Abstract
This study examined the removal of phenol from aqueous solutions through electro–Fenton and electro–persulfate processes using iron electrodes. The effect of operational parameters such as initial pH, current density, initial concentration of phenol, hydrogen peroxide dose, and persulfate (PS) concentration on the removal of phenol were investigated. The results showed that the efficiency of phenol removal was directly related to the initial concentrations of hydrogen peroxide and PS, and was inversely correlated with a highly alkaline pH and elevated concentration of phenol. The efficiency of phenol removal was significantly increased by increasing PS and H2O2 concentrations from 0.1 to 0.4 mM, but there was little influence on removal efficiency with greater quantities of PS and H2O2. Ultimately, phenol was almost completely removed after 45 min in both processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water scarcity is one of the greatest concerns of the twenty-first century. Freshwater shortages now affect more than 25 % of the world's population, and consequently, 2.2 million people die every year, as reported by the World Health Organization. However, a large amount of water is contaminated by domestic and industrial activities in developed countries; therefore, evaluation of wastewater quality is essential to avoid further contamination of the environment. The need for reuse of water resources is important in efforts to reduce freshwater consumption [1]. Overpopulation and the consequent increase in industrial activity results in the generation of high concentrations of pollutants, leading to contamination of aquatic environments. For example, phenol and its derivatives can be found in waste disposal of various industries, including the manufacture of resins, plastics, and paper, and coal conversion. Although the toxicity and environmental impact of phenolic compounds is dependent on the number, type and position of substitute groups on aromatic compounds, these chemicals have been found to be toxic for various organisms, including humans, animals, and plants. Hence, the removal of phenolic compounds is regarded as one of the greatest global concerns [2, 3]. There are several methods of treating wastewater containing phenol, the most important of which are advanced oxidation, chemical oxidation, adsorption, biological filtration, or a combination of these procedures [4]. High cost, long retention time, and generation of toxic by-products are drawbacks for the widespread use of some of these elimination strategies [5, 6]. Advanced oxidation processes have attracted much attention today due to their ease of use, low cost, and high efficiency [7–9]. Among the advanced oxidation processes, electro–Fenton and electro-persulfate processes are notable. The Fenton process involves the concurrent use of Fe2+ ions and hydrogen peroxide to decompose and eliminate pollutants. With this method, the presence of Fe2+ ions, hydrogen peroxide and radical hydroxyls make it possible for oxidation to take place. If the Fe2+ ions are produced by an electrolytic technique using iron electrodes, it is called an electro–Fenton (EF) process. The EF process has attracted much attention due to its characteristic low cost, facile operation, and high efficiency [10]. The Fenton process has been proven successful in the degradation of various types of organic compounds without producing any toxic substances in aquatic environments, and the whole process is very efficient and cost-effective compared to conventional chemical methods [11]. In recent years, the use of sulfate radicals has been an effective means of removing organic contaminants. Like hydroxyl radicals (E = 2.8 V), sulfate radicals (E = 2.6 V) have high oxidation potential. Additionally, sulfate radicals have greater oxidation potential in compounds with high acidic pH compared to hydroxyl radicals, although the latter has a longer half-life. Sources of sulfate radicals possess high stability in room temperature and aquatic environments. Persulfate ions (E = 2.01 V) can be activated by heat, UV, or transition metal elements [12, 13]. The advantages of Fe2+ compared to other transition metals are that it is inexpensive and nontoxic, and can be widely applied in oxidation processes. The Fe2+/S2O8 2− reagent is similar to Fenton's reagent (Fe2+/H2O2). PS can be activated by Fe2+ and produces sulfate radicals, as expressed by Eq. (1).
H2O2 can also be activated by Fe2+ to produce hydroxyl radicals via Eq. (2).
Similar to conventional Fenton processes, an inverse behavior is observed here. However, Fe2+ cannot be reproduced after transformation to Fe3+ in the Fe2+/PS process. Thus a high dose of iron ions is required to activate PS, and as a result, a large amount of sludge is formed. Excess Fe2+, on the other hand, reacts with sulfate radicals via Eq. (3), resulting in reduced efficiency of the process.
If Fe2+ is produced by an electrochemical method, its concentration will be low, and quenching of radicals will not occur. In order to investigate the significance of the electro-persulfate (EPS) process in phenol removal, and due to the lack of comprehensive data for comparison between concurrent and sole application of EF–EPS, we aimed to compare the EF and EPS processes for the purpose of phenol removal. The effect of different parameters was also examined in both processes.
Materials and methods
Materials
All chemicals, including hydrogen peroxide (H2O2), phenol with 99.5 % purity (C6H5OH), sodium persulfate (Na2S2O8), and sulfuric acid (H2SO4), were purchased from Merck Millipore. Phenol absorbance was measured using an ultraviolet-visible (UV-Vis) spectrophotometer and pH was measured using a pH meter manufactured by HACH Company.
Procedure
A known amount of phenol solution (100 mg l−1) was poured into the reactor. Distilled water was used to prepare the solutions. Solution pH was adjusted using sulfuric acid (1N) and NaOH (1N). After voltage, hydrogen peroxide dose, and PS concentration were set, iron electrodes were placed in the reactor and the experiment was run. Sampling was carried out at different times, and samples were then centrifuged (6000 rpm for 10 min), and absorbed amounts of the remaining phenol in the samples were measured through direct photometry using UV-Vis spectrophotometry at a wavelength of 500 nm [14]. The efficiency of phenol removal was calculated as follows [15]:
where C 0 and C are the initial and residual concentrations of phenol, respectively.
Bench scale reactor
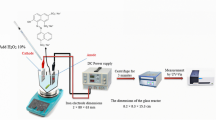
This experimental study was conducted using a batch processing reactor at the Hamadan University of Medical Sciences water and wastewater laboratory. The bench scale reactor, whose schematic representation is given in Fig. 1, is made of a 1000-ml glass beaker. Four iron electrodes, each 200 mm long, 20 mm wide, and 2 mm thick, float in the reactor at a distance of 1 cm. The electrodes are connected to a DC power supply, with alternative cathode and anode placement. A magnetic stirrer is used in order to achieve uniform mixing of the solution.
Results and discussion
Effect of pH
The Fenton process is heavily dependent on the pH of a solution [16]. In this study, we investigated the effect on EF and EPS processes of changes in pH over a range of 3 to 10, and results are shown in Fig. 2. It is clear that pH changes had a fairly significant effect on the removal of phenol, with the highest removal efficiency in pH = 3 of 95.18 and 93.99 % for the EPS and EF processes, respectively. Thus pH has a direct effect on the stability of H2O2and the amount of OH∙ produced, ultimately determining the type and state of iron in the solution [17–19].
Sulfate radicals react with H2O or OH− to generate hydroxyl radicals, based on Eqs. (5 and 6); each free H+ or consumed OH− can decrease pH, which in turn boosts removal efficiency [12]. Studies have shown that the oxidation potential of OH∙ increases as pH decreases [12, 20]. As mentioned above, the maximum phenol removal occurred at pH = 3: in acidic pH, the surface of iron over time is refreshed such that production of free electrons is faster than at other pH values [21]. The lowest phenol removal efficiency was observed at pH = 10. In alkaline pH, Fe2+ is oxidized and precipitates as Fe(OH)2 and is then removed from the catalytic cycle.
Effect of current density
In all electrochemical processes, current density (CD) is the primary parameter controlling the reaction rate [22]. According to Faraday’s law, intensifying the applied current increases the production of metal ions on a sacrificial anode surface [22]. In most electrochemical studies, a constant CD is used or potential difference is fixed [23]. To control the effect of iron concentration on the process, constant CD enters the electrode such that iron can be produced at a constant rate by the sacrificial anode and the system can be monitored under controlled conditions [24]. Increasing CD results a rapid ferrous generation and ferric reproduction, and ameliorates the decomposition of PS and the production of sulfate radicals, and thus the efficiency of phenol removal rises [12, 25]. When the CD increased from 0.12 to 0.17 mA cm−2 in the EPS and EF processes, phenol degradation increased from 50.63 and 47.54 % to 93.99 and 93.18 %, respectively. This in turn boosted the efficiency of phenol removal, such that maximum phenol removal efficiency was observed with a CD of 0.17 mA.cm−2. In other words, with higher CD, generation of sulfate radicals was enhanced by the combination of electron transfer reactions and quenching of radicals (Eq. 7) [23, 26]. As a result, as shown in Fig. 3, phenol removal efficiency declined at a CD of 0.24 mA cm−2.
Effect of PS concentration
Next, in order to optimize PS concentration, the effects of different concentrations of PS (0.1–0.5 mM) were surveyed at (pH = 3, CD = 0.17 mA cm−2 and [phenol]0 = 100 mg l−1). Results indicated that phenol removal efficiency increased linearly with increasing PS concentration. As shown in Fig. 4, phenol removal efficiency in the EPS process was increased from 53.31 to 93.99 % by increasing the PS concentration from 0.1 to 0.4 mM. As noted previously, the most important factor in sulfate radical production is the concentration of oxidizing agents, such that increasing the oxidant concentration will accelerate the production of sulfate radicals, and thus the reaction rate of phenol decomposition is increased as well [27, 28].
An increase in oxidant concentration to more than 0.5 mM led to phenol removal efficiency of 63.74 % after 45 min, as seen in Fig. 4. In this scenario, increasing the concentration of the oxidizing agent up to a certain level will result in an increase in the reaction rate. However, when the PS concentration was increased as much as 0.5 mM, an opposite trend in the removal of phenol was observed, according to Eq. (7). An excessive increase in oxidizing agent concentration above an optimal level leads to the scavenging of SO ·−4 by S2O8 2−, consequently reducing the process efficiency, based on Eqs. (7 and 8) [29].
Effect of hydrogen peroxide dose
In order to determine the optimal dose of hydrogen peroxide, different H2O2 concentrations ranging from 0.1 to 0.5 mM were added to each prepared sample, the pH was set to 3, and the experiment was performed. The amount of hydrogen peroxide is the main factor affecting the cost of the operation and the process efficiency [6]. As shown in Fig. 5, in the EF process, increasing the hydrogen peroxide concentration from 0.1 to 0.4 mM resulted in an increase in phenol removal efficiency from 49.71 to 93.18 %. It should be noted that H2O2 is the only source of OH∙ in the EF process. Increasing the amount of H2O2 up to a certain level, with the resulting increase in OH∙ concentration, will increase the process efficiency [30]. However, when the H2O2 concentration was increased as much as 0.5 mM, an opposite trend in the removal of phenol was observed. Therefore, when hydrogen peroxide concentration is elevated, it is converted into oxygen, water, and hydroxyl radicals, followed by a decline in process efficiency.
Effect of phenol concentration
The effect of the initial phenol concentration on EPS and EF processes was examined in a range of 50–200 mg l−1, with the results illustrated in Fig. 6. As shown in the figure, an increase in phenol concentration from 50 to 200 mg l−1 resulted in a reduction in removal efficiency from 93.18 to 15 % and from 93.99 to 53 % for the EF and EPS processes, respectively, after 45 min. It is clear that an increase in the initial concentration of pollutants necessitates greater oxidation potential, and thus with a constant amount of oxidant, the efficiency of the process is decreased. The intermediate compounds generated during the reaction would be characterized by high initial concentrations, which consume hydroxyl and sulfate radicals. This might lead to competition between pollutant molecules and intermediate compounds in reacting with hydroxyl and sulfate radicals. Therefore, the decomposition rate would drop with high initial concentrations [31].
Synergistic effect of process components
To determine the impact of EPS and EF process components, four samples were prepared and tested separately, which are presented in Table 1. In the first sample, the EPS process occurred under optimal conditions, resulting in a removal efficiency of 93.99 %. In the second sample, no CD was applied to the EPS process, and the efficiency rate was only 6.2 %, which is related to direct oxidation by PS ions. In the third sample, the EF process was carried out under optimal conditions, and the removal efficiency was 93.18 %. In the fourth sample, the EPS process was carried out without PS ions or electrolyte, but CD was applied, and no removal efficiency was seen. As shown in Table 1, electrolyte, electric CD, and PS must all be present to obtain maximum efficiency.
Conclusions
The present study compared EF and EPS processes using iron electrodes, with results indicating almost identical efficiency between the two processes in the removal of phenol. Under optimal conditions, removal efficiency for EF and EPS were 93.18 and 93.99 %, respectively. The results showed that phenol removal efficiency had a direct relationship with an increase in contact time and CD, and an inverse relationship with initial phenol concentration. Phenol removal efficiency was reduced when PS and hydrogen peroxide concentration were significantly higher than the optimal level. In order to reach maximum efficiency, electrolyte, electrical CD, and PS must all be present.
References
B. Bayarri, M.I. Maldonado, J. Giménez, S. Esplugas, O. González, J. Sol. Energy Eng. 129, 60 (2007)
P.S. González, E. Agostini, S.R. Milrad, Chemosphere 70, 982 (2008)
L. Jiang, M. Xuhui, Int. J. Electrochem. Sci. 7, 4078 (2012)
D.H. Bremner, A.E. Burgess, D. Houllemare, K.-C. Namkung, Appl. Catal. B Environ. 63, 15 (2006)
K. Verschueren, Handbook of environmental data on organic chemicals (Wiley, Baco Raton, 2001)
J. Virkutyte, V. Jegatheesan, Bioresour. Technol. 100, 2189 (2009)
F.J. Beltrán, F.J. Rivas, R. Montero-de-Espinosa, Water Res. 39, 3553 (2005)
B. Kasprzyk-Hordern, M. Ziółek, J. Nawrocki, Appl. Catal. B Environ. 46, 639 (2003)
T. Sreethawong, S. Chavadej, J. Hazard. Mater. 155, 486 (2008)
W.P. Ting, M.C. Lu, Y.H. Huang, J. Hazard. Mater. 156, 421 (2008)
H. Liu, X.Z. Li, Y.J. Leng, C. Wang, Water Res. 41, 1161 (2007)
J. Wu, H. Zhang, J. Qiu, J. Hazard. Mater. 215, 138 (2012)
Y.T. Lin, C. Liang, J.H. Chen, Chemosphere 82, 116 (2011)
APHA, Standard methods for the examination of water and wastewaters. 21std (American Public Health Association (APHA), Washington, 2005)
Y.S. Ng, N.S. Jayakumar, M.A. Hashim, Desalin. Water Treat. 278, 250 (2011)
A. Babuponnusami, K. Muthukuma, J. Chem. Eng. 2, 557 (2013)
M.R. Samarghandi, A. Shabanloo, K. Shamsi, J. Mehralipour, Y. Poureshgh, IJEHSE 4, 293 (2014)
M. Malakotian, M. Asadi, A. Mahvi, Iran. J. Health Environ. 5, 1 (2013)
E. Basturk, M. Karatas, Ultrason. Sonochem. 21, 1881 (2014)
S. Vinodha, S. Babithesh, P. Jegathambal, IBM J. Res. Dev. 5, 45 (2012)
A.R. Rahmani, M. Zarrabi, M.R. Samarghandi, A. Afkhami, H.R. Ghaffari, Iran. J. Chem. Eng. 7, 87 (2010)
J.B. Parsa, H.R. Vahidian, A. Soleymani, M. Abbasi, Desalination 278, 295 (2011)
B. Boye, M.M. Dieng, E. Brillas, Environ. Sci. 36, 3030 (2002)
A. Bagheri, G.H. Moussavi, A. Khavanin, IJEHSE 2, 143 (2012)
B. Kayan, B. Gözmen, M. Demirel, A.M. Gizir, J. Hazard. Mater. 177, 95 (2010)
T.W. Chan, N.J. Graham, W. Chu, J. Hazard. Mater. 181, 508 (2010)
S. Wang, N. Zhou, S. Wu, Q. Zhang, Z. Yang, Ultrason. Sonochem. 23, 128 (2015)
J. Saien, A.R. Soleymani, J.H. Sun, Desalination 279, 298 (2011)
L. Hou, H. Zhang, X. Xue, Sep. Purif. Technol. 84, 147 (2012)
R. Munter, Sci. Chem. 50, 59 (2001)
H. Sheme, K.G. Linden, J. Hazard. Mater. 136, 553 (2006)
Acknowledgments
This research was sponsored by the Research Department of Hamadan University of Medical Sciences. The researchers express their heartfelt gratitude to everyone involved in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahmani, A.R., Rezaeivahidian, H., Almasi, M. et al. A comparative study on the removal of phenol from aqueous solutions by electro–Fenton and electro–persulfate processes using iron electrodes. Res Chem Intermed 42, 1441–1450 (2016). https://doi.org/10.1007/s11164-015-2095-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2095-1