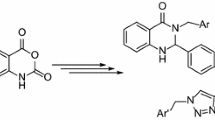
Abstract
A synthetic route has been proposed to 3-phenyl-1H-[1,4]oxazino[4,3-a]benzimidazol-1-one, which is the first representative of a new heterocyclic system. The transformation of the title compound to 4-phenyl-2,5-dihydro-1H-[1,2,5]triazepino[5,4-a]benzimidazol-1-one via reaction with hydrazine hydrate has been studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Interest in hetero-fused 1,2,5-triazepines is related to the broad spectrum of their biological activity [1]. However, design of drugs based thereon is restrained due to low accessibility of these compounds. They do not occur in nature, and organic synthesis is the main source of such structures. Unfortunately, no systematic studies on the assembly or fusion of 1,2,5-triazepine ring have been reported.
We previously proposed a synthetic approach to the first representative of a new heterocyclic system, 1,4-diphenyl-5H-[1,2,5]triazepino[5,4-a]benzimidazole [2], which is based on the reaction of 2-(2-benzoyl-1H-benzimidazol-1-yl)-1-phenylethanone with hydrazine hydrate, followed by thermal heterocyclization of the primary product. In continuation of our studies on the synthesis of benzimidazolo[1,2,5]triazepines, we presumed that, by analogy to 1,2-diazepine ring fusion, 1,2,5-triazepine ring can be fused to another heteroring via reaction of hydrazine with not only 1,5-dicarbonyl compounds containing the corresponding heterocycle [3] but also hetero-fused pyran-2-ones [1] and made an attempt to realize the latter approach.
Thus, the goal of the present work was to develop a preparative procedure for the synthesis of [1,4]oxazino[4,3-a]benzimidazole derivative and accomplish its transformation into the corresponding [1,2,5]triazepino[5,4-a]benzimidazole.
RESULTS AND DISCUSSION
The synthetic route to 3-phenyl-1H-[1,4]oxazino[4,3-a]benzimidazol-1-one (6) is outlined in Scheme 1. Methyl ester 2 was synthesized according to a procedure analogous to that described in [4]. Potassium 1-(2-oxo-2-phenylethyl)-1H-benzimidazole-2-carboxylate (4) was formed in the reaction of ester 2 with phenacyl bromide in the presence of potassium carbonate. This reaction can be carried out in acetone or acetonitrile, but the best result was obtained in moist acetonitrile. In this case, the product was a mixture of methyl 1-(2-oxo-2-phenylethyl)-1H-benzimidazole-2-carboxylate (3) and potassium 1-(2-oxo-2-phenylethyl)-1H-benzimidazole-2-carboxylate (4), but the latter was readily separated due to its poor solubility. Potassium salt 4 was then converted to sodium salt 5 by treatment with a saturated aqueous solution of sodium chloride.
Sodium 1-(2-oxo-2-phenylethyl)-1H-benzimidazole-2-carboxylate (5) was subjected to cyclization in thionyl chloride. The target product, 3-phenyl-1H-[1,4]oxazino[4,3-a]benzimidazol-1-one (6), was isolated by removal of excess thionyl chloride, followed by treatment of the residue with anhydrous methanol where compound 6 is almost insoluble. The yield of 6 was 50%; it was isolated as a pale yellow fine powder. The methanolic mother liquor contained a small amount of ester 3 and methyl 1-(1-chloro-2-oxo-2-phenylethyl)-1H-benzimidazole-2-carboxylate (9). Ester 3 is likely to be formed as a result of transesterification of 6 catalyzed by HCl generated from residual thionyl chloride and methanol. We failed to explain the formation of compound 9. Presumably, it resulted from radical chlorination of 3 on exposure to light.
Oxazinobenzimidazole 6 was also obtained by heating keto ester 3 in acetic anhydride for 8 h. It was isolated in 10% yield as yellowish needles, and its NMR spectra were completely identical to those of a sample isolated in the reaction of 5 with thionyl chloride. Prolonged heating of 3 in boiling acetic anhydride led to formation of tars. Compound 6 is the first representative of a new heterocyclic system, [1,4]oxazino[4,3-a]benzimidazoles. Acidification of an aqueous solution of sodium salt 5 gave acid 7 which underwent complete decarboxylation to 2-(1H-benzimidazol-1-yl)-1-phenylethanone (8) when dried at room temperature for 24 h (Scheme 1).
By analogy with the data of [5, 6, 7], we presumed that treatment of 6 with hydrazine hydrate will produce 4-phenyl-2,5-dihydro-1H-[1,2,5]triazepino[5,4-a]benzimidazol-1-one (11). However, instead of expected 11 we isolated 1-(2-oxo-2-phenylethyl)-1H-benzimidazole-2-carbohydrazide (10). Furthermore, no hydrazone at the phenacyl group was formed under these conditions. The same result was obtained in the reaction of 3 with hydrazine hydrate (Scheme 2). Hydrazide 10 in acetic acid was converted to triazepinone derivative 11. This cyclization conforms to the mechanism proposed previously [8].
It is known that triazepine ring tends to undergo contraction by the action of mineral acids [9]. No such ring contraction was observed in the case of triazepinone 11. By heating compound 11 in boiling aqueous HCl for 5 min we obtained benzimidazole 8 as a result of hydrolysis followed by decarboxylation.
Keto ester 3 is capable of reacting not only with hydrazine but also with other nitrogen nucleophiles. The reaction of 3 with excess formamide under reflux for 30 min quantitatively afforded 3-phenylpyrazino[1,2-a]benzimidazol-1(2H)-one (13) which is stabilized as aromatic tautomer, 3-phenylpyrazino[1,2-a]benzimidazol-1-ol (14). When ester 3 was heated in N-methylformamide for 30 min, the product was N-methyl-1-(2-oxo-2-phenylethyl)-1H-benzimidazole-2-carboxamide (15). In this case, stabilization of 2-methyl-3-phenylpyrazino[1,2-a]benzimidazol-1(2H)-one (16) via tautomerization is impossible, and compound 16 is not formed (Scheme 2).
As shown in [5, 6], recyclization of 6-phenyl-2H-pyran-2-one 17 with hydrazine to diazepinone 18 begins with the attack of hydrazine on C6, since the C5=C6 double bond is polarized so that the C6 atom bears a partial positive charge (Scheme 3). This mechanism [7–9] is typical of lactones with a π-amphoteric or π-rich aromatic ring. Structure 17 possesses one more reaction center, C2; however, its electrophilicity is reduced due to conjugation of the carbonyl group with the neighboring aromatic ring.
The imidazole aromatic system of oxazinone 6 is π-deficient, and the C5=C6 bond therein is not polarized. Therefore, nucleophile reacts at C2 to give hydrazide 10. Further cyclization of 10 to triazepinone 11 follows the mechanism proposed in [10].
An example of opening of a lactone ring in which the C5=C6 bond is not polarized was described in [11]. 1-phenyl-3H-pyrano[3,4-b][1]benzofuran-3-one (19) reacted with hydrazine hydrate to give 2-{2-[(E,Z)-hydrazinylidene(phenyl)methyl]-1-benzofuran-3-yl}acetohydrazide (20) rather than diazepinone 21 (Scheme 4). In this case, the attack of hydrazine was directed at C2. Molecule 19 possesses one more reaction center, C4, and compound 19 can be regarded as a 3-phenylacrylic acid ester which is capable of reacting with hydrazine according to Michael to produce 4a-hydrazinyl-1-phenyl-4,4a-dihydro-3H-pyrano[3,4-b][1]benzofuran-3-one (22). Reaction of the latter with excess hydrazine could give rise to 2-{3-hydrazinyl-2-[(E,Z)-hydrazinylidene(phenyl)methyl]-2,3-dihydro-1-benzofuran-3-yl}acetohydrazide (23). Presumably, compound 23 remained in the mother liquor after recrystallization of the products obtained in the reaction of 19 with hydrazine. Of particular interest is cyclization of hydrazone 23 under the same conditions as for hydrazone 20.
EXPERIMENTAL
The 1H and 13C NMR spectra were recorded on a Bruker Avance II spectrometer at 400 and 100 MHz, respectively, using DMSO-d6 as solvent and tetramethylsilane as internal standard. The melting points were measured on a Boetius type melting point apparatus and are uncorrected.
Methyl 1-(2-oxo-2-phenylethyl)-1H-benzimidazole-2-carboxylate (3). A mixture of 10 g (47 mmol) of compound 2, 14 g (70 mmol) of ω-bromoacetophenone, and 16 g (0.116 mol) of finely powdered potassium carbonate in 140 mL of acetonitrile was refluxed for 6 h. The mixture was cooled and filtered, and the precipitate was washed with acetonitrile. The solvent was distilled off to dryness, and the residue was recrystallized from propan-2-ol. Yield 2.6 g (19%), colorless crystals, mp 165–166°C. 1H NMR spectrum, δ, ppm: 3.88 s (3H, CH3), 6.23 s (2H, CH2), 7.34 t (1H, CH, J = 6.8 Hz), 7.39 t (1H, CH, J = 7.2 Hz), 7.60 t (2H, CH, J = 7.6 Hz), 7.65–7.75 m (2H, CH), 7.81 d (1H, CH, J = 7.6 Hz), 8.12 d (2H, CH, J = 7.6 Hz). 13C NMR spectrum, δC, ppm: 51.6 (CH2), 52.1 (CH3), 111.1 (CH), 120.9 (CH), 123.0 (CH), 124.9 (CH), 128.0 (2C, CH), 128.5 (2C, CH), 133.6 (CH), 134.4, 136.4, 140.6, 141.2, 159.9, 192.3 (CO). Found, %: C 69.34; H 4.83; N 9.57. C17H14N2O3. Calculated, %: C 69.38; H 4.79; N 9.52. M 294.31.
Sodium 1-(2-oxo-2-phenylethyl)-1H-benzimidazole-2-carboxylate (5). The solid product separated from the reaction mixture in the synthesis of 3 was added to 200 mL of a saturated solution of sodium chloride, and the mixture was stirred at 80°C for 30 min. The mixture was cooled, and the precipitate was filtered off, washed with brine, and dried.
2-(1H-Benzimidazol-1-yl)-1-phenylethanone (8). A solution of 0.5 g of sodium salt 5 in 5 mL of water was acidified to pH 6 with acetic acid, and the precipitate was filtered off and washed with water. Yield 260 mg (95%), colorless crystals, mp 120–121°C. 1H NMR spectrum, δ, ppm: 5.96 s (2H, CH2), 7.15–7.24 m (2H, CH), 7.35–7.44 m (1H, CH), 7.59 t (2H, CH, J = 7.6 Hz), 7.36–7.66 m (1H, CH), 7.70 t (1H, CH, J = 7.2 Hz), 8.08 s (1H, CH), 8.11 d (2H, CH, J = 7.6 Hz). 13C NMR spectrum, δC, ppm: 50.5 (CH2), 110.1 (CH), 119.2 (CH), 121.0 (CH), 121.9 (CH), 128.0 (2C, CH), 128.5 (2C, CH), 133.5 (CH), 134.4, 143.1, 144.5 (CH), 192.6 (CO). Found, %: C 76.21; H 5.18; N 11.89. C15H12N2O. Calculated, %: C 76.25; H 5.12; N 11.86. M 236.28.
3-Phenyl-1H-[1,4]oxazino[4,3-a]benzimidazol-1-one (6). Five to six drops of dimethylformamide were added with stirring to 100 mL of thionyl chloride, and sodium salt 5 was then added in portions. The mixture was stirred for 15 min at room temperature and was then refluxed for 1 h. The mixture was cooled, excess thionyl chloride was distilled off, and anhydrous methanol was added to the residue with stirring. The mixture was stirred for 10 min, and the precipitate was filtered off and washed with anhydrous methanol and water. Yield 6.2 g (50%), fine yellow crystals, mp 248–249°C. 1H NMR spectrum, δ, ppm: 7.43 t (1H, CH, J = 7.2 Hz), 7.46–755 m (3H, CH), 7.67 t (1H, CH, J = 7.2 Hz), 7.88 d (2H, CH, J = 8.0 Hz), 7.91 d (1H, CH, J = 8.4 Hz), 8.21 d (1H, CH, J = 8.4 Hz), 8.91 s (1H, CH). 13C NMR spectrum, δC, ppm: 103.4 (CH), 112.6 (CH), 121.3 (CH), 124.0 (2C, CH), 125.3 (CH), 125.7 (CH), 128.7 (2C, CH), 129.1 (CH), 129.9, 130.4, 134.6, 141.2, 143.0, 152.5 (CO). Found, %: C 73.18; H 3.87; N 10.71. C16H10N2O2. Calculated, %: C 73.27; N 3.84; N 10.68; O 12.20. M 262.27.
Methyl 1-(1-chloro-2-oxo-2-phenylethyl)-1H-benzimidazole-2-carboxylate (9). The methanolic filtrate obtained in the synthesis of 6 was evaporated, and the residue was recrystallized from propan-2-ol. The crystalline product, 1.35 g, was a mixture of compounds 3 and 9 at a ratio of 2:3. Repeated crystallization from 8 mL of methanol gave 0.5 g of 9 as colorless cubic crystals with mp 140–141°C. 1H NMR spectrum, δ, ppm: 4.02 s (3H, CH3), 7.37 t (1H, CH, J = 7.2 Hz), 7.43 t (1H, CH, J = 7.2 Hz), 7.51 t (2H, CH, J = 8.0 Hz), 7.60–7.67 m (2H, CH), 7.79 d (1H, CH, J = 8.0 Hz), 7.98 d (2H, CH, J = 8.0 Hz), 8.93 s (1H, CHCl). 13C NMR spectrum, δC, ppm: 52.8 (CH3), 66.0 (CHCl), 113.9 (CH), 121.4 (CH), 123.9 (CH), 125.7 (CH), 128.5 (2C, CH), 128.6 (2C, CH), 132.8, 133.7 (CH), 134.1, 139.9, 141.4, 159.8 (COO), 187.1 (CO). Found, %: C 62.07; H 4.04; Cl 10.77; N 8.56. C17H13ClN2O3. Calculated, %: C 62.11; H 3.99; Cl 10.78; N 8.52. M 328.76.
1-(2-Oxo-2-phenylethyl)-1H-benzimidazole-2-carbohydrazide (10). A mixture of 0.8 mmol of compound 3 or 6, 3 mL of methanol, and 2 mmol of hydrazine hydrate was refluxed for 1 h. The mixture was cooled and diluted with 8 mL of water, and the precipitate was filtered off. Yield 130 mg (58%), fine colorless crystals, mp 177–178°C. 1H NMR spectrum, δ, ppm: 6.29 s (2H, CH2), 7.25–7.35 m (2H, CH), 7.55–7.65 m (3H, CH), 7.69 t (1H, CH, J = 7.2 Hz), 7.73–7.79 m (1H, CH), 8.12 d (2H, CH, J = 7.6 Hz), 10.09 br.s (1H, NH). 13C NMR spectrum, δC, ppm: 51.2 (CH2), 110.7 (CH), 119.9 (CH), 122.6 (CH), 123.7 (CH), 128.0 (2C, CH), 128.5 (2C, CH), 133.4 (CH), 134.6, 136.4, 140.9, 142.9, 157.8 (CONH), 192.5 (CO). Found, %: C 65.37; H 4.83; N 19.07. C16H14N4O2. Calculated, %: C 65.30; H 4.79; N 19.04. M 294.32.
4-Phenyl-2,5-dihydro-1H-[1,2,5]triazepino[5,4-a]benzimidazol-1-one (11). A mixture of 162 mg (0.6 mmol) of compound 10, 0.1 mL of acetic acid, and 3 mL of water was refluxed for 30 min. The mixture was cooled, and the precipitate was filtered off. Yield 150 mg (98%), fine colorless crystals, mp 248–249°C. 1H NMR spectrum, δ, ppm: 5.60 s (2H, CH2), 7.30 t (1H, CH, J = 6.4 Hz), 7.35–7.51 m (4H, CH), 7.74 d (1H, CH, J = 7.6 Hz), 7.96–8.12 m (3H, CH), 11.70 s (1H, NH). 13C NMR spectrum, δC, ppm: 39.8 (CH2), 110.8 (CH), 120.6 (CH), 122.9 (CH), 124.5 (CH), 126.8 (2C, CH), 128.5 (2C, CH), 130.4 (CH), 132.9, 133.7, 142.0, 144.1, 157.0, 157.6. Found, %: C 79.59; H 4.42; N 20.31. C16H12N4O. Calculated, %: C 79.55; H 4.38; N 20.28. M 276.30.
3-Phenylpyrazino[1,2-a]benzimidazol-1(2H)-one (13). A mixture of 200 mg (0.7 mmol) of compound 3 and 2 mL of formamide was refluxed for 30 min. The mixture was cooled, and the colorless crystals were filtered off and washed with water. Yield 165 mg (93%), mp 342–343°C. 1H NMR spectrum, δ, ppm: 7.39–7.54 m (5H, CH), 7.79 d (2H, CH, J = 7.2 Hz), 7.86–7.92 m (1H, CH), 8.21 m (1H, CH), 8.34 s (1H, CH), 11.78 br.s (1H, NH or OH). 13C NMR spectrum, δC, ppm: 103.6 (CH), 112.6 (CH), 120.7 (CH), 124.0 (CH), 126.4 (2C, CH), 127.4, 128.6 (2C, CH), 128.8 (CH), 130.3, 131.4, 139.7, 142.8, 154.8 (CO). Found, %: C 73.63; H 4.29; N 16.12. C16H11N3O. Calculated, %: C 73.55; H 4.24; N 16.08. M 261.29.
N-Methyl-1-(2-oxo-2-phenylethyl)-1H-benzimidazole-2-carboxamide (15). A mixture of 200 mg (0.7 mmol) of compound 3 and 2 mL of N-methylformamide was refluxed for 30 min. The mixture was cooled and diluted with 5 mL of water, and the precipitate was filtered off, washed with water, dried, and recrystallized from methanol. Yield 73 mg (35%), colorless crystals, mp 172–173°C. 1H NMR spectrum, δ, ppm: 2.79 d (3H, CH3, J = 4.6 Hz), 6.28 s (2H, CH2), 7.25–7.35 m (2H, CH), 7.55–7.62 m (3H, CH), 7.69 t (1H, CH, J = 7.6 Hz), 7.72–7.77 m (1H, CH), 8.11 d (2H, CH, J = 7.6 Hz), 8.80 q (1H, NH, J = 4.6 Hz). 13C NMR spectrum, δC, ppm: 25.5 (CH3), 51.3 (CH2), 110.8 (CH), 119.8 (CH), 122.6 (CH), 123.7 (CH), 128.0 (2C, CH), 128.4 (2C, CH), 129.5, 133.3 (CH), 134.6, 136.6, 140.7, 159.4 (CON), 192.4 (CO). Found, %: C 69.65; H 5.19; N 14.38. C17H15N3O2. Calculated, %: C 69.61; H 5.15; N 14.33. M 293.33.
CONCLUSIONS
A preparative procedure has been developed for the synthesis of 3-phenyl-1H-[1,4]oxazino[4,3-a]benzimidazol-1-one (6), and its transformation into 4-phenyl-2,5-dihydro-1H-[1,2,5]triazepino[5,4-a]benzimidazol-1-one has been studied. A plausible mechanism of this transformation has been proposed. Both 4-phenyl-2,5-dihydro-1H-[1,2,5]triazepino[5,4-a]benzimidazol-1-one and other cyclic derivatives based on benzimidazole-2-carboxylic acid can also be synthesized from methyl 1-(2-oxo-2-phenylethyl)-1H-benzimidazole-2-carboxylate without intermediate preparation of the corresponding lactone.
REFERENCES
Elattar, K.M., Abozeid, M.A., and Etman, H.A., Synth. Commun., 2016, vol. 46, p. 93. https://doi.org/10.1080/00397911.2015.1109126
Kharaneko, A.O., Russ. J. Org. Chem., 2019, vol. 55, p. 115. https://doi.org/10.1134/S1070428019010147
Kharaneko, A.O., Russ. J. Org. Chem., 2016, vol. 52, p. 892. https://doi.org/10.1134/S1070428016060221
Copeland, R.A.B. and Day, A.R., J. Am. Chem. Soc., 1943, vol. 65, p. 1072. https://doi.org/10.1021/ja01246a019
Kharaneko, A.O. and Kharaneko, O.I., Russ. J. Org. Chem., 2018, vol. 54, p. 742. https://doi.org/10.1134/S1070428018050111
Shablikіna, O.V., Krekhova, O.F., Konovalenko, A.S., Moskvіna, V.S., and Khilya, V.P., Dopov. Nats. Akad. Nauk Ukr., 2018, no. 12, p. 71. https://doi.org/10.15407/dopovidi2018.12.071
Kharaneko, A.O., Pekhtereva, T.M., and Kharaneko, O.I., Russ. J. Org. Chem., 2020, vol. 56, p. 654. https://doi.org/10.1134/S1070428020040144
Kharaneko, A.O. and Bogza, S.L., Russ. J. Org. Chem., 2016, vol. 52, p. 1043. https://doi.org/10.1134/S1070428016070228
Khabarov, K.M., Kharaneko, O.I., and Bogza, S.L., Chem. Heterocycl. Compd., 2009, vol. 45, p. 468. https://doi.org/10.1007/s10593-009-0280-0
Kharaneko, O.I., Russ. J. Org. Chem., 2017, vol. 53, p. 738. https://doi.org/10.1134/S1070428017050153
Eresko, A.B., Tolkunov, V.S., and Tolkunov, S.V., Chem. Heterocycl. Compd., 2010, vol. 46, p. 1127. https://doi.org/10.1007/s10593-010-0637-4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Additional information
Translated from Zhurnal Organicheskoi Khimii, 2021, Vol. 57, No. 3, pp. 426–432 https://doi.org/10.31857/S0514749221030113.
Rights and permissions
About this article
Cite this article
Kharaneko, A.O., Pekhtereva, T.M. & Kharaneko, O.I. Synthesis of 3-Phenyl-1H-[1,4]oxazino[4,3-a]benzimidazol-1-one and Its Transformation into 4-Phenyl-2,5-dihydro-1H-[1,2,5]triazepino[5,4-a]benzimidazol-1-one. Russ J Org Chem 57, 396–401 (2021). https://doi.org/10.1134/S1070428021030118
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428021030118