Abstract
In this study, an environmentally friendly complexing agent, S,S′-ethylenediamine-N,N′-disuccinic acid (EDDS), was applied in Fe(III)-mediated activation of persulfate (PS), and the degradation performance of trichloroethylene (TCE) was investigated. The effects of PS concentration, Fe(III)/EDDS molar ratio, and inorganic anions on TCE degradation were evaluated, and the generated reactive oxygen species responsible for TCE removal were identified. The results showed that nearly complete TCE degradation was achieved with PS of 15.0 mM and a molar ratio of Fe(III)/EDDS of 4:1. An increase in PS concentration or Fe(III)/EDDS molar ratio to a certain value resulted in enhanced TCE degradation. All of the anions (Cl−, HCO3 −, SO4 2−, and NO −3 ) at tested concentrations had negative effects on TCE removal. In addition, investigations using radical probe compounds and radical scavengers revealed that sulfate radicals (SO ·−4 ), hydroxyl radicals (·OH), and superoxide radical anions (O ·−2 ) were all generated in the Fe(III)–EDDS/PS system, and ·OH was the primary radical responsible for TCE degradation. In conclusion, the Fe(III)–EDDS-activated PS process is a promising technique for TCE-contaminated groundwater remediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The chlorinated solvent trichloroethylene (TCE) has been widely used in chemical manufacturing as a cleaning and degreasing agent. As a result of the extensive usage and improper operation during production, application, transportation, and storage, TCE has become one of the most frequently detected organic pollutants in contaminated groundwater and at hazardous waste sites. Recently, in situ chemical oxidation by means of persulfate (PS) has received considerable attention for contaminated groundwater and soil remediation [1–3]. PS is stable at room temperature and can be activated by various methods, including heat, ultraviolet irradiation, transition metals, hydrogen peroxide, and alkaline, generating sulfate radicals (SO ·−4 , E 0 ≈ 2.6 V) and other reactive species [4–8]. Upon activation, PS is capable of degrading a wide variety of contaminants, including chlorinated aliphatics, chlorinated aromatics, fuel hydrocarbons, polycyclic aromatic hydrocarbons, pesticides, dyes, and pharmaceuticals and personal care products. [9–12].
Among the transition metals, ferrous iron [Fe(II)] has been commonly used as PS activator, as it is relatively inexpensive, nontoxic, and effective. Nevertheless, the homogeneous reaction of Fe(II) with PS creates an obstacle to maintaining iron availability in the solution, because the Fe(III) formed in the Fe(II)/PS process is stable and precipitates at pH > 4, and the initiation reaction then stalls (Eq. 1) [13]. Moreover, the Fe(II)/PS process requires a high concentration of Fe(II), which further consumes the produced SO ·−4 , as shown in Eq. 2. Therefore, to mitigate this effect, chelating agents are commonly applied in iron-activated PS processes to improve iron availability and to enhance contaminant degradation. Ethylenediaminetetraacetic acid (EDTA) and citric acid are both frequently used for chelating iron. Liang et al. [14, 15] reported that citric acid was superior to EDTA for the removal of benzene, toluene, ethylbenzene, and xylene (BTEX) and TCE in an iron-activated PS system, while sulfamethoxazole degradation was most efficiently achieved with EDTA [16]. As an alternative source of Fe(II), zero-valent iron (ZVI) has also been employed as a PS activator for the degradation of many organic contaminants, with promising results [17–19]. Moreover, industrial iron waste showd sutanined reaction stoichiometric efficiency and less sludge formation than commercial iron during PS activation [19].
The low biodegradability of EDTA, however, has led to its classification as an emerging contaminant [20, 21]. Alternatively, a new complexing agent for iron, S,S′-ethylenediamine-N,N′-disuccinic acid (EDDS), has recently attracted scientific interest [22]. EDDS is a structural isomer of EDTA and is known to be readily biodegradable. To date, EDDS-chelated iron-activated PS has been applied in only a few studies. Rastogi et al. [23] compared the effectiveness of EDDS in Fe(II)-mediated activation of PS, PMS (peroxymonosulfate), and H2O2, and found that EDDS was the most effective in the Fe(II)/H2O2 system, but was not effective in dissociating PS. Similarly, EDDS showed no promoting effect on Fe(II)/PS-mediated degradation of ciprofloxacin or sulfamethoxazole [16], or Orange G (OG) at low initial concentrations [24]. In contrast, Han et al. [25] reported that EDDS exhibited the best iron chelation activity in comparison to citric acid, oxalic acid, and tartaric acid, and both PS decomposition and aniline degradation were observed under acidic conditions, with a molar ratio of PS/EDDS/Fe(II)/aniline at 20:5:10:1. In light of these conflicting results, therefore, further research is needed to determine the effectiveness of EDDS in iron-activated PS systems.
Fe(III) has been used as an alternative source of ferrous iron in Fenton-like reactions, and numerous studies have reported the application of Fe(III) complexes in modified Fenton processes for organic pollutant degradation. However, the use of Fe(III) or a Fe(III) complex for PS activation is not well studied, and the mechanism behind Fe(III) activation is much less clear. One possibility is that some products of the organic pollutants or chelating agents (i.e. R· or RH) may promote a slow back reaction of Fe(III) to Fe(II) (Eqs. 3, 4), thus facilitating PS activation [25, 26]. Rodriguez et al. [27] found that OG was efficiently removed by the Fe(III)/PS process, and proposed that quinone intermediates generated during OG degradation acted as electron shuttles, enhancing the redox cycle of Fe(III)/Fe(II). Liang et al. [26] investigated the impact of solution pH on TCE oxidation using the Fe(III)–EDTA/PS process, and demonstrated that increasing pH resulted in enhanced TCE degradation. To date, no studies have explored the ability of the Fe(III)–EDDS complex to promote PS activation. The objective of this study, therefore, was (1) to investigate the degradation performance of TCE in the Fe(III)–EDDS/PS system with various PS concentrations and Fe(III)/EDDS molar ratios, (2) to evaluate the influence of different inorganic anions on TCE degradation, and (3) to examine the potential reactive oxygen species responsible for TCE removal.
Materials and methods
Materials
The followed reagents were purchased from Jingchun Reagent Co., Ltd. (Shanghai, China) and used without further purification: trichloroethylene (TCE, 99.0 %), carbon tetrachloride (CT, 99.5 %), nitrobenzene (NB, 99.0 %), anisole (AN, 99.0 %), isopropanol (IPA, 99.5 %), tert-butyl alcohol (TBA, 99.0 %), hexane (97.0 %), 1,10-phenanthroline monohydrate (98 %), sodium persulfate (98.0 %), ferric sulfate (99.0 %), sodium chloride (99.5 %), sodium sulfate (99.0 %), sodium bicarbonate (99.5 %), sodium nitrate (99.0 %), and potassium iodide (99.5 %). S,S′-ethylenediamine-N,N′-disuccinic acid trisodium salt (EDDS, 35 % in water) was obtained from Sigma-Aldrich (Shanghai, China). Ultrapure water (PURELAB Classic DI; ELGA, Marlow, UK) was used for preparing aqueous solutions.
Experimental procedures
A TCE stock solution was prepared by allowing the neat liquid TCE to equilibrate with Milli-Q water in the dark overnight under gentle stirring, and later diluted to the desired concentration (initial TCE concentration = 0.15 mM). The stock solution of probe chemicals (NB, AN, and CT) were prepared using the same procedure. All experiments were conducted in a 250-mL cylindrical glass reactor. The results from the control test with the addition of TCE solution alone confirmed less than a 3 % loss of TCE during the experimental period under all tested conditions. In TCE degradation experiments, the predetermined amounts of Fe2(SO4)3 and EDDS were added to the TCE solution and thoroughly mixed with a magnetic stirrer, followed by the addition of PS to start the reaction. The pH of the solution was unadjusted, and the temperature was kept constant at 20 °C with a cooling water jacket using a thermostatic circulating water bath (SCIENTZ SDC-6, Zhejiang, China). In chemical probe tests, the degradation performance of NB, AN, and CT was investigated individually without TCE. Aqueous samples were taken at desired time intervals and analyzed immediately. All experiments were conducted in triplicate, and mean values are reported.
Analytical methods
Aqueous sample was analyzed followed extraction with hexane. The concentrations of TCE and CT were quantified by a gas chromatograph (Agilent 7890A; Palo Alto, CA, USA) equipped with an electron capture detector and a DB-VRX column (60-m length, 320-μm i.d., 1.4-μm thickness). The injection volume of sample was 1 μL with a split ratio of 20:1. The temperatures of the injector and detector were 240 and 260 °C, respectively, and the oven temperature was isothermal at 75 °C. The recovery of TCE through the above procedure was in the range of 85 to 96 %. The concentrations of NB and AN were analyzed by the same gas chromatograph equipped with a flame ionization detector and HP-5 column (30-m length, 250-μm i.d., 0.25-μm thickness). The temperatures of injector, oven, and detector were set at 200, 150, and 250 °C, respectively. The concentration of PS was quantified using a spectrophotometric method following the procedures described by Liang et al. [28]. Ferrous iron was quantified with a spectrophotometer (DR6000; Hach, Loveland, CO) using 1,10-phenanthroline. The linear dynamic range for PS and ferrous iron measurement was 0–64 mM at 352 nm and 0.018–1.8 mM (1–100 mg/L) at 396 nm, respectively. The pH was measured with a pH meter (DELTA 320; Mettler-Toledo, Greifensee, Switzerland).
Results and discussion
Degradation performance of TCE in the Fe(III)–EDDS/PS process
To elucidate the effect of the Fe(III)–EDDS complex on TCE degradation in the presence of PS, we conducted several experiments for removal of TCE by PS, Fe(III)–EDDS, Fe(III)/PS, and Fe(III)–EDDS/PS processes, and the results are shown in Fig. 1. No observable removal of TCE was achieved over 60 min in the Fe(III)–EDDS or Fe(III)/PS processes, indicating that direct oxidation of Fe(III) and PS activation by Fe(III) were not sufficient for the degradation of TCE. In addition, 10.8 % removal of TCE was observed with the addition of PS alone, owing to the direct oxidation of PS. Similarly, Anipsitakis and Dionysiou [29] reported that 15 % of 2,4-dichlorophenol was removed after 4 h in the Fe(III)/PS system, and they attributed this to direct oxidation between Fe(III) and the substrate.
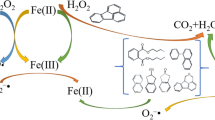
In the Fe(III)–EDDS/PS process, nearly complete degradation of TCE was achieved after 60 min of reaction. It is interesting to note that the removal of TCE was fast and efficient within the first minute (62.5 % removal), followed by gradual degradation over the remaining 59 min; other researchers have reported a similar two-stage process [30, 31]. Liang et al. [26] demonstrated that the byproducts of EDTA destruction by SO ·−4 or ·OH accelerated the reduction of Fe(III) to Fe(II), further enhancing PS activation. Huang et al. [22] showed that the presence of EDDS was thermodynamically favorable for improving the reduction of Fe(III)–EDDS to Fe(II)–EDDS. In addition, studies have reported that a superoxide (O ·−2 , E0 = − 2.4 V) was generated in a Fe(III)–EDDS-based Fenton process, and the ferric complex was reduced into its ferrous state by O ·−2 as well [22, 32, 33]. In the present study, we also confirmed the existence of O ·−2 (in the last section), which accelerated the Fe(III)/Fe(II) cycle and enhanced Fe(II) recovery. Therefore, the high efficiency of TCE degradation in the Fe(III)–EDDS/PS process is attributed to the generation of SO ·−4 or ·OH (hydroxyl radical), initiated by the complexation reaction of Fe(II) with PS. It should be noted that free ferrous iron was measured during the reaction, but the detected concentrations were all less than 1.5 mg/L, which can be explained by the rapid reaction of the recycled ferrous iron with PS.
The decomposition of PS and the variation in solution pH in the Fe(III)–EDDS/PS process were also investigated. As shown in Fig. 2, PS concentration was rapidly reduced from 15.0 to 12.7 mM within the first 30 s, and then gradually decreased to 11.4 mM after 60 min, which was fitted with TCE degradation performance. In addition, reaction stoichiometric efficiency (RSE) was introduced to estimate the utilization efficiency of PS, which was the ratio of the degraded TCE (Δ[TCE]) to the consumed PS (Δ[PS]) [34, 35]. As seen in the inset in Fig. 2, the RSE ranged from 4.2 to 5.1 % at selected times (0.5–60 min). Ghauch et al. [18] reported RSE of 5.2 % under optimum conditions for sulfamethoxazole removal in a micrometric ZVI/PS system. The initial pH value of the TCE solution with the addition of the Fe(III)–EDDS complex was 3.10, which dropped to 2.57 after 30 s, followed by PS addition, and a further gradual decrease to 2.30 after 60 min. The acidification may be due to the formation of acid products through TCE decomposition as well as the occurrence of acidic products of PS such as bisulfate (HSO −4 ) [36].
Effects of PS concentration and Fe(III)/EDDS molar ratio on TCE degradation
The effect of PS on the degradation of TCE in the Fe(III)–EDDS/PS process was examined by varying PS concentrations from 4.5 to 30.0 mM (corresponding to the molar ratios of PS/TCE from 30:1 to 200:1). The experimental results are shown in Fig. 3a. At a molar ratio of Fe(III)/EDDS = 4:1, increasing the concentration of PS from 4.5 to 15.0 mM resulted in an increase in TCE degradation from 77.1 to 99.7 % within 60 min. Increasing the PS concentration to 30.0 mM resulted in almost no further enhancement of TCE degradation, indicating an optimum PS concentration at a fixed Fe(II)/EDDS molar ratio. The results are consistent with the observations of other researchers who applied iron-activated PS oxidation for organic contaminant degradation [37, 38]. This phenomenon is also similar to a Fenton-like reaction where an excessive amount of H2O2 had a detrimental effect [39]. Therefore, the PS concentration in subsequent experiments was set at 15.0 mM.
Figure 3b shows the influence of the Fe(III)/EDDS molar ratio on the degradation of TCE. In general, increasing the molar ratio led to an increase in TCE degradation efficiency. When the molar ratio was increased from 1:1 to 4:1, TCE degradation efficiency increased from 19.2 to 99.6 %. A further increase of the molar ratio to 8:1 resulted in only a slight increase in TCE degradation efficiency within 30 min and similar removal of TCE (almost complete degradation) at the end of experiment. Liang et al. [26] found that an Fe(III)/EDTA molar ratio of 1:1 exhibited the highest rate of TCE degradation at pH 10 in the Fe(III)–EDTA/PS process, and further increasing the molar ratio became less effective. It should be noted that the molar ratio of Fe(III)/EDDS in the following experiments was set at 4:1.
Effects of inorganic anions on TCE degradation
Although many studies have documented the effects of anions on PS oxidation performance, to date, no studies have investigated their effects on TCE removal in the Fe(III)–EDDS/PS process. Therefore, in the present study, experiments were conducted with the addition of various anions (Cl−, HCO3 −, SO4 2−, and NO3 −). Figure 4 shows that all of the anions had scavenging effects on TCE degradation under the tested concentrations (1–100 mM) in the Fe(III)–EDDS/PS process, with decreasing TCE degradation efficiency observed as anion concentration in solutions increased.
As shown in Fig. 4a, as the Cl− concentration increased from 1 to 100 mM, TCE removal was reduced from 97.0 to 30.9 % after 60 min. Cl− at an elevated concentration reacted with the reactive oxygen species, thus resulting in competition with SO ·−4 or ·OH for reaction with TCE (Eqs. 5–7). The rate constants for Cl− reactions with SO ·−4 and ·OH were 4.7 × 108 M−1s−1 and 4.3 × 109 M−1s−1, respectively. The influence of HCO3 − can be observed in Fig. 4b, which shows that the effect of HCO3 − was not obvious at a low concentration (1.0 mM). However, significant inhibition occurred at concentrations of 10 and 100 mM, and possible chemical scavenging mechanisms are shown as Eqs. (8–10). In other studies [40, 41], PS activation was reportedly enhanced by the addition of Cl− or HCO −3 through production of reactive chlorine or carbonate species, as presented in Eqs. 5–10, but such enhancement was not observed in this study.
Figure 4c shows that an apparent inhibitory effect was observed at SO4 2− additions of 1–100 mM, although complete TCE degradation was still achieved within 60 min at 1 mM and 10 mM, whereas only 53.2 % of TCE was removed with the addition of 100 mM of SO4 2−. Compared with the above three anions, the inhibitory effect observed for NO3 − was much lower (Fig. 4d). The presence of SO4 2− and NO3 − is considered to impede the formation of Fe(III)–peroxo complexes, resulting in adverse effects on TCE removal. Such inhibitory activity has also been reported in Fe(III)–H2O2 processes, in which a reduction was observed in the rates of H2O2 and organic compound decomposition [42, 43]. In addition, SO ·−4 can also be consumed by NO3 − to form NO ·3 , with less reactivity (Eq. 11) [44]. In our previous study [45], the presence of SO4 2− and NO3 − at 1–100 mM had almost no influence on the degradation of 1,1,1-trichloroethane in a thermally activated PS process, indicating that the Fe(III)–EDDS/PS process is more sensitive to anions in aqueous solution.
Identification of reactive oxygen species generated in the Fe(III)–EDDS/PS process
In studies to date, the oxidative species SO ·−4 and ·OH are generally acknowledged to be key reactive species in PS activation systems and to play a major role in the degradation of organic contaminants. However, emerging evidence suggests that other reactive oxygen species, such as O ·−2 , may be important in PS chemistry as well. Furman et al. [7] studied the mechanism of base activation of persulfate and confirmed the generation of O ·−2 . Our previous study demonstrated the presence of SO ·−4 , ·OH, and O ·−2 in an Fe(II)–citric acid/PS system [31]. Thus, we hypothesized that both oxidation and reduction reactions may be responsible for TCE degradation in Fe(III)–EDDS/PS processes. Therefore, a chemical probe method and radical scavenger test were introduced to identify the yields of SO ·−4 , ·OH, and O ·−2 and their contribution to TCE degradation.
The generation of reactive oxygen species in the Fe(III)–EDDS/PS process was measured by the loss of probe compounds. Because nitrobenzene (NB) is known to react with ·OH at high rates (3.9 × 109 M−1s−1, approximately 3000–3900 times as high as its reaction with SO ·−4 ) [46], we chose NB as the probe compound for ·OH. Anisole (AN) was selected to identify the presence of both SO ·−4 and ·OH, due to its high reactivity with these two radicals [46]. Carbon tetrachloride (CT) is often used as a probe compound for O ·−2 because of its high reactivity with O ·−2 but high resistance to both SO ·−4 and ·OH [47, 48]. The initial concentrations of NB, AN, and CT were 2, 2, and 0.03 mM, respectively.
The results of the probe compound degradation in the Fe(III)–EDDS/PS process are presented in Fig. 5. In the control tests with NB/AN alone or in the presence of PS, minimal loss of NB or AN was observed, and the volatilization of CT was less than 5 % over 120 min (data not shown). As shown in Fig. 5, 47.2 % removal of NB was achieved, indicating that PS was activated by the Fe(III)–EDDS complex to form ·OH during the reaction. In addition, the degradation of AN (20.0 %) implies that ·OH and/or SO ·−4 were generated. Furthermore, when 1 M of tert-butyl alcohol [TBA, k ·OH = (3.8–7.6) × 108 M−1s−1, k SO4·− = (4.0–9.1) × 105 M−1s−1] was introduced as ·OH scavenger into AN degradation system, 10.3 % decomposition of AN was still observed, demonstrating the presence of SO ·−4 other than ·OH. In addition, 31.2 % of CT was degraded in the tested condition, suggesting that O ·−2 was present in the Fe(III)–EDDS/PS process as well.
Since the presence of SO ·−4 , ·OH, and O ·−2 was proven with the chemical probe method, radical scavenger tests were conducted to identify the contribution of these three radicals to TCE degradation. ·OH was scavenged by TBA as before, and both SO ·−4 and ·OH were scavenged by isopropanol (IPA, k ·OH = 2.8 × 109 M−1s−1, k SO4·− = 6.0 × 107 M−1s−1), while 1,4-benzoquinone (BQ) was selected as a quencher of O ·−2 (k O2·− = 9.6 × 108 M−1s−1) [49, 50]. Figure 6 shows the degradation of TCE after 60 min with the addition of excess TBA (100 mM), IPA (100 mM), and BQ (10 mM). Here, the removal of TCE decreases from 99.7 to 31.2 % in the presence of TBA, indicating that 68.5 % (=99.7 − 31.2 %) of TCE degradation was due to the activity of ·OH. The addition of IPA, on the other hand, reduced TCE removal by 82.2 % compared to the unscavenged system, and thus SO ·−4 contributed 13.7 % (=82.2 − 68.5 %) to the degradation of TCE. Moreover, with the addition of both IPA (100 mM) and BQ (10 mM), TCE degradation efficiency was almost completely inhibited. Hence, the complete degradation of TCE was attributed to the function of SO ·−4 , ·OH, and O ·−2 , and the contribution of O ·−2 was calculated as 17.8 % (=100 − 68.5 − 13.7 %). However, with the addition of BQ alone, TCE removal was reduced to 79.8 %, and the O ·−2 contribution was 19.9 % (99.7 − 79.8 %). The difference between the O ·−2 calculations can be explained by TCE volatilization or the slight reaction of BQ with SO ·−4 or ·OH.
Conclusion
The results of this study show that the Fe(III)–EDDS/PS process was effective for the removal of TCE, with almost complete degradation of TCE obtained after 60 min at a PS concentration of 15.0 mM and a molar ratio of Fe(III)/EDDS of 4:1. Increasing PS concentration from 4.5 to 15.0 mM and the molar ratio of Fe(III)/EDDS from 1:1 to 4:1 ensured a significant increase in TCE degradation, while a further increase in PS concentration and molar ratio of Fe(III)/EDDS produced no obvious additional enhancement of TCE removal. In addition, the effects of inorganic anions, including Cl−, HCO3 −, SO4 2−, and NO3 −, on the inhibition of TCE degradation were observed at different levels. The generation of SO ·−4 , ·OH, and O ·−2 was confirmed in an EDDS-chelated Fe(III)-activated PS system, and all were responsible for TCE degradation, with ·OH appearing to have a predominant effect.
References
L.W. Matzek, K.E. Carter, Chemosphere 151, 178 (2016)
B.T. Zhang, Y. Zhang, Y. Teng, M. Fan, Crit. Rev. Environ. Sci. Technol. 45, 1756 (2015)
A. Tsitonaki, B. Petri, M. Crimi, H. MosbÆK, R.L. Siegrist, P.L. Bjerg, Crit. Rev. Environ. Sci. Technol. 40, 55 (2010)
Y. Ji, C. Dong, D. Kong, J. Lu, Q. Zhou, Chem. Eng. J. 263, 45 (2015)
R. Zhang, P. Sun, T.H. Boyer, L. Zhao, C.H. Huang, Environ. Sci. Technol. 49, 3056 (2015)
C. Wang, J. Wan, Y. Ma, Y. Wang, Res. Chem. Intermed. 42, 481 (2015)
O.S. Furman, A.L. Teel, R.J. Watts, Environ. Sci. Technol. 44, 6423 (2010)
A.R. Rahmani, H. Rezaeivahidian, M. Almasi, A. Shabanlo, H. Almasi, Res. Chem. Intermed. 42, 1441 (2015)
B.G. Petri, R.J. Watts, A. Tsitonaki, M. Crimi, N.R. Thomson, A.L. Teel, in In Situ Chemical Oxidation for Groundwater Remediation, ed. by L.R. Siegrist, M. Crimi, J.T. Simpkin (Springer New York, New York, NY, 2011), p. 147
A. Ghauch, A.M. Tuqan, N. Kibbi, Chem. Eng. J. 197, 483 (2012)
A. Ghauch, A.M. Tuqan, N. Kibbi, Chem. Eng. J. 279, 861 (2015)
A. Ghauch, A.M. Tuqan, N. Kibbi, S. Geryes, Chem. Eng. J. 213, 259 (2012)
C. Liang, C.J. Bruell, M.C. Marley, K.L. Sperry, Chemosphere 55, 1213 (2004)
C. Liang, C.J. Bruell, M.C. Marley, K.L. Sperry, Chemosphere 55, 1225 (2004)
C. Liang, C.F. Huang, Y.J. Chen, Water Res. 42, 4091 (2008)
Y. Ji, C. Ferronato, A. Salvador, X. Yang, J.-M. Chovelon, Sci. Total Environ. 472, 800 (2014)
G. Ayoub, A. Ghauch, Chem. Eng. J. 256, 280 (2014)
A. Ghauch, G. Ayoub, S. Naim, Chem. Eng. J. 228, 1168 (2013)
S. Naim, A. Ghauch, Chem. Eng. J. 288, 276 (2016)
X. Xu, N.R. Thomson, Chemosphere 69, 755 (2007)
C.K. Schmidt, M. Fleig, F. Sacher, H.J. Brauch, Environ. Pollut. 131, 107 (2004)
W. Huang, M. Brigante, F. Wu, C. Mousty, K. Hanna, G. Mailhot, Environ. Sci. Technol. 47, 1952 (2013)
A. Rastogi, S.R. Al-Abed, D.D. Dionysiou, Water Res. 43, 684 (2009)
D. Han, J. Wan, Y. Ma, Y. Wang, M. Huang, Y. Chen, D. Li, Z. Guan, Y. Li, Chem. Eng. J. 256, 316 (2014)
D. Han, J. Wan, Y. Ma, Y. Wang, Y. Li, D. Li, Z. Guan, Chem. Eng. J. 269, 425 (2015)
C. Liang, C.P. Liang, C.C. Chen, J. Contam. Hydrol. 106, 173 (2009)
S. Rodriguez, L. Vasquez, D. Costa, A. Romero, A. Santos, Chemosphere 101, 86 (2014)
C. Liang, C.F. Huang, N. Mohanty, R.M. Kurakalva, Chemosphere 73, 1540 (2008)
G.P. Anipsitakis, D.D. Dionysiou, Environ. Sci. Technol. 38, 3705 (2004)
C. Tan, N. Gao, W. Chu, C. Li, M.R. Templeton, Sep. Purif. Technol. 95, 44 (2012)
X. Wu, X. Gu, S. Lu, M. Xu, X. Zang, Z. Miao, Z. Qiu, Q. Sui, Chem. Eng. J. 255, 585 (2014)
Y. Wu, M. Passananti, M. Brigante, W. Dong, G. Mailhot, Environ. Sci. Pollut. Res. 21, 12154 (2014)
J. Li, G. Mailhot, F. Wu, N. Deng, J. Photochem. Photobiol. A Chem. 212, 1 (2010)
A. Ghauch, A.M. Tuqan, Chem. Eng. J. 183, 162 (2012)
X. Wei, N. Gao, C. Li, Y. Deng, S. Zhou, L. Li, Chem. Eng. J. 285, 660 (2016)
S. Yang, P. Wang, X. Yang, L. Shan, W. Zhang, X. Shao, R. Niu, J. Hazard. Mater. 179, 552 (2010)
X.R. Xu, X.Z. Li, Sep. Purif. Technol. 72, 105 (2010)
S.Y. Oh, H.W. Kim, J.M. Park, H.S. Park, C. Yoon, J. Hazard. Mater. 168, 346 (2009)
Y. Wu, S. Zhou, F. Qin, K. Zheng, X. Ye, J. Hazard. Mater. 179, 533 (2010)
R. Yuan, S.N. Ramjaun, Z. Wang, J. Liu, J. Hazard. Mater. 196, 173 (2011)
L.R. Bennedsen, J. Muff, E.G. Søgaard, Chemosphere 86, 1092 (2012)
J. De Laat, G. Truong Le, B. Legube, Chemosphere 55, 715 (2004)
J. De Laat, T.G. Le, Environ. Sci. Technol. 39, 1811 (2005)
P. Neta, R.E. Huie, J. Phys. Chem. 90, 4644 (1986)
X. Gu, S. Lu, L. Li, Z. Qiu, Q. Sui, K. Lin, Q. Luo, Ind. Eng. Chem. Res. 50, 11029 (2011)
C. Liang, H.W. Su, Ind. Eng. Chem. Res. 48, 5558 (2009)
A.L. Teel, R.J. Watts, J. Hazard. Mater. 94, 179 (2002)
B.A. Smith, A.L. Teel, R.J. Watts, Environ. Sci. Technol. 38, 5465 (2004)
J. Bandara, J. Kiwi, New J. Chem. 23, 717 (1999)
J.M. Monteagudo, A. Durán, I. San Martin, A. Carnicer, Appl. Catal. B 106, 242 (2011)
Acknowledgments
This study was financially supported by grants from the National Natural Science Foundation of China (nos. 41373094, 51208199, and 21577033), Natural Science Foundation of Shanghai (16ZR1407200), China Postdoctoral Science Foundation (2015M570341), and the Fundamental Research Funds for the Central Universities (222201514339 and 22A201514057).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gu, X., Wang, Y., Miao, Z. et al. Degradation of trichloroethylene in aqueous solution by persulfate activated with Fe(III)–EDDS complex. Res Chem Intermed 43, 1–13 (2017). https://doi.org/10.1007/s11164-016-2601-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2601-0