Abstract
An efficient one-pot synthesis of new types of pyrrolo/pyrido[1,2-a][1,3]diazepines by using the seven-membered ring HKA, an activated methylene compound, and either arylglyoxal monohydrates or salicylaldehyde is described. This method has the advantages of mild reaction conditions and absence of catalyst and provides an entry point to pyrrolo/pyrido and diazepine ring structures.
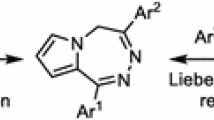
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diazepine nucleus is a pharmacophoric scaffold, and many diazepines have recently received great attention, because of their wide range of therapeutic and pharmacological properties [1,2,3]. Among them, pyrrolo[1,2-a][1,3]diazepines have attracted much attention as substances with pronounced central nervous system activity and anxiolytic, sedative and antiepileptic activities [4,5,6,7]. A number of pyrrolo[1,2-a][1,3]diazepinone derivatives possess analgesic action and act as antibacterial and antifungal agents with high specificity towards dermatophytes [8,9,10]. Selected bioactive compounds consisted of pyrrolodiazepinone and pyridodiazepines are shown in Fig. 1 [11]. The main approach to this structure includes a cyclization to the diazepine cycle with the pyrrole ring being already present. The popularity of this method can be explained by a facile electrophilic substitution in a pyrrole ring. An interesting method for the synthesis of the pyrrolo[1,2-a][1,3]diazepine fragment was recently reported by Ivachtchenko et al. [12]. They used bifunctional 1-(2-carboxyaryl) pyrrolecarbaldehyde and Ugi reaction conditions for this purpose.
Heterocyclic ketene aminals (HKAs) are push–pull alkenes with the electron-withdrawing NO2 and electron-donating amino groups [13, 14]. They are important building blocks in organic chemistry for synthesizing heterocyclic or fused heterocyclic compounds [15,16,17,18,19,20,21,22,23,24,25,26]. They have been used for the synthesis of a wide variety of heterocyclic systems and natural products, and precursors of chiral amines in asymmetric transformations [27,28,29,30].
We have recently started investigations on the one-pot reactions involving various ring sizes of HKAs for the synthesis of pyrido/pyrrolo[1,2-a][1,3]diazepines. To further explore the potentials of such kind of strategy for pyrido/pyrrolo[1,2-a][1,3]diazepines synthesis, we report our study on the one-pot reactions involving seven-membered ring HKA, an activated methylene compound, and either arylglyoxal monohydrates or salicylaldehyde under catalyst-free conditions in ethanol.
Results and discussion
Our studies were initiated by heating a solution of HKA 1, salicylaldehyde 2 and Meldrum’s acid 3 or malononitrile 4 in EtOH at reflux for 3 h. The reactions proceeded smoothly providing the new kinds of pyrido[1,2-a][1,3]diazepine 6 and 7. When we applied methyl cyanoacetate 5 instead of malononitrile 4, new chromene-3-carbonitrile derivatives 8 were obtained in the same condition. The yield of product 8b was very low in different conditions (Table 1).

To achieve the optimal conditions for the synthesis of pyrido[1,2-a][1,3]diazepine derivatives, we chose the reaction of 1, salicylaldehyde 2a and Meldrum’s acid 3 or malononitrile 4 as a model reaction to optimize the reaction conditions. Various solvents and catalysts were examined to develop standard reaction condition. The reaction proceeded with excellent yields when ethanol was used as the solvent at reflux. The results are summarized in Table 2.
The structures of compound 6 were deduced from their IR, 1H and 13C NMR and elemental analysis. In the IR spectrum of 6, absorption bands at 3450, 3055, 1724, 1451 and 1378 cm−1 were attributed to the OH, NH, C=O and NO2 stretching frequencies, respectively, indicating of the functional groups in the product. In this molecule, the CH2 protons are diastereotopic; the methine and methylene protons appear as an AMX system (2 J AM = 15.9 Hz, 3 J AX = 6.9 Hz, 3 J MX = 1.5 Hz, δ A = 2.66 ppm, δ M = 3.14 ppm, δ X = 4.78 ppm). Thus, the 1H NMR spectrum of 6 exhibited three dd (δ (H) 4.78, 3.14, 2.66) for the CH and CH2 protons. The 1H-decoupled 13C NMR spectrum of 6 showed 15 distinct resonances, in agreement with the proposed structure [31].
The structures of compounds 7a and 7b were deduced from their 1H NMR, 13C NMR and IR spectroscopy as well as mass spectrometry. For example, the 1H NMR spectrum of 7a clearly showed five singlets identified as amino groups (δ = 10.65 and 6.13), hydroxy (δ = 9.47), methine proton (δ = 4.85), along with characteristic multiplets for four CH2 groups (δ 3.65–4.05 and 1.48–2.05), and multiplets for the aromatic region (δ 6.66–6.98). The 1H-decoupled 13C NMR spectrum of 7a indicated 16 distinct resonances, which confirmed the suggested structure. The OH and amino protons resonance (at δ 10.65, 9.47 and 6.13), disappeared after addition of D2O to the DMSO solution of 7a.
The mechanism of these pyrido[1,2-a][1,3]diazepines is shown in Scheme 1. The reaction started with the condensation of Meldrum’s acid 3 or malononitrile 4 with the salicylaldehyde [32]. The resulting intermediate 9 reacted with HKA possibly via the aza-ene reaction and then imine–enamine tautomerization to form 10. This intermediate could cyclization by two paths. Product 7 is generated via attack of the NH group to the CN group, in path I. The NH group attacks carbonyl group or imino group to afford 11 which is decarboxylated to generate 6 or imine–enamine tautomerization to produce 7 in path II. Based on these results, Michael addition of 1–9 gives intermediate 12, followed by air oxidation to afford product 8 (Scheme 2).
The potential of this protocol for the synthesis of pyrrolo[1,2-a][1,3]diazepine was explored by using the barbiturates 13 as an activated methylene compound in a four-component reaction (Scheme 3). The reaction between diamines (1,2-ethanediamine, 1,3-propanediamine, 1,4-butanediamine), l,l-bis(methylthio)-2-nitroethene, barbiturates 13 and arylglyoxal 14 in ethanol under reflux condition leads to the formation of dihydropyrazine 15, hydroxy dihydropyrrole 16 and pyrrolodiazepines 17a and 17b in good yields, respectively. In this reaction, two new kinds of pyrrolo[1,2-a][1,3]diazepine (17a and 17b) were isolated by column chromatography in same condition. The total yield of them was 80% (17a, 35% and 17b, 45%).
It was interesting that when we used 1,2-ethanediamine, the 1,1-bis(methylsulfanyl)-2-nitroethene was not participated in this reaction. A number of efficient approaches for bicyclic pyrrole derivatives through six-membered ring of HKAs have been explored [33,34,35]. Depending on the various diamines that used in this reaction, diverse new hydroxy dihydropyrrole and dihydropyrazine rings have been prepared. The effect of ring size of HKAs appears in further reactivity of nitrogen lone pairs in six, or seven membered ring over the five-membered ring.
The structure of the product 13–17 was identified by their IR, 1H NMR, 13C NMR and mass spectra. For example, the 1H NMR spectrum of 15 showed two singlets for the NMe2 (δ = 2.95 ppm) and one signal for the OH group (δ = 11.82), they can be exchanged with deuterium, along with characteristic signals for the phenyl moiety (Fig. 2). The 13C NMR spectrum of 15 showed 14 resonances in agreement with the proposed structure. The C=C–CO appeared at δ = 90.7 and C=N appeared at δ = 155.9 ppm as key signals (Fig. 3). The C=N of 15 showed strong absorption band at about 1692 cm−1 in the IR spectrum. The 1H NMR spectrum of 16 showed three multiplets for the methylene protons at δ = 1.76–1.88 and 2.85–2.70 and four singlets for the CH, OH, NH and OH groups at δ = 4.02, 8.07, 9.86 and 10.39, respectively, with characteristic signal for the aromatic moiety. The 13C NMR spectrum of 16 showed 15 distinct resonances which was consistent with the proposed structure.
The 1H NMR spectrum of 17b exhibited three multiplets for the methylene protons at δ = 1.92–2.04 and 3.46–3.71 and three singlets for NMe2, CH and NH groups at δ = 3.31, 4.35 and 7.81 with characteristic signal for the aromatic moiety (Fig. 4). The 13C NMR spectrum of 17b showed 16 distinct resonances due to C–H, C–NO2, Cipso–CH and CNN appearing at δ = 45.9, 107.3, 133.0 and 151.7 ppm, respectively (Fig. 5). The mass spectrum of 17b displayed the molecular ion peak at m/z 411, in agreement with the proposed structure. The IR spectrum of this compound showed absorption bands due to the NH, CO, NO2, C–N groups at 3344, 1674, 1367, 1268 cm−1.
A plausible mechanism for the formation of compound 16 and pyrrolo[1,2-a][1,3]diazepine 17a and 17b is proposed in Scheme 4. The HKA can react with the arylglyoxal monohydrate 14 via an aza-ene reaction. Thereafter, 18 tautomerizes to 19 by an imine–enamine process, and then, the NH group attacks the intramolecular carbonyl group to afford 20. Subsequently, 21 is generated through the addition of 13–20. This intermediate can lose H2O to generate the stabilized pyrrol 17b or undergoes enolization to give 17a.
Conclusion
We concluded, when seven-membered ring HKA was used, various pyrido/pyrrolo[1,2-a][1,3]diazepines have been prepared. The reaction was shown to have attractive features and molecular diversities. These types of heterocycles contain a number of functional groups with possible biological activities. These one-pot reactions involving HKAs and other kinds of active methylene compounds are extendable for producing of other heterocyclic compounds and synthesis of different types of pyrrolo/pyrido[1,2-a][1,3]diazepines.
Experimental
General. All commercially available reagents were purchased from Fluka (Switzerland) and Merck (Germany) chemical Co. and used without further purification unless otherwise stated. NMR spectra were recorded with a Bruker DRX-300 AVANCE instrument (300 MHz for 1H and 75.4 MHz for 13C) with CDCl3 and DMSO-d6 as solvent. Chemical shifts are given in ppm (δ) relative to internal TMS, and coupling constant (J) is reported in hertz (Hz). Melting points were measured with an electrotherma1 9100 apparatus. Mass spectra were recorded with an Agilent 5975C VL MSD with Triple-Axis Detector operating at an ionization potential of 70 eV. IR spectra were measured with Bruker Tensor 27 spectrometer. Compound 1 was prepared according to the literature [21].
General procedure for the preparation of compounds 6, 7 and 8. A mixture of HKA 1 (1 mmol), salicylaldehyde 2 (1 mmol) was heated in EtOH (10 mL) at reflux. Then Meldrum’s acid 3 or malononitril 4 (1 mmol) was added to the reaction solution, and the mixture was stirred at reflux. After 3 h, the precipitate was filtered and washed with ethanol to afford the pure product.
General procedure for the preparation of compounds 15, 16 and 17. HKAs (1 mmol), arylglyoxal monohydrates 12 (1 mmol) and 1,3-dimethyl barbituric acid 11 (1 mmol) were dissolved in EtOH (10 mL), and the mixture was refluxed in a round-bottomed flask for 3 h. The solvent was removed under reduced pressure, and the residue was purified by silica gel (Merck 60, 70–230 mesh) column chromatography using hexane–ethyl acetate (5:1).
9-(2-Hydroxyphenyl)-10-nitro-2,3,4,5,8,9-hexahydropyrido[1,2-a][1,3]diazepin-7(1H)-one (6)
Yield: 0.203 g (67%). White powder. M.p. 265–267 °C. IR: 3450 (OH), 3055 (NH), 2990 (C–H), 1724 (C=O), 1599 (C=C), 1451, 1378 (NO2), 1268 (C–N), 1118 (C–O). 1H NMR (DMSO-d 6): δ 1.58–1.95 (m, 2CH2); 2.66 (dd, 2 J = 15.9 Hz, 3 J = 1.5 Hz, 1H); 3.14 (dd, 2 J = 15.9 Hz, 3 J = 6.9 Hz, 1H); 3.41–3.84 (m, 2 CH2NH); 4.21–4.25 (m, CH2N); 4.78 (dd, 3 J = 6.9 Hz, 3 J = 1.5 Hz, 1H); 6.64 (t, 3 J = 7.8 Hz, 1 arom. H); 6.70 (d, 3 J = 7.8 Hz, 1 arom. H); 6.81 (d, 3 J = 7.8 Hz, 1 arom. H); 7.03 (t, 3 J = 7.8 Hz, 1 arom. H); 9.69 (s, OH); 11.23 (br s, NH). 13C NMR (DMSO-d 6): δ 24.8, 25.2 (2CH2); 32.1 (CH2); 37.6 (CH); 45.2, 45.7 (2 CH2N); 112.2 (C–NO2); 115.8 (1 arom. CH); 119.5 (1 arom. CH); 126.1 (1 arom. CH); 126.5 (Cipso); 128.5 (1 arom. CH); 155.5 (Cipso–OH); 158.9 (CNN); 170.3 (C=O). Anal. calc. for C15H17N3O4 (303.31): C 59.40, H 5.65, N 13.85; found: C 59.1, H 5.9, N 13.6.
7-Amino-9-(2-hydroxyphenyl)-10-nitro-1,2,3,4,5,9-hexahydropyrido[1,2-a][1,3]diazepine-8-carbonitrile (7a)
Yield: 0.294 g (90%); yellow powder; mp 236–238 °C (dec.). IR (KBr) ῡ = 3465, 3358, 3300, 2179, 1645, 1494, 1345, 1211, 1101 cm−1; 1H NMR (300 MHz, DMSO-d 6): δ = 10.65 (1H, s, NH), 9.47 (1H, s, OH), 6.98–6.66 (4H, m, ArH), 6.13 (2H, s, NH2), 4.85 (1H, s, CH), 4.05–3.65 (4H, m, 2 CH2NH), 2.05–1.48 (4H, m, 2 CH2); 13C NMR (75.4 MHz, DMSO-d 6): δ = 158.6 (CNN), 155.6 (C–OH), 154.9 (NCNH2), 129.3 (C of Ar), 129.2 (CH of Ar), 128.1 (CH of Ar), 121.1 (CN), 119.2 (CH of Ar), 115.9 (CH of Ar), 112.6 (CNO2), 64.1 (CCN), 53.3, 45.7 (2 CH2N), 36.9 (CH), 26.8, 25.7 (2 CH2); MS: m/z = 327 (M+, 4), 281 (12), 236 (3), 170 (94), 143 (100), 115 (40), 70 (20), 41 (16). Anal. calc. for C16H17N5O3 (327.34): C 58.71, H 5.23, N 21.39.
7-Amino-9-(2-hydroxy-3-methoxyphenyl)-10-nitro-2,3,4,5-tetrahydropyrido[1,2-a][1,3]diazepine-8-carbonitrile (7b)
Yield: 0.216 g (61%); light brown powder; mp > 350 °C (dec.). 1H NMR (300 MHz, DMSO-d 6): δ = 8.49 (1H, m, ArH), 7.70–7.56 (2H, m, ArH), 7.41 (2H, s, NH2), 6.98 (1H, br s, OH), 3.94 (3H, s, OMe), 3.70–3.60 (2H, m, 2 CH2NH), 2.25–1.53 (4H, m, 2 CH2); MS: m/z = 355 (M+, 2), 330 (100), 315 (16), 287 (94), 143 (22), 259 (12), 205 (12), 165 (13), 137 (9), 115 (5), 70 (18), 44 (32). Anal. calc. for C17H17N5O4 (355.35): C 57.46, H 4.82, N 19.71.
6-Hydroxy-1,3-dimethyl-5-(3-phenyl-1,2,5,6-tetrahydropyrazin-2-yl)pyrimidine-2,4(1H,3H)-dione (13)
Yield 78%; cream powder; mp 267–269 °C. IR (KBr): ῡ = 3448, 3100, 2883, 1692, 1595, 1444, 1344, 1137 cm−1. 1H NMR (300 MHz, DMSO-d 6): δ = 11.82 (1H, s, OH), 7.50 (2H, d, 3 J = 6.9 Hz, ArH), 7.28–7.20 (3H, m, ArH), 3.75–3.69 (2H, m, CH2), 3.30–3.36 (2H, m, CH2), 2.95 (6H, 2NMe); 13C NMR (75.4 MHz, DMSO-d 6): δ = 163.1 (=C–OH), 155.9 (2C=N), 151.6 (C=O), 139.3 (Cipso), 129.3 (CHpara), 128.1 (2CHortho), 125.7 (2CHmeta), 90.7 (C=C–OH), 46.7, 37.5 (2CH2N), 28.0, 27.7 (2NMe).
4-((1,3-diazepan-2-ylidene)(nitro)methyl)-2-oxo-2H-chromene-3-carbonitrile (8a)
Yield: 0.192 g (69%); brown powder; mp > 300 °C (dec.). IR (KBr): ῡ = 3938, 2145, 1639, 1422, 1316, 1211, 1150 cm−1; 1H NMR (300 MHz, DMSO-d 6): δ = 10.65 (2H, br s, 2 NH), 8.99 (1H, d, 3 J HH = 8.4 Hz, Ar), 7.73 (1H, t, 3 J HH = 8.1 Hz, Ar), 7.45–7.42 (2H, m, Ar), 3.10–2.95 (4H, m, 2CH2N), 1.99–1.75 (4H, m, 2CH2) ppm.
6-Hydroxy-5-(6-hydroxy-8-nitro-6-phenyl-1,2,3,4,6,7-hexahydropyrrolo[1,2-a]pyrimidin-7-yl)-2-thioxo-2,3-dihydropyrimidin-4(1H)-one (16)
Yield (60%); light brown powder; mp 310 °C (dec.). 1H NMR (300 MHz, DMSO-d 6): δ = 10.39 (1H, s, NH), 9.86 (1H, s, OH), 8.07 (1H, s, OH), 7.39–7.04 (5H, m, ArH), 4.02 (1H, s, CH), 2.85–2.70 (4H, m, 2CH2N), 1.88–1.76 (2H, m, CH2); 13C NMR (75.4 MHz, DMSO-d 6): δ = 165.0 (C=O), 163.7 (=C–OH), 163.5 (CNN), 153.5 (C=O), 139.2 (Cipso), 129.6 (2CHmeta), 128.8 (CHpara), 128.5 (2CHortho), 103.9 (=C–NO2), 96.2 (C–OH), 86.7 (C=C–OH), 38.8 (CH), 38.5 (2CH2N), 36.7 (2CH2NH), 18.6 (CH2).
6-Hydroxy-5-(7-hydroxy-9-nitro-7-phenyl-2,3,4,5,7,8-hexahydro-1H-pyrrolo[1,2-a][1,3]diazepin-8-yl)-1,3-dimethylpyrimidine-2,4(1H,3H)-dione (17a)
Yield 76%; yellow paste; mp 250–252 °C. 1H NMR (300 MHz, CDCl3): δ = 10.33 (1H, s, NH), 8.01 (1H, s, OH), 7.45–7.20 (5H, m, ArH), 7.26 (1H, s, CH), 3.32, 3.22 (6H, s, 2 NMe), 3.62–2.95 (4H, m, 2CH2N), 1.75–1.57 (2H, m, 2CH2); 13C NMR (75.4 MHz, CDCl3): δ = 166.5 (C=O), 164.2 (=C–OH), 163.9 (CNN), 151.9 (C=O), 138.7 (Cipso), 29.2 (CHpara), 128.3 (2CHmeta), 125.6 (2CHortho), 107.5 (=C–NO), 100.1 (C–OH), 87.9 (C=C–OH), 45.5 (CH), 44.0 (CH2N), 41.0 (CH2NH), 29.7, 28.0 (2NMe), 27.4, 26.5 (2CH2); MS (70 eV): m/z = 429 (M+, 0.2), 411 (20), 365 (93), 285 (3), 251 (14), 182 (13), 149 (19), 117 (9), 84 (100), 43 (65).
1,3-Dimethyl-5-(9-nitro-7-phenyl-2,3,4,5-tetrahydro-1H-pyrrolo[1,2-a][1,3]diazepin-8-yl)pyrimidine-2,4,6(1H,3H,5H)-trione (17b)
Yield: 89%; yellow paste; mp 264–266 °C; IR (KBr): ῡ = 3344, 2922, 1674, 1521, 1367, 1268 cm−1. 1H NMR (300 MHz, CDCl3): δ = 7.81 (1H, s, NH), 7.44–7.42 (5H, m, ArH), 4.35 (1H, s, CH), 3.71–3.46 (2H, m, CH2N), 3.31 (6H, s, 2NMe), 2.04–1.92 (2H, m, CH2); 13C NMR (75.4 MHz, CDCl3): δ = 167.0 (2C=O), 151.7 (CNN), 150.5 (C=O), 133.0 (=C–N), 130.4 (2CHortho), 129.3 (CHara), 129.2 (2CHmeta), 128.5 (Cipso), 116.6 (C–NO2), 107.3 (C=C–N), 48.2 (CH), 47.9 (CH2N), 45.9 (CH2NH), 28.9 (2NMe), 28.8, 26.8 (2CH2); MS (70 eV): m/z = 411 (M+, 54), 365 (100), 308 (1), 251 (17), 182 (5), 142 (2), 104 (11), 55 (11).
References
A.B. Movahed, M.H. Mosslemin, Chem. Sci. Trans. 1, 365 (2012)
A. Alizadeh, A. Rezvanian, Y. Deng, Tetrahedron 66, 9933 (2010)
M. Driowya, A. Saber, H. Marzag, L. Demange, K. Bougrin, R. Benhida, Molecules 21, 1032 (2016)
A.S. Tatyana, V.B. Alexander, K.V. Vladimir, A.N. Tatyana, D.K. Gennady, Synlett 7(1106), 2 (2007)
A.R. Katritzky, R. Jain, R. Akhmedova, Y.-J. Xu, Arkivoc ix, 4 (2003)
J.H. Lin, H.G. Ramjit, S.M. Pitzenberger, E.H. Ulm, Chem. Abstr. 113, 191344 (1990)
T. Hara, Y. Shikayama, K. Ito, T. Mori, H. Fujimori, T. Sunami, Y. Hashimoto, Y. Ishimoto, Chem. Abstr. 104, 186459 (1986)
F. Corelli, S. Massa, G. Stefancich, G. Ortenzi, M. Artico, G. Pantaleoni, G. Palumbo, D. Fanini, R. Giorgi, Eur. J. Med. Chem. 21, 445 (1986)
A.V. Ivashchenko, V.Y. Vvedensky, A.P. Ilyn, V.M. Kysel, A.V. Khvat, Y.A. Kuzovkova, S.A. Kutepov, I.G. Dmitrieva, D.A. Zolotarev, S.Y. Tkachenko, I.M. Okun, D.V. Kravchenko, V.V. Kobak, A.S. Trifilenkov, Y.S. Mishunina, M.V. Loseva, E.A. Rizhova, V.Z. Parchinsky, S.A. Tsirulnikov, A.S. Kyselev, Chem. Abstr. 143, 452852 (2005)
L. Meerpoel, J. Van Gestel, F. Van Gerven, F. Woestenborghs, P. Marichal, V. Sipido, T. Gilkerson, R. Nash, D. Corens, R.D. Richards, Bioorg. Med. Chem. Lett. 15, 3453 (2005)
X.C. Li, K.S. Babu, M.R. Jacob, S.I. Khan, A.K. Agarwal, A.M. Clark, ACS Med. Chem. Lett. 2, 391 (2011)
A.P. Ilyn, A.S. Trifilenkov, J.A. Kuzovkova, S.A. Kutepov, A.V. Nikitin, A.V.J. Ivachtchenko, J. Org. Chem. 70, 1478 (2005)
L. Han, Y. Feng, M. Luo, Z. Yuan, X. Shao, X. Xu, Z. Li, Tetrahedron Lett. 57, 2727 (2016)
K.-M. Wang, S.-J. Yan, J. Lin, Eur. J. Org. Chem. 2014, 1129 (2014)
P.-H. Yang, Res. Chem. Intermed. 42, 5617 (2016)
C.-Y. Yu, P.-H. Yang, M.-X. Zhao, Z.-T. Huang, Synlett 12, 1835 (2006)
B. Zhou, Z.-C. Liu, W.-W. Qu, R. Yang, X.-R. Lin, S.-J. Yan, J. Lin, Green Chem. 16, 4359 (2014)
F. Sun, X. Shao, Z. Li, RSC Adv. 6, 15382 (2016)
M. Bayat, F.S. Hosseini, B. Notashm, Tetrahedron 73, 1196 (2017)
M. Bayat, F.S. Hosseini, B. Notashm, Tetrahedron Lett. 57, 5439 (2016)
M. Bayat, F.S. Hosseini, Tetrahedron Lett. 58, 1616 (2017)
M. Bayat, M. Rezaei, Monatsh. Chem. (2017). https://doi.org/10.1007/s00706-017-2033-6
M. Bayat, S. Nasri, Tetrahedron Lett. 58, 3107 (2017)
A.A. Mohammadi, S. Taheri, A. Amouzegar, J. Heterocycl. Chem. 53, 805 (2016)
S. Kazemi Movahed, M. Dabiri, A. Bazgir, Helv. Chim. Acta 96, 525 (2013)
A. Alizadeh, T. Firuzyar, A. Mikaeili, J. Heterocycl. Chem. 50, 676 (2013)
T.M. Lin, D.C. Evans, M.W. Warrick, R.P.J. Pioch, Pharmacol. Exp. Ther. 239, 406 (1986)
S. Kagabu, K. Moriya, K. Shibuya, Y. Hattori, S. Tsuboi, K. Shiokawa, Biosci. Biotechnol. Biochem. 56, 362 (1992)
X.-S. Shao, Z.P. Xu, X.F. Zhao, X.Y. Xu, L.M. Tao, Z. Li, X.H.J. Qian, Agric. Food Chem. 58, 2690 (2010)
X.-B. Chen, X.-M. Liu, R. Huang, S.-J. Yan, J. Lin, Eur. J. Org. Chem. 2013, 4607 (2013)
M. Bayat, M. Rezaei, J. Heterocycl. Chem. 54, 2748 (2017)
G. Brahmachari, ACS Sustain. Chem. Eng. 3, 2350 (2015)
X.-B. Chen, X.Y. Wang, D.D. Zhu, S.J. Yan, J. Lin, Tetrahedron 70, 2014 (1047)
A. Alizadeh, A.H. Vahabi, A. Bazgir, H.R. Khavasi, Z. Zhu, L.-G. Zhu, Tetrahedron 72, 1342 (2016)
I. Savych, S.A. Ejaz, S.J.A. Shah, V.O. Iaroshenko, A. Villinger, V.Y. Sosnovskikh, J. Iqbal, A. Abbasi, P. Langer, Eur. J. Org. Chem. 2017, 186 (2017)
Acknowledgements
Financial support of this research from Imam Khomeini International University, Iran, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bayat, M., Rezaei, M. Synthesis of new types of pyrrolo/pyrido[1,2-a][1,3]diazepines based on seven-membered ring HKA via a one-pot three-component reaction. J IRAN CHEM SOC 15, 769–777 (2018). https://doi.org/10.1007/s13738-017-1275-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1275-x