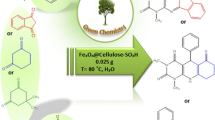
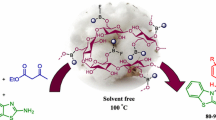
Abstract
In this work, a facile protocol for the preparation of a new cellulose-based functionalized magnetic nanocomposite catalyst is described. The structure and morphology of the prepared nanomaterial was characterized by scanning electron microscopy, energy-dispersive X-ray analysis, X-ray diffraction patterns, and Fourier transform infrared spectroscopy analyses. Then, its catalytic activity was investigated in the synthesis of 2,4-dihydropyrano[2,3-c]pyrazole and spiro[indoline-3,4′-pyrano[2,3-c]pyrazole derivatives through a one-pot four-component reaction between isatin or aldehydes or acetophenones, ethylacetoacetate, hydrazine hydrate, and malononitrile or ethylcyanoacetate in short reaction times, high yields, and mild conditions. The nanocomposite was simply separated by an external magnet and reused several times without remarkable loss of activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multicomponent reactions (MCRs) are an important tool to efficiently synthesis of various complex molecules in one-pot conditions starting from at least three simple and readily available raw materials. In the recent years, researchers have increased their interest in MCRs and used this strategy for pharmaceutical and drug discovery research [1, 2].
One of the most important challenges in pharmaceutical chemistry is the synthesis of biologically active molecules via economical protocols [3]. Dihydropyrano[2,3-c]pyrazoles play an important role in the synthesis of biologically active compounds. These compounds have many properties such as antimicrobial [4], insecticidal [5], and anti-inflammatory activities [6].
In the synthesis of pyranopyrazoles, three-component (3-CR) or four-component (4-CR) reactions are often used [7,8,9,10,11]. In the 4-CR, carbonyl compounds, hydrazine hydrate, β-keto-ester, and malononitrile condensed in the presence of a catalyst. Different catalysts have been reported for these reactions such as tetraethylammonium [12], [(CH2)4SO3HMIM][HSO4] [13] and Al2O3 [14].
The indole moiety is one of the most important heterocycles which is involved in a variety of natural products and medicinal agents [15,16,17,18,19]. Compounds carrying the indole moiety exhibit antibacterial and antifungal activities [15,16,17,18,19]. Furthermore, it has been reported that C-3 spiroindoline derivatives highly enhance biological activity of chemical compounds [16,17,18,19].
Most of the reported protocols for the synthesis of both these products, especially in the case of spiroindoline–pyranopyrazoles, were suffering from disadvantages such as expensive reagents, long reaction times, low yields, toxic reagents, and harsh reaction conditions. Hence, development of an effective method for the synthesis of these scaffolds is significant. The preparation of an efficient catalyst is also necessary. In the past decade, functionalized magnetic catalysts have been the center of attentions due to their diverse advantages such as a broad range of applications, reusability, high stability, availability, and easy separation [20]. As a result, nanocatalysts are reliable and a promising option for chemists to be utilized in organic reactions.
In the past decades, functionalized magnetic catalysts have been the center of attention due to their diverse advantages such as a broad range of application, reusability, high stability, availability, and easy separation [21]. In connection with our previous works on the introduction of novel protocols in MCRs and bionanostructure catalysts [22,23,24,25,26], herein, the preparation and characterization of γ-Fe2O3@cellulose-OSO3H as a new bionanocomposite that catalyzes the effective synthesis of 2,4-dihydropyrano[2,3-c]pyrazole (Fig. 1, path A) and spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] (Fig. 1, path B) at room temperature are described. This nanocatalyst can remove simply after completion of the reaction by an external magnetic field.
Synthesis of 2,4-dihydropyrano[2,3-c]pyrazoles 1 and spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives 2 through four-component reactions of isatin or aldehyde or acetophenone derivatives, malononitrile or ethylcyanoacetate, ethyl acetoacetate and hydrazine hydrate catalyzed by γ-Fe2O3@cellulose-OSO3H
Reported methods hint different disadvantages, such as expensive reagents, long reaction times, low yields, and use of the toxic reagents in both of the reactions, especially, for spiroindoline–pyranopyrazoles production. Therefore, the use of effective catalysts will be an important approach to reduce these issues.
Results and discussion
Catalyst characterization
Initially, γ-Fe2O3 nanoparticles were synthesized by co-precipitation method using cellulose microcrystals. As indicated in Fig. 2, the size and morphology of the nanoparticles were studied by scanning electron microscopy (SEM). SEM images proved the homogeneous structure of the catalyst. The cellulose layer was coated homogeneously on the surface of the nanoparticles. The average size of nanoparticles was about 25–55 nm.
Figure 3 shows the Fourier transform infrared (FT-IR) spectra of γ-Fe2O3@cellulose-OSO3H in comparison with cellulose and cellulose-OSO3H. A broad band at 3350 cm− 1 was observed which is attributed to σ O−H stretching vibrations of OH groups on cellulose structure that due to the structure of each spectrum, the substrate cellulose nanocomposite structure proved to be designed. Hydrogen bonding is the reason of the broadness of this band. Due to the presence of nanoparticles between hydrogen bonds in the cellulose or cellulose-OSO3H structure, the OH band of the nanocomposite is lower than them. In the IR spectrum of nanocomposite, a new band at 437 cm− 1 is related to Fe–O bonds in Fe2O3 nanoparticles that can prove the nanoparticle synthesis on cellulose surface. In addition, the decrease in the height of OH broadband also is another reason for why Fe2O3 nanoparticles have been placed on the cellulose surface. Other important bands, which must be appeared at 580 and 634 cm− 1, are not distinguished because of overlapping with cellulose bands. The stretching vibrations of S=O bands should have been seen at 1032 cm− 1 related to SO3H is not seen due to the overlapping with cellulose bands located at 1046 cm− 1.
To determine the elements composition in the nanocomposite structure, the γ-Fe2O3@cellulose–OSO3H was analyzed by energy-dispersive X-ray (EDX) analysis. As shown in Fig. 4, elements C, O, F, and S were indicated. Cellulosic surface structure confirms the elements C and O as well as Fe and O in the synthesis of nanoparticles on the surface. In addition, existence of SO3H groups on the nanocomposite structure was confirmed by sulfur element on this analysis.
X-ray powder diffraction (XRD) pattern was also used to determine the structure of the nanocatalyst. The XRD pattern of the γ-Fe2O3@cellulose-OSO3H nanocomposite is shown in Fig. 5. The peak in region 2θ = 20 degree, which is a curved peak, represents the cellulose substrate and cellulose XRD. The sharp peaks in the region of 30–65° are related to γ-Fe2O3 nanoparticles on its surface. This could easily be proven by comparing with the γ-Fe2O3 pattern. The XRD pattern of the γ-Fe2O3, along with its relationship with the crystalline planes of each signal, corresponds perfectly with nanocomposite crystalline signals. These results showed that γ-Fe2O3@cellulose-OSO3H has been successfully synthesized. The γ-Fe2O3 peaks at XRD pattern are as follows: 2 2 0, 3 3 1, 4 0 0, 4 2 2, 5 1 1, and 4 4 0. The XRD pattern peaks of the obtained nanocomposite are as follows: 2θ = 30.3°, 35.8°, 43.35°, 53.7°, 57.3°, and 62.91° and XRD pattern of cellulose fibers shows two peaks at 2θ = 22.7° and 16.7°. According to the above results, it can be figured out that the patterns resulted from γ-Fe2O3@cellulose–OSO3H nanocomposite was in accordance with cellulose and γ-Fe2O3 patterns. This confirms the synthesis of γ-Fe2O3 nanoparticles inside cellulose sulfuric acid matrix. The average size of the nanoparticles is about 32 nm which is obtained via Scherrer equation:
Inspection of the catalytic activity of γ-Fe2O3@cellulose–OSO3H in the synthesis of pyranopyrazole and spiro-indole-pyranopyrazole derivatives
Synthesis of pyranopyrazole compounds using γ-Fe2O3@cellulose–OSO3H as catalyst
To optimize the reaction conditions, we considered the reaction of 3-nitrobenzaldehyde, ethylacetoacetate, malononitrile, and hydrazine hydrate (molar ratios of 1:1:1:1) as a model reaction (Fig. 6). First, this reaction was attempted under catalyst-free conditions, but after 1 h, the product was obtained in low yield. Therefore, the presence of catalyst for completion of this reaction is necessary. A number of catalysts such as Al2O3, [14] tetraethylammonium bromide [12], [(CH2)4SO3HMIM] [HSO4] [13], and meglumine [37] were reported. Some of these catalysts need heating at refluxing and provide low yields after long reaction times. In comparison with those catalysts, new nanocomposite (γ-Fe2O3@cellulose–OSO3H) proved to be the most efficient one which gave higher yield (96%) in 3 min with a very small amount of catalyst. The results are shown in Table 1. The progress of the reaction was monitored by TLC. In summary, the reaction time and yield of model reaction at room temperature and ethanol as a solvent in the presence of 0.005 g nanocatalyst were the best condition. Therefore, some other pyranopyrazole derivatives were synthesized with aforementioned mole ratio and in optimized condition utilized from the model reaction. These results persuaded us to other carbonyl compounds (aldehyde, acetophenone, and malononitrile derivatives (malononitrile or ethylcyanoacetate) for the synthesis of pyranopyrazole compounds. This reaction has the advantages of easy separation of catalyst by an external magnet, use of acetophenone derivatives, the first report of using ethylcyanoacetate instead of malononitrile, high yield and short reaction time, excellent recyclability of catalyst, use of the eco-friendly solvent, and easy workup.
We investigated aldehydes and acetophenones containing both electron-withdrawing and electron-donating groups and also malonitrile derivatives (malononitrile and ethylcyanoacetate). Due to very short reaction time in aldehyde derivatives, effects of these groups are not comprehensible and in reactivity of acetophenons, steric hindrance effects of groups are not salient, either. However, in case of acetophenones, compounds which contain electron-withdrawing groups react better and require short reaction time when compared to those containing of electron-donating groups. Because of the presence of methyl group in acetophenones and steric hindrance of this group, reaction time with acetophenone is longer than aldehydes. In the case of malononitrile derivatives (malononitrile or ethylcyanoacetate), no differences were observed in reaction time.
Table 2 clearly shows that the reaction of various aromatic aldehydes or acetophenones, malononitrile, ethyl acetoacetate, and hydrazine hydrate in ethanol as a solvent and the nanocomposite provided the corresponding pyranopyrazole in high yields and short reaction time. Some of these derivatives were produced for the first time, and for ensure of formation of them, these compounds were analyzed with melting point, IR, and NMR spectral data.
To show the efficiency of γ-Fe2O3@cellulose–OSO3H as appropriate catalyst for pyranopyrazole synthesis, we compared our results with other results which have been reported using various catalysts in Table 3. To compare the catalytic activity of γ-Fe2O3 nanoparticles or SO3H, cellulose sulfuric acid and γ-Fe2O3@cellulose were synthesized and applied in model reaction. The results showed, in the presence both these catalysts, that reaction time was longer and reaction yield was lower. However, in the presence of this new nanocomposite [with Lewis acid (γ-Fe2O3) and Brønsted–Lowry acid (SO3H)], the results were better.
Synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives using γ- Fe2O3@cellulose–OSO3H
To optimize the reaction conditions, we considered the reaction of isatin, ethylacetoacetate, malononitrile, and hydrazine hydrate (mole ratios 1:1:1:1) as a model reaction (Table 4). The progress of the reaction was monitored by TLC. According to results that were showed in this table, because of trace yield of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole], the presence of catalyst is necessary for the effective reaction progress and gaining high yield. A few reports observed for this reaction. Triethanolamine, meglumine, piperidine, 4-DMAP, and NaBr/electricity passed (F/mol) are some of catalysts applied for this reaction. Almost all of these catalysts were used once, so they do not have reusability in organic synthesis and organocatalysts, such as piperidine, are poison, too. Some of these catalysts need difficult conditions such as electricity passed, ultrasonic, high temperature, or low yields in long reaction time. Hence, a new catalyst that eliminates these problems is necessary. γ-Fe2O3@cellulose–OSO3H is effective and catalyzed this reaction in high yield and low reaction time and its separation from reaction pot, by an external magnetic field is very simple. In the pilot reaction, according to the results that showed conditions, the efficiency and yields of the products in ethanol as a green solvent at room temperature were higher than other solvent. 0.01 g of the catalyst was an optimized amount of the catalyst for this reaction. More or lower amounts of catalyst decrease the yield in the same reaction time.
Then, we decided to develop this optimized conditions to other isatins and ethylcyanoacetate as a malononitrile derivative for the synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives (Table 5). We investigated isatins containing NO2 or Cl on benzene ring and N–R derivatives of isatins. According to results that are listed in Table 5, in the presence of electron-withdrawing groups on the benzene of isatin, its chemical activity increases but with larger group on nitrogen of isatin, its activity decreases. In general, isatins which contain electron-withdrawing groups require shorter reaction time in comparison with those with N–R derivatives of isatin. This is also related to steric hindrance effects of alkyl groups.
To develop the optimized conditions, other isatins and ethylcyanoacetate as a malononitrile derivative were used for the synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives. According to results shown in Table 5, isatins which contain electron-withdrawing groups require shorter reaction times than those with N–R derivate of isatin. This may also be related to steric hindrance effects of alkyl groups.
To show the ability of γ-Fe2O3@cellulose–OSO3H as a useful catalyst for the synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives through one-pot four-component reaction, we compared our outcomes with other results which has been reported using various catalysts under different conditions (Table 6).
To investigate the recoverability and reusability of γ-Fe2O3@cellulose–OSO3H catalyst, it was tested in the reaction of 3-nitrobenzaldehyde, ethylacetoacetate, malononitrile, and hydrazine hydrate (mole ratio 1:1:1:1) and isatin, ethylacetoacetate, malononitrile, and hydrazine hydrate (mole ratio 1:1:1:1) as pilot reactions. After completion of the reaction, the nanocatalyst was simply separated by an external magnet. Then washed with hot ethanol and dried at room temperature. The catalyst was used and recycled five times without significant loss of activity (Fig. 7).
Conclusions
In summary, a new protocol was developed for the preparation of γ-Fe2O3@cellulose–OSO3H bionanostructure catalyst. The structure and morphology of the prepared nanocomposite were well confirmed by SEM, EDX, XRD, and FT-IR analyses. Then, its catalytic activity was investigated in two important syntheses of pyranopyrazoles and spiroindoline–pyranopyrazoles. This nanocomposite was easily separated by an external magnet and was effective reusable at least five times without remarkable loss of catalytic activity. The syntheses of pyranopyrazoles and spiroindoline–pyranopyrazoles were carried out through one-pot four-component reactions between isatin or aromatic aldehyde or acetophenone derivatives, ethylacetoacetate, hydrazine hydrate, and malononitrile or ethylcyanoacetate. This protocol had many advantages such as short reaction times, high yields, mild reaction conditions, and low requirement of the catalyst amount.
Experimental section
General
All solvents, chemicals, and reagents were purchased from Merck or Aldrich and used as received. Melting points were determined using an Electrothermal 9100 apparatus. FT-IR spectra were recorded as KBr pellets on a Shimadzu FT-IR-8400S spectrometer. Analytical TLC was carried out using Merck 0.2 mm silica gel 60 F-254 Al-plates. 1H and 13C NMR spectra were obtained using Bruker DRX-500 Avance spectrometers. The powder X-ray diffraction pattern was recorded using a X-PERT-PRO diffractometer with Cu Kα, (λ = 1.54 Å) irradiation, in the range of 5 to 80 (2θ) with a scan step of 0.026. The morphology of catalyst was studied with scanning electron microscopy using SEM (AIS2300C SEI) on gold-coated samples. Elemental analysis of the nanocomposite was provided by TESCAN-VEGA II energy-dispersive X-ray (EDX) analysis.
Preparation of γ-Fe2O3@cellulose-OSO3H
At first, the cellulose microcrystal (5.00 g) was added to 20 mL of CHCl3 and stirred vigorously. Then, 1.00 g of chlorosulfonic acid diluted in 5 mL of chloroform was added dropwise at 0 °C during 2 h. When the addition was completed, the mixture was stirred for 2 h until HCl was removed from reaction vessel. Finally, the mixture was filtered and washed with methanol (3*10 mL) and dried at room temperature to afford cellulose sulfuric acid. In second, an aqueous solution of NaOH, urea, and H2O was prepared with material ratios 7:12:81, respectively. Then, the temperature was decreased to -12 °C by use of ice and salt (NaCl). After that, 2.0 g of the synthesized cellulose sulfuric acid was added. Next, 0.5 g of FeCl2.4H2O and 1.0 g of FeCl3. 6H2O were dissolved in 50 mL of deionized water and added dropwise to the cellulose sulfuric acid solution. Finally, the resulted mixture was filtered and washed with water, ethanol and acetone and dried at room temperature to afford γ-Fe2O3 nanoparticles coated on cellulose sulfuric acid as a brown composite powder.
General procedure for the synthesis of pyranopyrazole derivatives
A mixture of an aldehyde 1 (1 mmol), malononitrile 2 (1 mmol), ethylacetoacetate 3 (1 mmol), hydrazine hydrate 4 (1 mmol), and γ-Fe2O3@cellulose-OSO3H (0.005 g) in 2 mL of ethanol was vigorously stirred in a 5 mL round bottom flask at room temperature. The reaction progress was monitored by TLC. After completion of the reaction, the catalyst was separated by an external magnet. The pure products were obtained by recrystallization from ethanol.
General procedure for the synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives
A mixture of isatin 1 (1 mmol), malononitrile or ethylcyanoacetate 6 (1 mmol), ethyl acetoacetate 7 (1 mmol), hydrazine hydrate (1 mmol), and γ-Fe2O3@cellulose–OSO3H (0.01 g) in 2 mL of ethanol was stirred vigorously in a 5 mL round bottom flask at room temperature. Reaction progression was monitored by TLC. The reaction progress was monitored by TLC. After completion of the reaction, the catalyst was separated by an external magnet. The catalyst was washed by hot ethanol, dried and reused in subsequent reactions. The pure products were obtained by recrystallization from ethanol.
Spectral data
Cellulose microcrystalline
IR (KBr) cm− 1: 3338, 2898, 1656, 1429, 1369, 1163, 1110, 1058, 667, 615.
Cellulose–OSO3H
IR (KBr) cm− 1: 3350, 2900, 1645, 1429, 1371, 1163, 1110, 1058, 667, 613, 561.
γ-Fe2O3@cellulose–OSO3H
IR (KBr) cm− 1: 3353, 2894, 1633, 1423, 1367, 1201, 1155, 1062, 1027, 663, 565, 437.
6-Amino-3,4-dimethyl-4-(4-nitrophenyl)-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (Table 2, Entry 27)
White powders (94%): mp 193–195 °C. IR (KBr) cm− 1: 3481, 3223, 3122, 2189, 1641, 1596, 1490, 1470, 1352. 1H NMR (500.13 MHz, DMSO-d6) δ: 1.7 (3H, s, CH3), 1.7 (3H, s, CH3), 6.9 (2H, br s, NH2), 7.5 (2H, d, J = 8.9 Hz, H–Ar), 8.1 (2H, d, J = 8.9 Hz, H–Ar), 12.1 (1H, s, NH).
6-Amino-4-(4-methoxy-3-(phenoxymethyl)phenyl)-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (Table 2, Entry 24)
White powders (94%): mp 189–191 °C. 1H NMR (500.13 MHz, DMSO-d6) δ: 1.7 (3H, s, CH3), 3.7 (3H, s, OCH3), 4.5 (1H, s, CH), 5.0 (2H, s, CH2), 6.8–7.4 (10H, m, H–Ar and NH2). 12.065 (1H, s, NH). 13C NMR (125.61 MHz, DMSO-d6) δ: 9.7, 35.7, 39.5, 55.5, 57.3, 69.9, 97.6, 111.9, 113.21, 120.0, 127.9, 128.3, 135.6, 136.8, 137.0, 147.3, 148.0, 154.7, 160.7.
6′-Amino-5-chloro-3′-methyl-5′-propionyl-2′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazol]-2-one (Table 5, Entry 10)
White powder (92%); mp 280–282 °C; IR (KBr) cm− 1: 3417, 3311, 3240, 1695, 1666, 1475, 1292, 1199. 1H NMR (500.13 MHz, DMSO-d6) δ: 0.7 (3H, t, J = 7.1 Hz, CH3), 1.6 (3H, s, CH3), 3.6–3.8 (2H, m, CH2), 6.8 (1H, d, J = 8.2 Hz, H–Ar), 6.8 (1H, d, J = 1.8 Hz, H–Ar), 7.1 (1H, dd, J = 5.1 and 2.1 Hz, H–Ar), 8.0 (2H, br s, NH2), 10.5 (1H, s, NH), 12.2 (1H, s, NH).
References
A. Maleki, Ultrason. Sonochem. 40, 460 (2018)
A. Maleki, Tetrahedron 68, 7827 (2012)
A. Maleki, R. Firouzi-Haji, Z. Hajizadeh, Int. J. Biol. Macromol. 116, 320 (2018)
H.M. Al-Matar, K.D. Khalil, A.Y. Adam, M.H. Elnagdi, Molecules 15, 6619 (2010)
Z.H. Ismail, G.M. Aly, M.S. El-Degwi, H.I. Heiba, M.M. Ghorab, Egypt. J. Biotechnol. 13, 73 (2003)
M.E. Zaki, H.A. Soliman, O.A. Hiekal, A.E. Rashad, Z. Naturforsch. C. 61, 1 (2006)
R. Moosavi-Zare, M.A. Zolfigol, E. Noroozizadeh, M. Tavasoli, V. Khakyzadeh, A. Zare, New J. Chem. 37, 4089 (2013)
A. Maleki, A.A. Jafari, S. Yousefi, Carbohydr. Polym. 175, 409 (2017)
A. Maleki, M. Ghassemi, R. Firouzi Haji, Pure Appl. Chem. 90, 387 (2018)
A. Maleki, R. Ghalavand, R. Firouzi-Haji, Iran. J. Catal. 8, 221 (2018)
A. Maleki, Polycycl. Aromat. Compd. 38, 402 (2018)
G.S. Kumar, C. Kurumurthy, B. Veeraswamy, P.S. Rao, P.S. Rao, B. Narsaiah, Org. Prep. Proced. Int. 45, 429 (2013)
J. Ebrahimi, A. Mohammadi, V. Pakjoo, E. Bahramzade, A. Habibi, J. Chem. Sci. 124, 1013 (2012)
H. Mecadon, M.R. Rohman, M. Rajbangshi, B. Myrboh, Tetrahedron Lett. 52, 2523 (2011)
R.J. Sundberg, Indoles. (Academic Press, New York, 1996)
J.F. Da Silva, S.J. Garden, A.C. Pinto, J. Braz. Chem. Soc. 12, 273 (2001)
K.C. Joshi, P. Chand, Pharmazie 37, 1 (1982)
K.C. Joshi, R. Jain, K. Sharma, S.K. Bhattacharya, R.K. Goel, J. Indian Chem. Soc. 115, 202 (1988)
A.H. Abdel-Rahman, E.M. Keshk, M.A. Hanna, S.M. El-Bady, Bioorg. Med. Chem. 12, 2483 (2004)
A. Maleki, Helv. Chim. Acta 97, 587 (2014)
A. Maleki, Tetrahedron Lett. 54, 2055 (2013)
A. Maleki, A.A. Jafari, S. Yousefi, V. Eskandarpour, C.R. Chim. 18, 1307 (2015)
A. Maleki, V. Eskandarpour, J. Rahimi, N. Hamidi, Carbohydr. Polym. 208, 251 (2019)
A. Maleki, A.A. Jafarim, S. Yousefi, J. Iran. Chem. Soc. 14, 1801 (2017)
A. Maleki, M. Aghaei, R. Paydar, J. Iran. Chem. Soc. 14, 485 (2017)
Z. Hajizadeh, A. Maleki, Mol. Catal. 460, 87 (2018)
H.V. Chavan, S.B. Babar, R.U. Hoval, B.P. Bandgar, Bull. Korean Chem. Soc. 32, 3963 (2011)
K. Kanagaraj, K. Pitchumani, Tetrahedron Lett. 51, 3312 (2010)
S.N. Darandale, J.N. Sangshetti, D.B. Shinde, J. Korean Chem. Soc. 56, 328 (2012)
M.A. Nasseri, S.M. Sadeghzadeh, Monatsh. Chem. 144, 151 (2013)
K. Ablajan, W. Liju, A. Tuoheti, Y. Kelimu, Lett. Org. Chem. 9, 639 (2012)
P.P. Bora, M. Bihani, G. Bez, J. Mol. Catal. B 92, 24 (2013)
Y.M. Litvinov, A.A. Shestopalov, L.A. Rodinovskaya, A.M. Shestopalov, J. Comb. Chem. 11, 914 (2009)
M. G.Dekamin,M.Alikhani,A.Emami,H.GhafuriandS.Javanshir,J. Iran. Chem. Soc., 2016,13,591.
Y. Zou, Y. Hu, H. Liu, D. Shi, ACS Comb. Sci. 14, 38 (2011)
R.G. Redkin, L.A. Shemchuk, V.P. Chernykh, O.V. Shishkin, S.V. Shishkina, Tetrahedron 63, 11444 (2007)
R.Y. Guo, Z.M. An, L.P. Mo, S.T. Yang, H.X. Liu, S.X. Wang, Z.H. Zhang, Tetrahedron 69, 9931 (2013)
J. Feng, K. Ablajan, A. Sali, Tetrahedron 70, 484 (2014)
S. Ahadi, Z. Yasaei, A. Bazgir, Heterocycl. Chem. 47, 109 (2010)
K. Ablajan, L.J. Wang, Z. Maimaiti, Y.T. Lu, Monatsh. Chem. 145, 491 (2014)
H. Sachdeva, R. Saroj, Sci. World J., (2013). https://doi.org/10.1155/2013/680671
Acknowledgements
The authors gratefully acknowledge partial support from the Research Council of the Iran University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maleki, A., Eskandarpour, V. Design and development of a new functionalized cellulose-based magnetic nanocomposite: preparation, characterization, and catalytic application in the synthesis of diverse pyrano[2,3-c]pyrazole derivatives. J IRAN CHEM SOC 16, 1459–1472 (2019). https://doi.org/10.1007/s13738-019-01610-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01610-9