Abstract
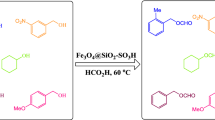
Some derivatives of nitroenamines were synthesized in moderate to good yields in the presence of sulfonic acid supported on silica-modified maghemite (γ-Fe2O3@SiO2–OSO3H) as a highly efficient and magnetically separable catalyst. Both up to five times recyclability and the magnetic separation are salient features of this catalytic system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to both economical and environmental reasons, heterogeneous catalysts are gradually replacing homogeneous catalysts in the most fields. Heterogeneous systems possess the inherent aptitude of recyclability, a matter that has recently gained a great deal of attention. In this context, assembling the catalyst on a suitable support is the most facile way to render it as heterogeneous. In recent years, magnetic nanoparticles have taken up a special position in organic syntheses either as support or as catalyst [1–4]. Using them as support results in the straightforward separation of the catalyst from the reaction mixture (this is only achieved using an external magnet). Therefore, we have turned our attention to these systems [5–8]. This time, we report the synthesis of the nitroenamines facilitated by γ-Fe2O3@SiO2–OSO3H as an efficient catalyst. The foregoing catalyst has recently been prepared in our laboratory and used in the synthesis of miscellaneous organic compounds [9].
Nitroenamines, olefins with an electron-donating amino group attached to one side and the strongly electron-withdrawing nitro group to the other, are indispensable compounds in organic synthesis. They show substantial pharmacological properties [10, 11], and are also utilized as synthetic intermediates [12–17]. Nevertheless, there are only a few synthetic methods, reported in literatures, for the synthesis of these compounds. Some of the recent methods entail the reaction of β-halo- [18–20], β-alkylthio- [21], β-alkylsulfinyl- [22] or β-alkoxynitroethenes [23, 24] with an amine. Other methods are transamination [25], nitration of imines [26, 27] rearrangement of N-nitroenamines [28] and the reaction of phenyl acetylene with aniline in the presence of mercury (II) chloride [29]. Despite all merits, the above-mentioned methods suffer from major drawbacks such as the requirement for the pre-preparation of the appropriate precursors, the toxicity of the catalyst and low efficiency. Amongst all reports, the condensation reaction of the nitromethane with orthoformates and amines in the presence of p-toluene sulfonic acid as catalyst seems to be superior, due to easily accessible starting materials [30]. However, it still suffers from the shortcoming of low reaction yields. Also this procedure does not benefit from the feature of reusability and recovery of the catalyst. This prompted us to innovate a heterogeneous, and reusable catalyst which would easily be separated from the reaction medium and also able to improve the yield, relative to the methods using the homogeneous catalyst.
Experimental
General procedure for the direct synthesis of nitroenamines in the presence of γ-Fe2O3@SiO2–OSO3H nanoparticles as catalyst
The amine (1 mmol) was added to a mixture of γ-Fe2O3@SiO2–OSO3H (20 mg, 1.2 mol % of H+) and triethyl orthoformate (2 mmol). The mixture was stirred for 5 min and then nitromethane (5 mmol) was added. The mixture was stirred at 80 °C under Ar atmosphere until completion of the reaction [monitored by TLC (n-hexane/ethyl acetate, 80:20)]. Afterward, the catalyst was separated from the reaction mixture using an external magnet, washed with water and ethanol, respectively, and then oven dried at 80 °C overnight for reuse in a subsequent run. The mixture was concentrated under vacuum, and the crude residue was purified by column chromatography on silica gel eluting with n-hexane/ethyl acetate (80:20, 500 ml) to afford the corresponding nitroenamine. The products were identified by melting point (in some cases), 1H-NMR, Mass, and IR spectra. The known products were characterized by comparison of their spectroscopic data and their melting points with reported values.
4-Methyl-N-(2-nitrovinyl) aniline f. Brown solid; 0.12 g, 70 % yield; mp 102–104 °C; IR (KBr) ν 1180, 1263, 1353, 1637, 2934, 3271 cm−1; 1H-NMR (250 MHz, CDCl3): δ 2.36 (s, 3H, CH3), 6.65 (d, 3 J = 5 Hz, 1H, CHNO2), 7.04 (d, 3 J = 6.8 Hz, 2H, Ar), 7.2 (d, 3 J = 6.8 Hz, 2H, Ar), 7.26–7.30 (m, 1H, CHNH), 10.72 (brs, 1H, CHNO2). MS (EI, 70 eV): m/z (%) = 178 (M+, 100), 130 (55), 118 (40), 91 (52).
N-Benzyl-N-methyl-2-nitroethenamine g. Yellowish brown solid; 0.15 g, 80 % yield; mp 70–72 °C; IR (KBr) ν 1180, 1260, 1312, 1622, 2925, 3121 cm−1; 1H-NMR (250 MHz, CDCl3): δ 2.77 (s, 3H, CH3), 4.48 (s, 2H, CH2), 6.68 (d, 3 J = 12.5 Hz, 1H, CHNO 2 ), 7.08–7.41 (m, 5H, Ar), 8.33 (d, 3 J = 10 Hz, 2H, CHNMe). MS (EI, 70 eV): m/z (%) = 192 (M+, 19), 176 (11), 146 (89), 131 (14), 91 (100).
N-Benzyl-2-methyl-N-(2-nitrovinyl) propan-2-amine h. Brown solid; 0.19 g, 80 % yield; mp 95–97 °C; IR (KBr) ν 1185, 1265, 1313, 1612, 2924, 3448 cm−1; 1H-NMR (250 MHz, CDCl3) δ 1.45 (s, 9H, (CH3)3), 4.48 (s, 2H, CH2), 6.46 (d, 3 J = 8.9 Hz, 1H, CHN), 7.22–7.38 (m, 5H, Ar), 8.65 (d, 3 J = 8.9 Hz, 1H, CHNO2); MS (EI, 70 eV): m/z (%) = 235 (M+, 14), 188 (28), 132 (51), 91 (100).
4-(2-Nitrovinyl) morpholine i. Yellowish brown solid; 0.1 g, 60 % yield; mp 128–130 °C; IR (KBr) ν: 1195, 1253, 1319, 1629,2920, 3437 cm−1; 1H -NMR (250 MHz, CDCl3) δ 3.32 (brs, 4H, (CH2)2N), 3.74 (brs, 4H, (CH2)2O), 6.69 (d, 3 J = 7.4 Hz, 1H, CHNO2), 7.05 (d, 3 J = 7.3 Hz, 1H, CHN); MS (EI, 70 eV): m/z (%) = 158 (M+, 66), 141 (54), 125 (16), 111 (71), 95 (64), 83 (100).
1-Methyl-3-(2-nitrovinyl) -1H-indole j. Brown solid; 0.1 g, 50 % yield; mp 125–127 °C; IR (KBr) ν 1195, 1247, 1302, 1616, 2923, 3440 cm−1; 1H-NMR (250 MHz, CDCl3) δ 3.87 (s, 3H, CH3), 7.26 (s, 1H, CHNMe), 7.4 (brs, 2H, Ar), 7.52 (s, 1H, CHCHNO2), 7.74–7.79 (m, 2H, Ar), 8.26 (d, 3 J = 13.2 Hz, 1H, CHNO2); MS (EI, 70 eV): m/z (%) = 202 (M+, 100), 185 (23), 172 (6), 155 (87), 144 (26), 115 (50).
Results and discussion
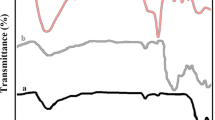
Silica-coated uniform maghemite (γ-Fe2O3) core–shell nanoparticles were synthesized by a chemical co-precipitation technique of ferric and ferrous ions in alkaline solution and then sulfonic acid functionalization of the iron oxide was achieved by tetraethyl orthosilicate (TEOS) subsequently by chlorosulfonic acid [to γ-Fe2O3@SiO2 (1 g), chlorosulfonic acid (1 g) was added dropwise at room temperature over 15 min] and the loading of impregnated acid was measured by back titration (0.60 mmol g−1). The synthesized nanocatalyst (γ-Fe2O3@SiO2–OSO3H) was characterized by FT-IR, SEM and XRD (see Supplementary data).
In the first step, to establish the optimum conditions, the reaction of diphenylamine, triethyl orthoformate and nitromethane was chosen as the model reaction. The variable parameters investigated to reach the best result were: the type of solvent, the reaction temperature, the amount of the catalyst and nitromethane. The results have been depicted in Table 1. As a start, the reaction was conducted in some organic solvents such as CH2Cl2, EtOH, CHCl3 and toluene under the conditions shown in Table 1, but no satisfactory results were obtained (Table 1, entries 1–4). Surprisingly, performing the reaction under neat conditions at 80 °C led to the best result (Table 1, entry 5). At the next stage, the effect of the amount of nitromethane on the reaction was considered. It was revealed that restricting the amount of nitromethane to 3 mmol, led to a decline in the yield. By contrast, no tangible change was observed by increasing the amount of this reagent to 10 mmol (Table 1, entries 6 and 7). The similar results were observed by changing the amount of the catalyst in the same mode (Table 1, entries 8 and 9). Finally, the impact of the temperature on the reaction was evaluated. By elevating the temperature up to 100 °C the yield remained unchanged. However, no product was formed when the reaction was carried out at room temperature (Table 1, entries 10 and 11). Note that the product was not formed in the absence of catalyst even in trace amounts. Meanwhile, in the presence of γ-Fe2O3@SiO2 nanoparticles as the catalyst, a 20 % efficiency was obtained which it indicates that the presence of sulfonic acid is essential as the catalyst for increasing the reaction yield (Table 1, entry 12).
Thus, the following conditions were used for the synthesis of the other nitroenamines: amine (1 mmol), triethyl orthoformate (2 mmol), nitromethane (5 mmol), catalyst (20 mg, 1.2 mol % of H+) at 80 °C under argon atmosphere (Scheme 1).
Our findings from these experiments were as follow: the primary and secondary aromatic amines were well coupled with triethyl orthoformate and nitromethane to give the corresponding nitroenamine (Table 2). As shown, both aniline and benzyl amine derivatives were converted to the corresponding nitroenamine with moderate to good yields. Also, N-methyl cyclohexyl amine led to the expected product in a reasonable yield (Table 2, entry 5). To show the broad scope of this reaction, morpholine as the cyclic amine was also used as the substrate and gave rise to the corresponding product (Table 2, entries 9). Another case examined was N-methyl indole which olefinated at C3 position (Table 2, entry11). Note that the primary amines had a less efficiency than the secondary amines probably because they were more prone to oxidation and side reactions. Worthy of note is that according to the previously reported reactions [30, 31] and based on the coupling constants, it seems that there is a possibility of formation of both isomers (E or Z), depending on the substrate. Since the experiments have not been done on our behalf to prove the type of isomer, it is reasonable to draw the structure of the products as a whole.
Finally, the reusability of the catalyst was explored in the model reaction. To this end, after the completion, the catalyst was recovered using an external magnet, washed with H2O and ethanol and oven dried at 80 °C overnight. A new reaction was then performed with fresh reactants under identical conditions. Using this approach, our catalyst was reused for at least five times without any further treatment while no appreciable loss in the catalytic activity was observed (Fig. 1).
Conclusion
In conclusion, a simple and magnetically separable catalytic system was introduced for the synthesis of nitroenamine derivatives. Different types of primary and secondary amines were tested and the corresponding nitroenamines were prepared in acceptable yields. A series of nitroenamines, that had previously been reported, were synthesized in the presence of this heterogeneous catalytic system with greater efficiency and shorter reaction time. In addition, the new products in this family of compounds were synthesized. Due to the magnetic properties, the catalyst was easily separated from the reaction mixture using an external magnet and up to five times reused without any significant decrease in the activity.
Supporting information
Providing information about the preparation and characterization of the catalyst and the FT-IR, Mass and 1H-NMR spectra of new products are given as supporting information.
References
R. Arundhathi, D. Damodara, P.R. Likhar, M.L. Kantam, P. Saravanan, T. Magdaleno, S.H. Kwon, Adv. Synth. Catal. 353, 2875 (2011)
S. Yang, C. Wu, H. Zhou, Y. Yang, Y. Zhao, C. Wang, W. Yang, J. Xu, Adv. Synth. Catal. 355, 53 (2013)
R. Cano, M. Yus, J. Ramón, ACS Catal. 2, 1070 (2012)
T. Zeng, W. Chen, C.M. Cirtiu, A. Moores, G. Song, C.G. Lee, Green Chem. 12, 570 (2010)
M. Sheykhan, L. Ma’mani, A. Ebrahimi, A. Heydari. J. Mol. Catal. A Chem. 335, 253 (2011)
L. Ma’mani, M. Sheykhan, A. Heydari, Appl. Catal. A Gen. 395, 34 (2011)
L. Ma’mani, A. Heydari, M. Sheykhan, Appl. Catal. A Gen 384, 122 (2010)
M. Sheykhan, M. Mohammadquli, A. Heydari, J. Mol. Struct. 1027, 156 (2012)
S. Rostamnia, K. Lamei, M. Mohammadquli, M. Sheykhan, A. Heydari, Tetrahedron Lett. 53, 5257 (2012)
R.N. Brogden, A.A. Carmine, R.C. Heel, T.M. Speight, G.S. Avery, Drugs 24, 267 (1982)
R.N. Brogden, A.A. Carmine, R.C. Heel, T.M. Speight, G.S. Avery, Chem. Abstr. 97, 207505s (1982)
S. Rajappa, Tetrahedron 37, 1453 (1981)
H. Uno, T. Kinoshita, K. Matsumoto, T. Murashima, T. Ogawa, N. Ono, J. Chem. Res. (S), 76 (1996)
K. Nishide, R. Kurosaki, K. Hosomi, H. Imazato, T. Inoue, M. Node, T. Ohmori, K. Fuji, Tetrahedron 51, 10857 (1995)
A. Gómez-Sánchez, F.J. Hidalgo, J.L. Chiara, J. Heterocycl. Chem. 24, 1757 (1987)
D. Dauzonne, A. Fleurant, P. Memerseman, Synth. Commun. 20, 3339 (1990)
S. Rajappa, Tetrahedron 55, 7065 (1999)
V.I. Potkin, V.I. Gogolinskii, N.I. Nechai, V.A. Zapol’skii, R.V. Kaberdin, Zh. Org. Khim. 31, 1816 (1995)
V.I. Potkin, V.I. Gogolinskii, N.I. Nechai, V.A. Zapol’skii, R.V. Kaberdin, Chem. Abstr. 125, 113914u (1996)
N.V. Nguyen, K. Baum, Tetrahedron Lett. 33, 2949 (1992)
J.L. Vidaluc, F. Calmel, D. Bigg, E. Carilla, A. Stenger, P. Chopin, M. Briley, J. Med. Chem. 37, 689 (1994)
R.C. Young, R.C. Mitchell, T.H. Brown, C.R. Ganellin, R. Griffiths, M. Jones, K.K. Rana, D. Saunders, I.R. Smith, N.E. Sore, T.J. Wilks, J. Med. Chem. 31, 656 (1988)
A. Gomez-Sanchez, F.J. Hidalgo-Garcia, J.L. Chiara, J. Bellanato, An. Ouim. Ser. C 81, 139 (1985)
A. Gomez-Sanchez, F.J. Hidalgo-Garcia, J.L. Chiara, J. Bellanato Chem. Abstr. 105, 24542r (1986)
A. Krówczynski, L. Kozerski, Synthesis 489 (1983)
A.I. Fetell, H. Feuer, J. Org. Chem. 43, 497 (1978)
H. Feuer, R.M. McMillan, Ibid. 44, 3410 (1979)
G. Biichi, H. Wiiest, Ibid. 44, 4116 (1979)
J. Barluenga, F. Aznar, R. Liz, M. Bayod, Synthesis 159 (1983)
M. Faulques, L. Rene, R. Royer, Synthesis 260 (1982)
J. Weng, Y. Li, R. Wang, G. Lu, Chem. Cat. Chem. 4, 1007 (2012)
Acknowledgments
The authors would acknowledge the financial support provided by the Tarbiat Modares University for carrying out this research.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahdudi, S., Saberi, D. & Heydari, A. Green synthesis of nitroenamines by γ-Fe2O3@SiO2–OSO3H nanoparticles as a highly efficient and magnetically separable catalyst. J IRAN CHEM SOC 12, 903–907 (2015). https://doi.org/10.1007/s13738-014-0554-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-014-0554-z