively high CO2 conversion possesses highest productivity.
4 CONCLUSIONS
In this paper, iron promoted catalysts were synthesized using incipient wet impregnation method. The calcined catalysts were characterized using XRD, BET, H2-TPR and TEM techniques. The catalysts conducted in a fixed-bed reactor. The activity of the catalysts were evaluated in CO2 hydrogenation at pressure, temperature, H2/CO2 ratio and GHSV of 20 atm, 300°C, 3,3 NL/(h gCat) respectively. The catalysts were active in CO2 hydrogenation reaction with conversions of approximately 15.6–35.6%. The RWGS reaction can be effectively catalyzed by iron-based catalysts. The addition of promoters such as K and Ce remarkably enhanced the CO2 conversion. The promotional effect is ascribed to the strong interaction between Fe and K/Ce, altering the electronic density of iron and thus facilitating CO2 activation on the catalytic surface. As compared with individual promoter of K or Ce, double promoters of K and Ce significantly improved CO2 conversion and improved hydrocarbon yield, which are due to the synergistic effect of the promoters.
REFERENCES
Larcher, D. and Tarascon, J.M., Nat. Chem., 2015, vol. 7, pp. 19–29.
Lund, H., Energy, 2007, vol. 32, pp. 912–919.
Edwards, P.P., Kuznetsov, V.L., David, W.I.F., and Brandon, N.P., Energy Policy, 2008, vol. 36, pp. 4356–4362.
Chu, S. and Majumdar, A., Nature, 2012, vol. 488, pp. 294–303.
Chuasomboon, N., Numpilai, T., and Chareonpanich, K.M., Fuel, 2019, vol. 241, pp. 695–703.
Ren, S.J., Fan, X., Shang, Z.Y., Shoemaker, W.R., Ma, L., and Wu, T.P., J. CO2 Util., 2020, vol. 36, pp. 82–95.
Li, B., Duan, Y., Luebke, D., and Morreale, B., Appl. Energy, 2013, vol. 102, pp. 1439–1447.
Frusteri, F., Bonura, G., Cannilla, C., Cordaro, M., Ferrante, D., and Aloise, A., Appl. Catal., B, 2015, vols. 176–177, pp. 522–531.
Frusteri, F., Cordaro, M., Cannilla, C., and Bonura, G., Appl. Catal., B, 2015, vol. 162, pp. 57–65.
Pijolat, M., Perrichon, V., Primet, M., and Bussiére, P., J. Mol. Catal., 1982, vol.17, pp. 367–380.
Schulz, H., Schaub, G., Claeys, M., and Riedel, T., Appl. Catal., A, 1999, vol. 186, pp. 215–227.
Fujimoto, K. and Shikada, T., Appl. Catal., 1987, vol. 3, pp. 13–23.
Lee, J.F., Chern, W.S., Lee, M.D., and Dong, T.Y., Can. J. Chem. Eng., 2009, vol.70, pp. 511–515.
Prasad, P.S.S., Bae, J.W., Jun, K.W., and Lee, K.W., Catal. Surv. Asia, 2008, vol. 12, pp. 170–183.
Schulz, H., Appl. Catal., A, 1999, vol. 186, pp. 3–12.
Van Der Laan, G.P. and Beenackers, A.A.C.M., Catal. Rev. Sci. Eng, 1999, vols. 255–318, p. 41.
Williams, K.J., Boffa, A.B., Salmeron, M., Bell, A.T., and Somorjai, G.A., Catal. Lett., 1991, vol. 9, pp. 415–426.
Riedel, T., Schaub, G., Jun, K., and Lee, K., Ind. Eng. Chem. Res., 2001, vol. 40, pp. 1355–1363.
Wang, J., You, Z., Zhang, Q., Deng, W., and Wang, Y., Catal. Today, 2013, vol. 215, pp. 186–193.
Shahroudbari, I., Sarrafi, Y., Hoseinzadeh, R., and Zamani, Y., Pet. Coal, 2020, vol. 62, no. 4, pp. 1572–1576.
Arsalanfar, M., Mirzaei, A. A., Atashi, H., Bozorgzadeh, H.R., Vahid, S., and Zare, A., Fuel Process. Technol., 2012, vol. 96, pp. 150–159.
Mirzaei, A.A., Pourdolat, A., Arsalanfar, M., Atashi, H., and Samimi, A.R., J. Ind. Eng. Chem., 2013, vol. 19, pp. 1144–1152.
Zamani, Y., Bakavoli, M., Rahimizadeh, M., Mohajeri, A., and Seyedi, S.M., Chin. J. Catal., 2012, vol. 33, pp. 1119–1124.
Farias, F.E.M., Neto. R.C.R., Baldanza, M.A.S., Schmal, M., and Fernandes, F.A.N., Braz. J. Chem. Eng, 2011, vol. 28, pp. 495–504.
Sánchez De La Torre, F., Rivera De La Rosa, J., Kharisov, B.I., and Lucio-Ortiz, C.J., Materials, 2013, vol. 6, pp.4324–4344.
Kishan, G., Lee, M.W., Nam, S.S., Choi, M J., and Lee, K.W., Catal. Lett., 1998, vol. 56, pp. 215–219.
Nam, S.S., Kim, H., Kishan, G., Choi, M.J., and Lee, K.W., Appl. Catal., A, 1999, vol. 179, pp. 155–163.
Riedel, T., Claeys, M., Schulz, H., Schaub, G., Nam, S.S., Jun, K.W., Choi, M.J., Kishan, G., and Lee, K.W., Appl. Catal., A, 1999, vol. 186, pp. 201–213.
Jozwiak, W.K., Goralski, J., and Domka, F., Appl. Catal., A, 2000, vol. 190, pp. 107–115.
Jin, Y. and Datye, A.K., J. Catal., 2000, vol. 196, no. 1, pp. 8–17.
Wan, H.J., Wu, B.S., and Zhang, C.H., Catal. Commun., 2007, vol. 8, no. 10, pp. 1538–1545.
Bukur, D.B. and Sivaraj, C., Appl. Catal., A, 2002, vol. 231, nos. 1–2, pp. 201–214.
Zhang, H., Ma, H., Zhang, H., Ying, W., and Fang, D., Catal. Lett., 2012, vol. 142, no. 1, pp. 131–137.
Li, S., Ding, W., Meitzner, G.D., and Iglesia, E., J. Phys. Chem. B, 2002, vol. 106, pp. 85–91.
Riedel, T., Claeys, M., Schulz, H., Schaub, G., Nam, S.S., Jun, K.W., Choi, M.J., Kishan, G., and Lee, K.W., Appl. Catal., A, 1999, vol. 186, pp. 201–213.
Herranz, T., Rojas, S., Pérez-Alonso, F., J., Ojeda, M., Terreros, P., and Fierro, J. L.G., Appl. Catal., A, 2006, vol. 311, pp. 66–75.
Lisheng, G., Jian, S., Qingjie, G., and Noritatsu, T., J. Mater. Chem. A, 2018, vol. 6, pp. 23244–23262.
Nawapat, C., Thanapha, N., Chin, K.C., Nuchanart, S., Thana S., Chularat W., Jumras, L., and Thongthai, W., Fuel, 2021, vol. 283. https://doi.org/10.1016j.fuel.2020.119248
Jiang, F., Liu, B., Geng, S., Xu, Y., and Liu, X., Catal. Sci. Technol., 2018, vol. 8, no. 16, pp. 4097–4107.
Zhang, A., Liu, M., Li, J., Song, F., Song, C., and Guo, X., RSC Adv., 2016, vol. 2016, pp. 10831–10836.
Nuttakorn, B., Xiao, J., Chunshan, S., and Pattarapan, P., Top. Catal., 2018, vol. 61, nos. 15–17, pp. 1–14.
Sun-Mi, H., Chundong, Z., Seung, J.H., Hae-Gu, P., Yong, T.K., Sunkyu, Y., Ki-Won, J., and Seok, K.K., J. CO2 Util., 2020, vol. 37, pp. 65–73.
Patzlaff, J., Liu, Y., Graffmann, C., and Gaube, J., Appl. Catal., A, 1999, vol. 186, pp. 109–119.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The paper is published in the original.
Rights and permissions
About this article
Cite this article
Isa Shahroudbari, Sarrafi, Y. & Zamani, Y. Study of Carbon Dioxide Hydrogenation to Hydrocarbons Over Iron-Based Catalysts: Synergistic Effect. Catal. Ind. 13, 317–324 (2021). https://doi.org/10.1134/S2070050421040085
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070050421040085