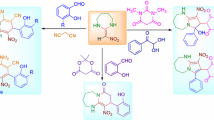
Abstract
A convenient and efficient method is described for the synthesis of novel tricyclic scaffolds incorporating an imidazole ring and medium-sized benzoxazepine ring, via the reaction of 1,2-diketons, 2-formyl phenoxy acetic acids and ammonium acetate in acetic acid under reflux conditions, and then the addition of thionyl chloride to produce lactam in the absence of a catalyst.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Compounds containing a fused seven-membered benzoxazepine ring have been of pivotal interest over the past few years due to their wide range of biological activities and pharmacological properties [1–5]. In particular, 1,4-benzoxazepine derivatives are of pharmaceutical interest because of their activity on the central nervous system as enzyme inhibitors, analgesics, and sedatives, and for their antitumor activity [6–9]. Substituted fused benzoxazepine rings have been identified as PI3-kinase inhibitors for cancer. For instance, 5,6 dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepine scaffolds (Fig. 1) are very promising therapeutics for the treatment of various types of cancers. The synthesis of 2,3,4,5-tetrahydro-1,4-benzoaxazepines often involves the reduction of carbonyl groups as in 5-oxo-2,3,4,5-tetrahydro-1,4-benzoxazepine, or the reduction of a double bond as in 2,3-dihydro-1,4-bezoxazepine [10].
Alternatively, 2,3,4,5-tetrahydro-1,4-benzoxazepines can be prepared using one of the following benzoxazepine synthetic methods: (1) reaction of 2-aryloxyethylamines with 2-formylbenzoic acid to form aminonaphtalides followed by cyclization; (2) rearrangement of methyl 2-(8-methoxy-2,3-dihydro-1,4-benzooxazepine-5-yl) benzoate using Bischler-Napieralski condition; and (3) scandium or copper triflate-catalyzed acylaminoalkylation of α-methoxy-isoindolones for the formation of 1,4-benzoxazepines [11–13]. Levan et al. [14, 15] reported the isolation and characterization of unsubstituted 1,2,3,4,5,6,11b-hexahydroimidazo[1,2-d][1,4]benzoxazepine as a by-product without mentioning the product yield.
Experimental
All commercially available chemicals of 1,2-diketones and solvents were purchased from Merck and Sigma–Aldrich and used without further purification. Melting points were taken on a Kofler hot-stage apparatus and are uncorrected. 1H and 13C NMR spectra were recorded on a Bruker FT-500, with TMS as internal standard. IR spectra were obtained on a Nicolet Magna FTIR 550 spectrophotometer in KBr. Mass spectra were determined on an Agilent Technology (HP) mass spectrometer operating at an ionization potential of 70 eV. Elemental analysis was performed with an Elementar Analysensystem VarioEL in CHNS mode.
General procedure for the synthesis of [2-(4,5-diaryl-1H-imidazole-2-yl) phenoxy] acetic acid (4)
Mixtures of 1,2-diketons (1; 1 mmol) and 2-formyl phenoxy acetic acids (2; 1 mmol) and ammonium acetate (3; excess) were heated at refluxed for 5 h in acetic acid. Upon completion of the reaction, checked by TLC, the reaction mixture was poured into ice-water with stirring. The precipitated products were recrystallized from ethanol to give pure products (4).
General procedure for the synthesis of 2,3-diarylbenzo[f]imidazo[1,2-d][1,4]oxazepin-5(6H)-one derivatives (5)
Thionyl cholride or oxalyl chloride (1.5 mmol) was added dropwise to a mixture of 2-(2(4, 5-diaryl 1H imidazole-2-yl) phenoxy) acetic acid (1 mmol) and triethylamine (3 mmol) in dry CH2Cl2 (5 ml) and the mixture was stirred overnight at room temperature. The reaction mixture was washed twice with saturated NaHCO3 and brine. The organic layer was dried by sodium sulfate, filtered and the solvent removed to give the crude product which was then purified using plate chromatography, eluting with petroleum ether/ethyl acetate (9:1).
2,3-diphenylimidazo[1,2-d][1,4]benzoxazepin-5(6H)-one (5a, C23H16N2O2)
Yield (85%). Pale yellow crystals. M.p. 139–141°. IR (KBr, cm−1): 17,416, 1239, 1152. 1H NMR: 4.90 (s, 2H of CH2); 7.14 (t, J = 8.0 Hz, CH); 7.19 (d, J = 8.0 Hz, CH); 7.30 (t, J = 7.2 Hz, CH); 7.36–7.40 (m, 4H of Ph); 7.55–7.57 (m, 3H of Ph); 8.20 (d, J = 7.7 Hz, CH). 13C NMR: 66.2; 113.9; 118.5; 121.8; 127.1; 127.6; 127.8; 128.4; 123.8; 129.7; 142.8; 154.1; 171.4 ppm Anal. Calcd for C23H16N2O2 (352.12): C 78.39, H 4.58, N 7.95, Found: C 78.61, H 4.34, N 7.22.
2,3-bis(2-chlorophenyl)imidazo[1,2-d][1,4]benzoxazepin-5(6H)-one (5b)
Yield (85%). Pale yellow crystals. M.p. 157–159°. IR (KBr, cm−1): 1747, 1221, 1107 1H NMR: 4.92 (s, 2H of CH2); 7.13 (t, J = 7.5 Hz, CH); 7.18 (d, J = 8.3 Hz, CH); 7.30–7.34 (m, 4H of Ph); 7.36–7.40 (m, 3H of Ph); 7.46 (d, J = 7.7 Hz, CH); 8.20 (d, J = 7.7 Hz, CH). 13C NMR: 66.4; 114.4; 119.0; 122.3; 127.4; 128.2; 129.9; 130.1; 130.3; 132.5; 132.9; 143.4; 154.6; 171.9 Anal. Calcd for C23H14Cl2N2O2 (421.28): C 65.58, H 3.35, N 6.65; Found: C 65.67, H 3.61, N 6.22.
2,3-bis(4-fluorophenyl)imidazo[1,2-d][1,4]benzoxazepin-5(6H)-one (5c)
Yield (90%). Pale yellow crystals; M.p. 283–285°. IR (KBr, cm−1): 1725, 1259, 1024 cm−1. 1H NMR: 4.92 (s, 2H of CH2), 7.15 (d, J = 8.8 Hz, CH); 7.46 (t, J = 8.7 Hz, CH); 7.52–7.56 (m, 6H of Ph); 8.04 (t, J = 8.3 Hz, CH); 8.28 (d, J = 7.7 Hz, CH). 13C NMR: 66.0; 113.8; 115.4; 115.6; 116.7; 116.9; 118.3; 121.9; 127.9; 129.7; 129.9; 133.0; 133.1; 142.8; 154.1; 160.5; 162.4; 171.4 Anal. Calcd for C23H14F2N2O2 (388.10): C 71.13, H 3.63, N 7.21; Found: C 70.87, H 3.71, N 7.34.
2,3-bis(4-fluorophenyl)imidazo[1,2-d][1,4]benzoxazepin-5(6H)-one (5d)
Yield (80%). Pale yellow crystals. M.p. 301–303°. IR (KBr, cm−1): 1751, 1250, 1112. 1H-NMR: 4.92 (s, 2H of CH2), 7.14 (t, J = 7.6 Hz, CH); 7.18 (d, J = 8.2 Hz, CH); 7.37–7.41 (m, 1H of Ph); 7.48–7.59 (m, 8H of Ph); 7.84–7.59 (m, 1H of Ph, CH); 8.20 (dd, J = 8 Hz, 1.75 Hz, CH). 13C-NMR: 66.4; 113.9; 118.5; 118.8; 120.3; 121.6; 127.8; 129.5; 129.8; 131.4; 132.5; 143.0; 154.3; 171.5. MS: m/z (%) = 340 [m]+.(100), 342[m + 2]+ (91), 325(14), 261(44), 233(50), 210(19). Anal. Calcd for C23H14Br2N2O2 (507.94): C 54.15, H 2.66, N 5.49. Found: C 54.02, H 2.74, N 5.38.
2,3-bis(4-methoxyphenyl)imidazo[1,2-d][1,4]benzoxazepin-5(6H)-one (5e)
Yield (70%). Pale yellow crystals. M.p. 146–149°. IR (KBr, cm−1): 1740, 1215, 1035. 1H NMR: 3.77 (s, 3H of OCH3); 3.87 (s, 3H of OCH3); 4.88 (s, 2H of CH2); 6.94 (d, J = 8.4 Hz, CH); 7.13–7.20 (m, 3H of Ph), 7.37 (t, J = 7.4 Hz, 1H of Ph), 7.55 (d, J = 8.4, CH); 7.86 (d, J = 8.4, CH); 8.17 (d, J = 7.4, CH); 13C NMR: 55.4; 56.3; 66.9; 114.3; 114.6; 115.3; 118.8; 122.2; 125.4; 125.8; 128.2; 129.3; 130.2; 131.1; 132.5; 142.4; 154.6; 158.8; 165.2; 171.9; 172.5; 194.0. Anal. Calcd for C25H20N2O4 (412.14): C 72.80, H 4.89, N 6.79; Found: C 72.63, H 5.01, N 6.58.
2,3-bis(3-methoxyphenyl)imidazo[1,2-d][1,4]benzoxazepin-5(6H)-one (5f)
Yield (70%). Pale yellow crystals. M.p. 136–139°. IR (KBr, cm−1): 1728, 1225, 1035. 1H NMR: 3.73 (s, 3H of OCH3); 3.84 (s, 3H of OCH3); 4.92 (s, 2H of CH2); 6.94 (d, J = 8.4 Hz, CH); 7.13–7.20 (m, 3H of Ph), 7.37 (t, J = 7.4 Hz, 1H of Ph), 7.55 (d, J = 8.4, CH); 7.86 (d, J = 8.4, CH); 8.17 (d, J = 7.4, CH); 13C NMR: 55.9; 56.4; 66.5; 113.0; 113.4; 114.3; 118.7; 120.5; 122.2; 128.4; 129.9; 129.3; 130.3; 131.2; 132.5; 134.3; 143.1; 154.6; 159.6; 171.9. Anal. Calcd for C25H20N2O4 (412.14): C 72.80, H 4.89, N 6.79; Found: C 72.63, H 5.01, N 6.58.
2,3-di(thiophen-2-yl)imidazo[1,2-d][1,4]benzoxazepin-5(6H)-one (5g)
Yield (77%). Brown crystals; M.p. 139–141°. IR (KBr, cm−1):1751, 1112, 1250 cm−1. 1H NMR: 4.92 (s, 2H of CH2), 7.12–7.15 (m, 5H of thiophen); 7.16–7.18 (m, 3H of Ph); 8.17 (d, J = 7.0 Hz, CH); 8.30 (d, J = 7.0 Hz, CH). 13C NMR: 66.0; 113.8; 115.4; 115.6; 116.7; 116.9; 118.3; 121.9; 127.9; 129.7; 129.9; 133.0; 133.1; 142.8; 154.1; 160.5; 162.4; 171.4 Anal. Calcd for C23H14F2N2O2 (388.10): C 71.13, H 3.63, N 7.21. Found: C 70.87, H 3.71, N 7.34.
2,3-di-p-tolylimidazo[1,2-d][1,4]benzoxazepin-5(6H)-one (5h)
Yield (68%). Pale yellow crystals; M.p. 156–159°. IR (KBr, cm−1): 1747, 1225, 1113 cm−1. 1H NMR: 2.3 (s, 3H of CH3), 2.4 (s, 3H of CH3); 4.90 (s, 2H of CH2); 7.13 (t, J = 7.5 Hz, CH); 7.18 (t, J = 7.5 Hz, 5H of Ph); 7.37 (t, J = 8.0 Hz, CH); 7.44 (d, J = 7.5 Hz, CH); 7.8 (d, J = 8.0 Hz, CH); 8.20 (d, J = 7.5 Hz, CH). 13C NMR: 21.3; 21.4; 66.1; 113.0; 117.5; 122.0; 125.3; 127.3; 127.9; 129.7; 129.9; 142.5; 154.2; 172.8 Anal. Calcd for C25H20N2O2 (380.15): C 78.93, H 5.30, N 7.36. Found: C 79.07, H 5.41, N 7.58.
10-bromo-2,3-bis(4-fluorophenyl)imidazo[1,2-d][1,4]benzoxazepin 5(6H)-one (5i)
Yield (90%). Pale yellow crystals; M.p. 283–285°. IR (KBr, cm−1): 16,751, 1250, 1226 cm−1. 1H NMR: 4.88 (s, 2H of CH2), 7.14 (d, J = 8.9 Hz, CH); 7.46 (t, J = 8.9 Hz, CH); 7.46 (t, J = 8.7 Hz, CH); 7.50–7.56 (m, 5H of Ph); 8.03 (d, J = 8.2 Hz, CH). 8.28 (d, J = 2.5 Hz, CH). 13C NMR: 66.4; 115.4; 115.6; 116.3; 116.7; 129.7; 131.9; 133.0; 129.9; 133.0; 133.1; 141.4; 153.3; 171.2 Anal. Calcd for C11H7BrN2O2 (279.09): C 59.12, H 2.80, N 6.00. Found: C 59.34, H 2.91, N 5.94.
10-bromoimidazo[1,2-d][1,4]benzoxazepin-5(6H)-one (5j)
Yield (85%). Pale yellow crystals. M.p. 139–141°. IR (KBr, cm−1): 1710, 12,528, 1029. 1H NMR: 4.88 (s, 2H of CH2); 7.12 (d, J = 7.5 Hz, CH); 7.27 (s, CH); 7.5 (d, J = 7.5 Hz, CH); 8.19 (s, CH). 13C NMR: 66.6; 113.2; 116.5; 120.3; 122.6; 129.7; 132.0; 141.0; 153.6; 170.8 Anal. Calcd for C11H7BrN2O2 (277.97): C 47.34, H 2.53, N 10.04; Found: C 47.56, H 2.74, N 9.82.
10-bromo-2,3-dimethylimidazo[1,2-d][1,4]benzoxazepin 5(6H)-one (5k)
Yield (85%). Pale yellow crystals. M.p. 139–141°. IR (KBr, cm−1): 1718, 1258, 1029. 1H NMR: 2.1 (s, 3H of CH3), 2.2 (s, 3H of CH3); 4.6 (s, 2H of CH2); 7.18 (d, J = 8.8 Hz, CH); 7.54 (d, J = 8.8 Hz, CH); 8.05 (d, J = 2.0 Hz, CH). 13C NMR: 11.0, 12.6, 66.6; 113.2; 116.5; 120.3; 122.3; 129.5; 131.0; 140.0; 152.6; 169.1 Anal. Calcd for C13H11BrN2O2 (306.00): C 50.84, H 3.61, N 9.12; Found: C 50.62, H 3.76, N 8.86.
Results and discussion
In continuation of our research on the synthesis of biologically active heterocycles, [16–19] and due to the fact that the synthesis of benzo[f]imidazo[1,2-d][1,4]oxazepines is quite infrequent, we present a new, reliable, and efficient route for the synthesis of compounds 5 in high yield, using a straightforward way in the absence of a catalyst (Scheme 1). The reaction was first carried out by condensation between 1,2-diketones 1, 2-formyl phenoxy acetic acids 2, and ammonium acetate (NH4OAc) (3) in acetic acid under reflux conditions for 5 h and the precipitated products were then filtered and dried. Then, thionyl chloride (or oxalyl chloride) was added as an acid activator to dry CH2Cl2 in the presence of triethyl amine to give lactam 5a–5k (Scheme 1). In order to discover the most appropriate reaction conditions, our initial efforts to obtain compounds 5 involved the reaction of benzil, 2-formylphenoxy acetic acid, and NH4OAc in solvents such as ethanol, dichloromethane, acetonitrile, toluene, and acetic acid, and under solvent-free conditions at various temperatures. As expected, acetic acid proved to be the best solvent under reflux conditions (Table 1). However, as 1,2-diketones of glyoxal and diacetyl (Table 3, entries 10 and 11) are highly reactive, the reaction was carried out under milder conditions in methanol for 2 h. To evaluate and optimize the lactamization of compound 4 by thionyl chloride, it was found that the combination of three equivalents of triethyl amine in dry dichloromethane at room temperature was the most effective and led to the formation of the corresponding lactam 5a in good yield (85%). Increasing the amount of the base and the temperature did not cause an increase in yield (Table 2).The application of ammonium acetate in acetic acid as a source of ammonia has been studied widely in the synthesis of N-heterocycles [20, 21]. Moreover, it was found that excess ammonium acetate should be used to obtain the highest yield of product for two reasons: first, it is water-soluble and the excess amount can be easily removed during workup, and second, it is a neutral salt and not a significant active species other than as ammonia source. To the best of our knowledge, there is no report on the synthesis of benzo[f]imidazo[1,2-d][1,4]oxazepines-5(6H)-one derivatives by this procedure.
The structure of 5b was elucidated by spectroscopic analysis. In the 1H NMR spectrum, signals for 12 aromatic protons were observed around 7.12–8.18 ppm along with a singlet at 4.92 ppm for methylene protons (–CH2–) attached to an oxygen and carbonyl group. The 13C NMR spectrum showed 12 distinct signals in the aromatic region and a resonance at δ = 171.9 ppm for the C=O group. To determine the most appropriate conditions, the reaction was conducted under microwave irradiation in a one-pot manner; however, the corresponding product 5a was obtained in lower yield even after 30 min. It is noteworthy that the procedure was effective for both electron-donating and electron-withdrawing substituents on the aromatic rings of the 1,2-diketone. Nevertheless, 2-formyphenoxy acetic acid with the electron-withdrawing group provided products in moderate yield with longer reaction times.
A possible mechanism for the formation of 5a is proposed in Scheme 2. The first step is the formation of imine intermediate 7 and α-imino ketone 8 via the nucleophilic attack of ammonia, derived from NH4OAc. The reaction of 7 and 8 leads to the formation of intermediate 9 which undergoes cyclization, dehydration, and a [1,5]-H shift to give [2-(4,5-diaryl-1H-imidazole-2-yl) phenoxy] acetic acid 4; then, the activation by thionyl chloride produces acyl chloride 10. In the last step, the nucleophilic attack of the ring nitrogen on the acyl chloride leads to the formation of products 5.
Conclusion
In this study, an efficient, rapid, and high-yielding procedure has been reported for the synthesis of potentially bioactive 2,3-benzo[f]imidazo[1,2d][1,4]oxazepine-5(6H)-one derivatives 5. The reactions were performed first by the condensation of aromatic and also aliphatic 1,2-diketones, 2-formyl phenoxy acetic acids, and ammonium acetate in acetic acid under reflux conditions, and then thionyl chloride was added dropwise in dry CH2Cl2 in the presence of triethyl amine to produce the corresponding lactams. The procedure is very simple and affords the derivatives in good yields in the absence of a catalyst.
References
G.L. Grunewald, V.H. Dahanukar, P. Ching, K.R. Crisclone, J. Med. Chem. 39, 3539 (1996)
G. Steiner, A. Franke, E. Hädicke, D. Lenke, H.J. Teschendorf, H.P. Hofmann, H. Kreiskott, W.J. Worstmann, J. Med. Chem. 29, 1877 (1986)
I.A. O’Neil, C.L. Murray, R.C. Hunter, S.B. Kalindjian, T.C. Jenkins, Synlett 1, 75 (1997)
Y. Liao, B.J. Venhuis, N. Rodenhuis, W. Timmerman, H. Wikström, E. Meier, G.D. Bartoszyk, H. Böttcher, C.A. Seyfried, S. Sundell, J. Med. Chem. 42, 2235 (1999)
F. Novelli, T. Bruno, F. Sparatore, IL Farmaco 52, 499 (1997)
G.N. Walker, R.T. Smith, J. Org. Chem. 36, 305 (1997)
K. Goutham, D.A. Kumar, S. Suresh, B. Sridhar, R. Narender, G.V. Karunakar, J. Org. Chem. 80, 11162 (2015)
L.M. Greene, G. Campiani, M. Lawler, D.C. Williams, D.M. Zisterer, Mol. Pharmacol. 73, 419 (2008)
A.M. McElligott, E.N. Maginn, L.M. Greene, S. McGuckin, A. Hayat, P.V. Browne, S. Butini, G. Campiani, M.A. Catherwood, E. Vandenberghe, D.C. Williams, D.M. Zisterer, M. Lawler, Cancer Res. 69, 8366 (2009)
M.E. Derieg, L.H. Sternbach, J. Heterocycl. Chem. 3, 237 (1996)
J. Pecher, A. Waefelaer, Bull. Soc. Chim. Belg. 87, 911 (1978)
H. Heaney, K.F. Shuhaibar, Tetrahedron Lett. 35, 2751 (1994)
M.T. ElGihani, H. Heaney, K.F. Shuhaibar, Synlett 9, 871 (1996)
K.R. Levan, C.A. Root, Inorg. Chem. 20, 3566 (1981)
K.R. Levan, C.A. Root, J. Org. Chem. 46, 2404 (1981)
M. Mahdavi, N. Foroughi, M. Saeedi, M. Karimi, H. Alinezhad, A. Foroumadi, A. Shafiee, T. Akbarzadeh, Synlett 25, 385 (2014)
M. Mahdavi, R. Hariri, M. Saeedi, M. Karimi, H. Alinezhad, A. Foroumadi, A. Shafiee, T. Akbarzadeh, Tetrahedron Lett. 56, 7082 (2015)
S. Farzipour, M. Saeedi, M. Mahdavi, H. Yavari, M. Mirzahekmati, N. Ghaemi, A. Foroumadi, A. Shafiee, Synth. Commun. 44, 481 (2013)
H. Alinezhad, M. Tajbakhsh, M. Zare, J. Fluor. Chem. 132, 995 (2011)
M. Weiss, J. Am. Chem. Soc. 74, 200 (1952)
H. Alinezhad, S.M. Tavakkoli, S. Res. Chem. Intermed. 41, 5931 (2015)
Acknowledgements
This research was supported by grants from the University of Mazandaran and the research council of Tehran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yavari, H., Alinezhad, H. & Tajbakhsh, M. Efficient synthesis of novel benzo [f]imidazo[1,2-d][1,4]oxazepine-5(6H)-one derivatives. Res Chem Intermed 43, 3283–3290 (2017). https://doi.org/10.1007/s11164-016-2825-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2825-z