Abstract
A cellulose-based magnetic nano-composite supported SO3H group was synthesized and characterized using Fourier transform infrared spectroscopy, energy-dispersive X-ray spectroscopy, thermogravimetric analysis, X-ray diffraction, and scanning electron microscopy. Thereafter, its capability to promote the one-pot, three-component synthesis of pyrimido [4,5-b] quinolone and pyrido [2,3-d] pyrimidine derivatives was evaluated. The obtained results are indicative of excellent yields and short reaction times. The biopolymer based catalyst is readily recovered and reused several times without a decrease in yield.
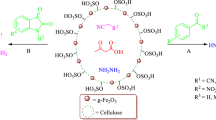
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multi-component reactions are known as versatile synthesis tools for the assembly of novel and structurally complex molecules from simple starting materials in a minimum of reaction steps [1]. These reactions have emerged as a valuable method for the formation and breakage of carbon–carbon and carbon-heteroatom bonds in a one-pot and simple work-up procedure. The synthesis of highly functionalized quinolines illustrates the proper application of multi-component reactions because the quinoline moiety possesses promising biological activities, such as antimalarial, anticancer, antimicrobial [2], antiallergy, and anti-inflammatory activities, which essentially associate to the nature and the place of the substituents [3]. In addition to the abovementioned activities, pyridopyrimidins show a broad spectrum of biological and pharmacological activity, including antifloate, tyrosine kinase inhibitors, anti-aggressive [4], antitumor, cardiotonic, heptoprotactive, antifungal, antibronchitic [5], and calcium antagonist [6].
Recently, effort has been made in order to replace the traditional liquid inorganic homogeneous acid, with heterogeneous ones, because of their environmental, economical and industrial aspects. To address the aforementioned issues, the covalently immobilized –SO3H group onto the surface of a solid support is one of the most common methods to develop the novel heterogeneous acid catalysts realm [7–11]. To date, numerous solid supports have been used as anchors for sulfonic acid groups such as nano-cellulose [12], silica [13], Fe3O4@SiO2 [14], mesoporous silica [15], γ-Fe2O3@SiO2 [16], nano-Fe3O4–TiO2 [17], nano-WO3 [18], nano–ZrO2 [19], CuFe2O4 [20], and PEG [21]. Therefore, considering the green chemistry aspects, biopolymers have attracted much attention as supports for catalytic applications. Among them, cellulose, as one of the most important biopolymers, has received growing attention as a non-toxic, renewable, and biocompatible polymer. It has many properties, such as biocompatible, broad chemical modifying capacity, hydrophilicity, and large surface area [22–26]. Recently, it has been introduced as a versatile support in many catalysts [27–31]. Cellulose sulfuric acid as a recyclable solid acid catalyst was prepared by Shaabani’s group [32] and has been applied in various organic transformations [33]. Exploring facile economical methods for separation of catalysts, magnetite nano-particles (Fe3O4) has attracted much interest in recent years because they can be easily recovered from the reaction mixture using an external magnet. Concerning their specific features, such as low-toxicity, high degree of chemical stability, they have found diverse application in chemical, biomedical, and material science [34–40]. In addition, they are adaptable to large-scale production. Considering all of these advantages and in continuation of our efforts to develop environmental benign synthetic methodology [14, 20, 41–48], we report the preparation and characterization of Fe3O4@Cellulose sulfuric acid as a superior heterogeneous acid catalyst (Scheme 1) in the synthesis of structurally diverse functionalized pyrimido[4,5-b]quinolines and indeno fused pyrido[2,3-d]pyrimidines via one-pot condensation of 6-amino-1,3-dimethyl uracil, 1,3-dicarbonyl compounds with aromatic aldehydes in water at 80 °C.
Experimental
All chemicals were purchased from Sigma-Aldrich and Merck company and used without further purification. The progress of the reaction was monitored by TLC on commercial aluminum-backed plates of silica gel 60 F254, visualized using ultraviolet light. Melting points were determined in open capillaries using an Electrothermal 9100 without further corrections. 1H NMR and 13C NMR spectra were recorded using a Bruker DRX-400 spectrometer at 400 and 100, respectively. Elemental analyses were performed on a Perkin-Elmer CHN analyzer, 2400 series II. Nano-Fe3O4@Celloluse-SO3H was characterized by FT-IR spectra, which were obtained from potassium bromide pellets in the range of 400–4000 cm−1 using a Shimadzu 8400 s spectrometer. X-ray diffraction (XRD) was detected by Philips using Cu-Ka radiation of wavelength 1.54 Å; Scanning electron Microscopy, FE-SEM-EDX, analysis was performed using Tescanvega II XMU Digital Scanning Microscope. Samples were coated with gold at 10 mA for 2 min prior to analysis. The magnetic property of the catalyst was confirmed by using a vibrating sample magnetometer (VSM, Lakeshore7407) at room temperature. Thermogravimetric analyses (TGA) was obtained using a LINSEIS model STS PT 16,000 thermal analyzer under air atmosphere at a heating rate of 5 °C min−1.
Preparation of Fe3O4 nanoparticles
MNPs were synthesized by co-precipitation method according to our earlier report [14].
Preparation of Fe3O4@Cellulose
The nano-Fe3O4@Cellulose composite was prepared by adding a suspension of cellulose (2 wt%) to a suspension of the MNPs in water (10 mg/mL). The obtained mixture was heated at 70 °C for 2 h. The nano-Fe3O4@Cellulose were isolated with external magnetic field and washed with deionized water two to three times, and then dried in an oven [49].
Preparation of Fe3O4@Cellulose-SO3H
A flask equipped with a constant-pressure dropping funnel and a gas inlet tube for conducting HCl gas over an adsorbing solution was charged with Fe3O4@Cellulose (1 g). Then, neat chlorosulfonic acid (0.022 mL, 3 mmol) was added drop-wise over a period of 15 min in an ice bath (0–5 °C). HCl gas immediately evolved from the reaction vessel. After that, the mixture was shaken for 1 h at room temperature and Fe3O4@Cellulose-SO3H was collected as a dark brown solid. The catalyst was washed with EtOH three times and dried at room temperature [13].
Loading of H+
The optimum concentration of H+ of Fe3O4@Cellulose-SO3H was determined by acid–base potentiometric titration of the aqueous suspension of catalyst with standard NaOH solution [19]. At first, 0.1 g catalyst was dispersed in 20 mL H2O by ultrasonic bath for 60 min at room temperature. The amount of the acid was neutralized by addition of standard NaOH solution (0.093 N) to the equivalence point of titration. The required volume of NaOH to this point was 4.3 mL. This is equal to a loading of 3.9 mmol H+/g.
General procedure for the synthesis of pyrimido[4,5-b]quinolones and pyrido[2,3-d]pyrimidines
Fe3O4@Cellulose-SO3H (0.025 g, 0.097 mmol H+) was added to a mixture of benzaldehyde (1.0 mmol), 6-amino-1,3-dimethyluracil (1.0 mmol), and 1,3-diketone (1.0 mmol) in distilled water (1 mL). The reaction mixture was heated in an oil bath at 80 °C with stirring for the appropriate time upon completion of the reaction, monitored by TLC, and then the reaction mixture was allowed to cool to room temperature. The obtained solid was dissolved in hot EtOH and the catalyst was separated using an external magnet. The pure products were afforded in 80–95 % yields after recrystallization in EtOH, and characterized by mp, IR, and NMR analysis.
Spectral data of new products
5-(4-bromophenyl)-1,3-dimethyl-5,8,9,10-tetrahydropyrimido[4,5-b]quinoline-2,4,6(1H,3H,7H)-trione (5b)
mp > 300 °C; IR (KBr): (v max/cm−1): 3365, 1703, 1595; 1H NMR(400 MHz, DMSO-d6): δ 1.94 (m, 2H), 2.24 (m, 2H), 2.51 (m, 2H), 3.1 (s, 1H), 3.4 (s, 3H), 3.45 (s, 3H), 7–7.4 (m, 4H), 9.1 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ 21.15, 26.94, 28.11, 30.73, 33.96, 37.06, 90.10, 112.78, 119.38, 130.45, 131.04, 144.27, 146.37, 150.98, 152.22, 161.20, 195.31 ppm. Anal. Calced for C17H18BrN3O3: C 52.05, H 4.59, N 10.71; found: C 51.92, H 4.45, N 10.57.
5-(4-methoxyphenyl)-1,3-dimethyl-5,8,9,10-tetrahydropyrimido[4,5-b]quinoline-2,4,6(1H,3H,7H)-trione (5c)
mp 294–297 °C; IR (KBr, cm−1): 3346, 2956, 1701, 1664; 1H NMR(400 MHz, DMSO-d6): δ 1.95 (m, 2H), 2.24 (m,2H), 2.55 (m,2H), 3.1 (s,1H), 3.46 (s,1H), 3.49 (s,1H), 3.67 (s,3H),6.7–7.1 (m,4H), 9.07 (S,1H); 13C NMR (100 MHz, DMSO-d6): δ 21.20, 26.93, 28.09, 30.63, 33.13, 37.14, 55.34, 90.89, 113.52, 113.58, 129.01, 139.34, 143.99, 151.00, 151.73, 157.92, 161.26, 195.39 ppm. Anal. Calced for C18H21N3O4: C 62.97, H 6.12, N 12.24; found: C 62.85, H 5.96, N 12.09.
Result and discussion
The route leading to nano-Fe3O4@Cellulose-SO3H is illustrated in Scheme 2.
The magnetized catalyst was prepared from low cost commercially available materials. Initially, the nano-Fe3O4 magnetic particles have been prepared by a chemical co-precipitation in basic solution [14]. Then, the nano-Fe3O4@Cellulose composite was prepared simply by dispersing of the nano-Fe3O4 particles in aqueous cellulose solution. The resulting magnetic composite was functionalized using chlorosulfonic acid and fully characterized by FT-IR, FE-SEM, EDX, XRD, TGA, and VSM techniques. The FT-IR spectrum of the synthesized nano-Fe3O4@Cellulose-SO3H in comparison to that of nano-Fe3O4 and nano-Fe3O4@Cellulose is shown in Fig. 1. The presence of major bands around 550 cm−1 is attributed to the vibration of Fe–O [50]. Typical wave numbers of the hydroxyl groups of cellulose, such as –CH–OH and –CH2–OH stretching are in the range of 3400–3300 cm−1. The methylene groups stretching, from the incorporated molecule, are located at 2900 cm−1 and the band in the 3000–2800 cm−1 range is attributed to the –C–H groups. The OH bending vibration of the cellulose surface is located at 1639 cm−1, the primary and secondary hydroxyl bending appeared in the range of 1500–1200 cm−1, and C–O stretching vibration is observed at 1100 cm−1 [51, 52].
After functionalization of nano-Fe3O4@cellulose composite with SO3H group, The characteristic peaks of the OSO3H group of chlorosulfonic acid were observed at 3300, 1581, 1288, and 1064 cm−1, that corresponds to O–H, S=O, and S–O groups, respectively, indicating the presence of –OSO3H group bonded to magnetic composite [53].
The presence of magnetite crystal phase in cellulose sulfuric acid was identified with the X-ray diffraction powder (Fig. 2). The characteristic XRD peaks of Fe3O4 appeared at 2θ = 31.1°, 39°, 45.2°, 50.43°, 56.6°, and 65.84° corresponding to the (220), (311), (400), (422), (511), and (440) planes of Fe3O4 crystal, that match well with standard spectra [54]. According to Debye–Scherrer formula, the average crystal size of magnetite particles was calculated to be 8.91 nm. As can be seen in the XRD pattern of Fe3O4@Cellulose, the position and the relative intensities of observed peaks are similar to those of nano-Fe3O4 and cellulose. This result confirms the presence of the Fe3O4 crystalline structure in the final product. Notably, the same peaks are also observed in Fe3O4@cellulose-SO3H XRD spectra, which indicate the structural ability of Fe3O4@cellulose with –SO3H modification [51].
The morphology and structure of synthesized composites were determined with scanning electron microscopy (SEM) (Fig. 3). The SEM image of Fe3O4@Cellulose composite showed that the Fe3O4 nanoparticles have been dispersed in a cellulose matrix (Fig. 3a). In addition the SEM image of Fe3O4@Cellulose-SO3H has definitely been altered upon functionalization as shown in Fig. 3b.
The EDX spectrum of Fe3O4@Cellulose and Fe3O4@Cellulose-SO3H as an exact elemental analysis is depicted in Fig. 4 and shows the presence of C, O, and Fe of Fe3O4@Cellulose composite (Fig. 4a) and C, O, Fe, and S of Fe3O4@Cellulose-SO3H acid catalyst, that clearly indicate the efficient preparation of Fe3O4@Cellulose composite. The appearance of Au elements in EDX analysis is the result of the coating of materials by the layer of Au element. In addition, an elemental map of the catalyst showed the presence of S, O, C, and Fe, too.
To determine the thermal stability of the catalyst, thermogravimetric analysis (TGA) was used. TG curves of Fe3O4@Cellulose and Fe3O4@Cellulose-SO3H are shown in Fig. 5. The weight loss of Fe3O4@Cellulose is about 60 % at 315–400 °C, corresponding to the thermal decomposition of cellulose chains. The pattern of mass loss was changed in TGA analysis of Fe3O4@Cellulose-SO3H (Fig. 5b), which confirms the functionalization of magnetic cellulose with –SO3H [17]. Moreover, the catalyst was stable up to 200 °C, indicating its safe use at temperature range 80–150 °C in related organic reactions.
One of the most important advantages of the magnetic catalysts is their super-paramagnetic behavior, making their separation feasible by an external magnet. For this reason the magnetic property of as-synthesized acid catalyst was evaluated by VSM analysis (Fig. 6). In VSM analysis, good magnetization saturation (21.2 emu g−1) and no hysteresis were ascribed to super-paramagnetic behavior of the Fe3O4@Cellulose-SO3H. So it can be simply recovered using an external magnet.
To check if it is able to catalyze the reactions under study, we have considered the one-pot, three-component synthesis of functionalized pyrimido[4,5-b]quinolines and indeno fused pyrido[2,3-d]pyrimidines derivatives. In order to optimize the reaction condition, the reaction of benzaldehyde (1 mmol), uracil (1 mmol), and dimedone (1 mmol) for the synthesis of corresponding product 1,3,8,8-tetra methyl-5-phenyl-5,8.9,10-tetrahydropyrimido[4,5-b]quinolone-2,4,6(1H,3H,7H)-trione (4a) was selected as the model reaction (Scheme 3). In this regard, various reaction parameters such as solvent, temperature and the amount of catalyst were investigated. In addition, with particular reference to the importance of green chemistry, the model reaction was tried in water and solvent-free state as green conditions (Table 1). As Table 1 shows, in the absence of the catalyst, a low yield of the products were obtained (Table 1, entry 1).
To clarify the dependency of the catalytic activity to the presence of –SO3H group, the model reaction was conducted with Fe3O4 and Fe3O4@Cellulose. As the desired product is formed slightly in these cases, it seems to be reasonable to conclude that the catalytic effect is risen from the –SO3H group (Table 1, entries 12 and 13). Finally, the best results are obtained with the following reaction condition, 0.025 g catalyst (0.097 mmol/g H+) at 80 °C in water (Table 1, entry 3). The keto-enol tatumerization equilibrium of the 1,3-dicarbonyl compound has been recognized as an important factor for the success of this reaction and water is a very favorable option for a solvent if a cyclic dicarbonyl reactant is employed [55, 56].
To establish the efficiency of the novel acid catalyst in previously mentioned kinds or reactions, we dealt with the reaction of the substituted aryl aldehydes and the other diketones such as 1,3-cyclohexandione and indanedione in optimized reaction condition (Scheme 4), and the results depicted in Table 2. All the investigated arylaldehydes with either electron-donating or electron-withdrawing substituent afforded corresponding products in high yields and short reaction time.
The proposed mechanism presented sequences, including condensation, addition, cyclization, and dehydration, was reported in the literature (Scheme 5) [57, 59–61]. On the basis of the mechanism, the adduct (I) is created through the Fe3O4@Cellulose-SO3H-catalyzed Knoevenagel condensation between the aldehyde and cyclic 1,3-diketone. Then, the 6-amino-1,3-dimethyluracil attacks to the adduct (I) via a Michael addition to produce an open chain intermediate (II). Subsequently, intermediate (II) undergoes intra molecular cyclization by the reaction of nucleophilic amino function to carbonyl group followed by dehydration to generate product (III). The aromatization take place in synthesis of indeno fused pyrido[2,3-d]pyrimidines (6a–f).
In order to show the advantages of the new catalyst, a comparison of the use of nano-Fe3O4@Cellulose-SO3H with the other reported catalysts for the synthesis of the target products has been shown in Table 3. These results demonstrate that the new catalyst promotes the reaction more effective in terms of short reaction times and simple conditions. Furthermore, the yield of products in the presence of nano-Fe3O4@Cellulose-SO3H is comparable to the reported catalysts.
The reusability and recovery of the catalyst are important issues for the catalytic reactions which make the method economically valuable, industrially profitable and environmentally sustainable. So the model reaction under optimal condition was selected to investigate the turns that the catalyst may keep holds its catalytic activity. After completion the reaction, the mixture decanted and hot ethanol added to it. The catalyst was easily separated using an external magnet, washed with ethanol, dried at 70 °C, and used for the next cycle. The recovered catalyst was added to fresh substrates of the mentioned reaction for at least five times without any noticeable reduction in catalytic activity. The yields of the five runs were found to be 95, 93, 90, 88, and 85 %, respectively. The slight decrease in the yield could probably be due to the gradual loss of the catalyst during the work-up procedure. The acidity of the understudy catalyst after five runs was measured using potentiometric titration and was obtained 3.9 mmol H+/g that was equal to the fresh catalyst. In addition the FT-IR of this recovered sample was recorded which was identical with that of taken for the characterization of the novel prepared catalyst (Fig. 7).
Conclusion
In summary, an eco-friendly, efficient and heterogeneous acid catalyst, Fe3O4@Cellulose-SO3H was prepared, characterized and applied for one-pot, three-component synthesis of pyrimido[4,5-b]quinolones and pyrido[2,3-d]pyrimidines derivatives. Application of cellulose as a green and recoverable biopolymer, use of water as solvent, easy recovery of catalyst, and low catalyst loading are some merits of this methodology.
References
J. Safari, S. Gandomi-Ravandi, Z. Akbari, J. Adv. Res. 4, 6 (2013)
I.R. Siddiqui, P. Rai, R. Rahila, H. Sagir, P. Singh, RSC Adv. 5, 35 (2015)
R.M. Singh, N. Sharma, R. Kumar, M. Asthana, S. Upadhyay, Tetrahedron Lett. 68, 50 (2012)
J.M. Khurana, A. Chaudhary, B. Nand, A. Lumb, Tetrahedron Lett. 53, 24 (2012)
I. Devi, B.S.D. Kumar, P.J. Bhuyan, Tetrahedron Lett. 44, 45 (2003)
N.A. Hassan, M.I. Hegab, F.M. Abdel-Motti, S.H.A. Hebah, F.M.E. Abdel-Megeid, A.I. Hashem, J. Heterocycl. Chem. 44, 4 (2007)
A. Shaabani, Z. Hezarkhani, M.T. Faroghi, Monatsh. Chem. (2016). doi:10.1007/s00706-016-1717-7
M. Shamsi-Sani, F. Shirini, M. Abedini, M. Seddighi, Res. Chem. Intermed. 42, 2 (2016)
E. Kolvari, N. Koukabi, M.M. Hosseini, J. Mol. Catal. A: Chem. 397, 68 (2015)
A.R. Kiasat, L. Hemat-Alian, Res. Chem. Intermed. 41, 2 (2015)
M. Wu, Y. Wang, D. Wang, M. Tan, P. Li, W. Wu, N. Tsubaki, J. Porous Mater. 23, 1 (2016)
B. Sadeghi, M.H. Sowlat Tafti, J. Iran. Chem. Soc. 13, 8 (2016)
M.A. Zolfigol, Tetrahedron 57, 46 (2001)
F. Nemati, M.M. Heravi, R. Saeedi Rad, Chin. J. Catal. 33, 11–12 (2012)
E. Mehrasbi, Y. Sarrafi, A. Vahid, H. Alinezhad, Res. Chem. Intermed. 41, 7 (2015)
S. Mahdudi, D. Saberi, A. Heydari, J. Iran. Chem. Soc. 12, 5 (2015)
A. Amoozadeh, S. Golian, S. Rahmani, RSC Adv. 5, 57 (2015)
A. Amoozadeh, S. Rahmani, J. Mol. Catal. A: Chem. 396, 96–107 (2015)
E. Kolvari, N. Koukabi, M.M. Hosseini, M. Vahidian, E. Ghobadi, RSC Adv. 6, 9 (2016)
F. Nemati, A. Elhampour, M.B. Natanzi, S. Sabaqian, J. Iran. Chem. Soc. 13, 6 (2016)
R.H. Vekariya, H.D. Patel, RSC Adv. 5, 61 (2015)
A.M. Salgueiro, A.L. Daniel-da-Silva, A.V. Girão, P.C. Pinheiro, T. Trindade, Chem. Eng. J. 229, 276–284 (2013)
C. Esmaeili, M. Ghasemi, L.Y. Heng, S.H.A. Hassan, M.M. Abdi, W.R.W. Daud, H. Ilbeygi, A.F. Ismail, Carbohydr. Polym. 114, 253–259 (2014)
S. Rostamnia, B. Zeynizadeh, E. Doustkhah, A. Baghban, K.O. Aghbash, Catal. Commun. 68, 77–83 (2015)
Y. Habibi, L.A. Lucia, O.J. Rojas, Chem. Rev. 110, 6 (2010)
C. Johansson, J. Bras, I. Mondragon, P. Nechita, D. Plackett, P. Simon, D.G. Svetec, S. Virtanen, M.G. Baschetti, C. Breen, Bioresources. 7, 2 (2012)
Z. Ghasemi, S. Shojaei, A. Shahrisa, RSC Adv. 6, 61 (2016)
A. Shaabani, H. Nosrati, M. Seyyedhamzeh, Res. Chem. Intermed. 41, 6 (2015)
L. Edjlali, R.H. Khanamiri, J. Abolhasani Monatsh. Chem. 146, 8 (2015)
B. Liu, Z. Zhang, K. Huang, Cellulose 20, 4 (2013)
H.R. Shaterian, F. Rigi, Res. Chem. Intermed. 40, 5 (2014)
A. Shaabani, A. Rahmati, Z. Badri, Catal. Commun. 9, 1 (2008)
R.H. Vekariya, H.D. Patel, ARKIVOC 1, 136–159 (2015)
R.K. Sharma, S. Dutta, S. Sharma, R. Zboril, R.S. Varma, M.B. Gawande, Green Chem. 18, 11 (2016)
B. Liu, Z. Zhang, ACS Catal. 6, 326 (2016)
B. Liu, Y. Ren, Z. Zhang, Green Chem. 17, 1610 (2015)
Z. Zhang, J. Zhen, B. Liu, K. Lv, K. Deng, Green Chem. 17, 1308 (2015)
Sh Yin, J. Sun, B. Liu, Z. Zhang, J. Mater. Chem. A 3, 4992 (2015)
Z. Zhang, Z. Yuan, D. Tang, Y. Ren, K. Lv, B. Liu, Chem. Sus. Chem. 7, 3496 (2014)
N. Mei, B. Liu, J. Zheng, K. Lv, D. Tang, Z. Zhang, Catal. Sci. Technol. 5, 3194 (2015)
F. Nemati, A. Elhampour, H. Farrokhi, M. Bagheri, Natanzi. Catal. Commun. 66, 15 (2015)
F. Nemati, M.M. Heravi, A. Elhampour, RSC Adv. 5, 57 (2015)
F. Nemati, S.H. Nikkhah, A. Elhampour, Chin. Chem. Lett. 26, 11 (2015)
F. Nemati, A.G.G. Ghiyaei, B. Notash, M.H. Shayegan, V. Amani, Tetrahedron Lett. 55, 25 (2014)
F. Nemati, A. Elhampour, S. Zulfaghari, Phosphorus, Sulfur Silicon Relat. Elem. 190, 10 (2015)
F. Nemati, A. Elhampour, Res. Chem. Intermed. 42, 7611 (2016)
A. Elhampour, F. Nemati, M. Kaveh, Chem. Lett. 45, 223 (2016)
A. Elhampour, F. Nemati, J. Chin. Chem. Soc. 63, 653 (2016)
L.C. Fidale, M. Nikolajski, T. Rudolph, S. Dutz, F.H. Schacher, T. Heinze, J. Colloid Interface Sci. 390, 1 (2013)
M.H. Beyki, M. Bayat, S. Miri, F. Shemirani, H. Alijani, Ind. Eng. Chem. Res. 53, 39 (2014)
X. Sun, L. Yang, Q. Li, J. Zhao, X. Li, X. Wang, H. Liu, Chem. Eng. J. 241, 174–183 (2014)
P.V. Chavan, K.S. Pandit, U.V. Desai, M.A. Kulkarni, P.P. Wadgaonkar, RSC Adv. 4, 79 (2014)
H.R. Shaterian, M. Ghashang, M. Feyzi, Appl. Catal. A 345, 2 (2008)
S. Peng, H. Meng, Y. Ouyang, J. Chang, Ind. Eng. Chem. Res. 53, 6 (2014)
H.M. Savanur, R.G. Kalkhambkar, G. Aridoss, K.K. Laali, Tetrahedron Lett. 57, 3029 (2016)
J.H. Clark, D.J. Macquarrie, J. Sherwood, Chem. Eur. J. 19, 5174 (2013)
F. Nemati, R. Saeedirad, Chin. Chem. Lett. 24, 5 (2013)
K. Tabatabaeian, A.F. Shojaei, F. Shirini, S.Z. Hejazi, M. Rassa, Chin. Chem. Lett. 25, 2 (2014)
S.-J. Ji, S.-N. Ni, F. Yang, J.-W. Shi, G.-L. Dou, X.-Y. Li, X.-S. Wang, D.-Q. Shi, J. Heterocycl. Chem. 45, 3 (2008)
G.K. Verma, K. Raghuvanshi, R. Kumar, M.S. Singh, Tetrahedron Lett. 53, 4 (2012)
K. Mohammadi, F. Shirini, A. Yahyazadeh, RSC Adv. 5, 23586 (2015)
Acknowledgment
The authors gratefully acknowledge Semnan University Research Council for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Osanlou, F., Nemati, F. & Sabaqian, S. An eco-friendly and magnetized biopolymer cellulose-based heterogeneous acid catalyst for facile synthesis of functionalized pyrimido[4,5-b]quinolines and indeno fused pyrido[2,3-d]pyrimidines in water. Res Chem Intermed 43, 2159–2174 (2017). https://doi.org/10.1007/s11164-016-2752-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2752-z