Abstract
A simple and efficient procedure for the synthesis of benzo[4,5]imidazo[1,2-α]pyrimidine and 2-amino-4-substituted-1,4-dihydrobenzo[4,5]imidazolo[1,2-α]pyrimidine-3-carbonitrile derivatives using poly(vinylpyrrolidonium) perchlorate {[PVPH]ClO4} as a newly reported, modified polymeric catalyst is reported. Some of the advantages of this novel synthetic method are: easy preparation of the catalyst, simple and easy work-up procedure, short reaction times, high to excellent yields of the products and reusability of the catalyst.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dihydropyrimidine derivatives are among the most important classes of tricyclic compounds which show an interesting wide range of biological and pharmaceutical properties such as antiviral, antibacterial, anti-inflammatory, anti-hypertensive, anti-tubercular, anti-malarial and cytotoxic activities [1–5]. In addition, they can be used as calcium channel blockers and antihypertensive agents [6]. These significant properties have attracted the attention of many organic chemists to the synthesis of these types of compounds, so that their investigations have led to various synthetic methods using different types of catalysts. Some of these catalysts are: H3BO3 [7], 1,1,3,3-N,N,N′,N′-tetramethylguanidinium trifluoroacetate [8], tetrabutylammonium hydrogen sulfate [9], thiamine hydrochloride [10], Zn(ClO4)2·6H2O [11], [bmim]BF4 [12], H2NSO3H [13], ionic liquid supported-nanoporous silica (SBA-IL) [14], N,N,N′,N′-tetrabromobenzene-1,3-disulfonamide (TBBDA) [15] and p-toluenesulfonic acid [16]. The reaction was also investigated under catalyst-free conditions [17].
Although a number of these methods have their own advantages, some of them suffer from one or more disadvantages, such as use of large amounts of catalysts, long reaction times, unsatisfactory products yields, high temperatures and the use of toxic organic solvents; these disadvantages have limited the use of these methods. Therefore, it is necessary to find more convenient methods for the synthesis of dihydropyrimidines.
Experimental
Chemicals were purchased from Fluka, Merck, and Sigma-Aldrich chemical companies. Cross-linked poly(vinylpyrrolidone) was purchased from BASF Corp. (Germany). All products were characterized by comparison of their physical constants, infrared (IR) spectra and nuclear magnetic resonance (NMR) spectroscopy with authentic samples and those reported in the literature.
Preparation of catalyst [PVPH]ClO4 [18]
HClO4 (5 mmol, ≅0.43 mL, as a 70 % aqueous solution) was added to a suspension of 0.05 g of powdered poly(vinylpyrrolidone) [crosslinked poly(vinylpyrrolidone) with MW > 1,000,000] in 10 mL of dry CH2Cl2 over a period of 10 min in an ice bath. After the addition was completed, the mixture was stirred for 2 h and then filtered. The solid residue was washed with diethyl ether (10 mL) and dried at 80 °C to afford [PVPH]ClO4 as a white powder (Scheme 1) [18].
General procedure for the synthesis of the benzo[4,5]imidazo[1,2-α]pyrimidine (A) or 2-amino-4-substituted-1,4-dihydrobenzo[4,5]imidazolo[1,2-α]pyrimidine-3-carbonitrile (B) derivatives
A mixture of ethyl acetoacetate and/or acetylacetone (1 mmol) or malononitrile (1.1 mmol), 2-aminobenzimidazole (1 mmol), aldehyde (1 mmol) and [PVPH]ClO4 (30 mg, 6.84 mol %) was heated at 100 °C in an oil bath for the appropriate time. After completion of the reaction [monitored by thin layer chromatography (TLC): n-hexane: ethyl acetate (3:1)], the reaction mixture was cooled to room temperature and EtOH (5 mL) was added to it and filtered to separate the catalyst. After evaporation of the solvent from the filtrate, the crude solid product was recrystallized from ethanol to obtain pure A or B products.
The spectral [IR, proton ( 1H) NMR and 13C NMR] data of new compounds are presented below.
(Compound 4, Table 2): Ethyl 4-(4-methylthiophenyl)-2-methyl-1,4-dihydrobenzo[4,5]imidazo[1,2-a]pyrimidine-3-carboxylate
Yellow solid, m.p. 271–273 °C; IR (KBr): ν = 3232, 3103, 3031, 1695, 1611, 1568, 1449, 1251, 1014, 874, 829, 733 cm−1. 1H NMR [deuterated dimethyl sulfoxide (DMSO-d6), 400 MHz] σ: 1.17 (t, J = 7.2 Hz, 3H, CH3), 2.40 (s, 3H, CH3), 2.47 (s, 3H, CH3), 4.0–4.07 (m, 2H, CH2), 6.40 (s, 1H, CH), 6.96 (t, J = 7.2 Hz, 1H, ArH), 7.05 (t, J = 7.6 Hz,1H, ArH), 7.14 (d, J = 8.4 Hz, 2H, ArH), 7.25–7.36 (m, 3H, ArH), 7.8 (d, J = 8 Hz, 1H, ArH), 10.8 (brs, 1H, NH) ppm. 13C NMR (DMSO-d6, 100 MHz): 14.5, 14.8, 19.1, 55.9, 59.8, 98.3, 110.1, 117.26, 120.6, 122.2, 126.0, 128.1, 131.3, 138.2, 139.0, 142.7, 146.0, 146, 9, 165.6 ppm.
(Compound 10, Table 3): 2-Amino-4-(pyridin-3-yl)-1,4-dihydrobenzo[4,5]imidazolo[1,2-a]pyrimidine-3-carbonitrile
m.p. 242–244 °C; IR (KBr): v = 3452, 3315, 3056, 2180, 1663, 1590, 1465, 1399, 712 cm−1. 1HNMR (DMSO-d6, 400 MHz) σ: 5.37 (s, 1H), 6.97 (s, 2H, NH2), 7.03 (t, J = 7.6 Hz, 1H), 7.14 (t, J = 7.6 Hz, 1H), 7.26 (d, J = 7.6 Hz,1H), 7.40–8.54 (m, 5H), 8.64 (s, 1H, NH) ppm. 13CNMR (DMSO-d6, 100 MHz): 51.6, 61.4, 113, 116.6, 120.5, 123.9, 124.4, 129.7, 134.4, 136, 138.5, 143, 148, 149.5, 150, 152.
Very recently we reported the preparation of poly(vinylpyrrolidonium) perchlorate {[PVPH]ClO4} [18] and its applicability in the promotion of the synthesis of polyhydroquinoline derivatives via Hantzsch condensation. Herein and in continuation of this study, we wish to report the applicability of this reagent in the accelerated preparation of tricyclic dihydropyrimidine derivatives.
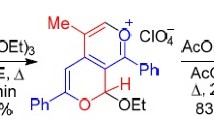
At the first step, optimization of the reaction conditions was done by studying the reaction of 2-aminobenzimidazole with 4-chlorobenzaldehyde and ethylacetoacetate in the presence of various amounts of [PVPH]ClO4 in the absence of solvent and also in different solvents such as dichloromethane, ethanol and water under thermal conditions (Table 1). We found that the reaction was rapid and gave excellent yields of the products in the presence of 30 mg of the catalyst at 100 °C in the absence of solvent (Scheme 2).
Results and discussion
Catalytic activity
After optimization of the reaction conditions and in order to show the general applicability of the method, different types of aldehydes were subjected to the same reaction under the determined conditions. The obtained results showed that in all cases the corresponding products were obtained in good to excellent yields in appropriate times (Table 2, entries 1–11). We have also found that the same results were obtained when acetylacetone was used in place of ethylacetoacetate (Table 2, entries 12–14). The results showed that in all cases, regardless of the substituent, the reaction gave the products in good to high yields within relatively short reaction times.
After successful synthesis of a series of benzo[4,5]imidazo[1,2-α] pyrimidine derivatives, we decided to study the preparation of 2-amino-4-substituted-1,4-dihydrobenzo[4,5]imidazolo[1,2-α]pyrimidine-3-carbonitriles by the reaction of malononitrile, 2-aminobenzimidazole and aldehydes in the presence of [PVPH]ClO4 under the above-mentioned reaction conditions (Scheme 2).
The results showed that various aldehydes were reacted with the other starting materials using this method to produce the desired products in excellent yields within very short reaction times (Table 3). It should be noted that only two methods for the preparation of these compounds are reported in the literature [16, 17].
In a plausible mechanism, at first, the aldehyde is activated by the proton of the catalyst. Then, the carbonyl group is attacked by ethylacetoacetate or malononitrile to form the Knoevenagel products (I). In continuation, 2-aminobenzimidazole is reacted with these intermediates via Michael addition reaction to produce the related intermediate (II). Finally, this intermediate is converted to the requested products via intermolecular cyclization and dehydration reactions (Scheme 3).
In continuation, we decided to examine the catalytic activity of the recycled catalyst for the synthesis of the title derivatives. After separation of the product, the catalyst was washed with EtOH, dried and reused for the same reaction. This process was carried out over three runs and all reactions led to the desired products with high efficiency (Figs. 1, 2). It should be noted that the Fourier transform infrared (FT-IR) and pH analysis of the recovered catalyst showed nearly the same loading of H+ as the freshly prepared catalyst. These results suggest that changing of the catalyst and/or leaching of the acid species does not occur during the course of the reaction.
Table 4 compares the efficiency of [PVPH]ClO4 with the other catalysts in the synthesis of tricyclic dihydropyrimidine derivatives of benzaldehyde. The presented results show that the same reactions are carried out in the presence of the larger amounts of the catalysts (Table 4, entries 1, 4, 8, 10) or the reaction times are longer compared with the present method (Table 4, entries 2, 3, 4, 8).
Conclusion
In this paper, we have developed an efficient and green procedure for the synthesis of two types of tricyclic compounds including benzo[4,5]imidazo[1,2-α]pyrimidines and 2-amino-4-substituted-1,4-dihydrobenzo[4,5]imidazolo[1,2-α]pyrimidine-3-carbonitriles using [PVPH]ClO4 as a new polymeric catalyst. These procedures have several advantages, including heterogeneous reaction conditions, easy work-up, simple procedure for the preparation of the catalyst, appropriate times and high to excellent yields of the products. Also, the catalyst could be successfully recovered and reused for at least three runs without significant loss in its activity.
References
B.B. Snider, Z. Shi, J. Org. Chem. 58, 3828 (1993)
L.E. Overman, M.H. Rabinowitz, P.A. Renhowe, J. Am. Chem. Soc. 117, 2657 (1995)
A.R. Trivedi, V.R. Bhuva, B.H. Dholariya, D.K. Dodiya, V.B. Kataria, V.H. Shah, Bioorg. Med. Chem. Lett. 20, 6100 (2010)
B.R.P. Kumar, G. Sankar, R.B.N. Baig, S. Chandrashekaran, Eur. J. Med. Chem. 44, 4192 (2009)
O. Alam, S.A. Khan, N. Siddiqui, W. Ahsan, S.P. Verma, S.J. Gilani, Eur. J. Med. Chem. 45, 5113 (2010)
K.S. Atwal, G.C. Rovnyak, S.D. Kimball, D.M. Floyd, S. Moreland, B.N. Swanson, J.Z. Gougoutas, J. Schwartz, K.M. Smillie, M.F. Malley, J. Med. Chem. 33, 2629 (1990)
H.M. Meshram, A.S. Kumar, G.S. Kumar, A. Swetha, B.C. Reddy, P. Rameshanjeeva, Der Pharma Chemica 4, 956 (2012)
A. Shaabani, A. Rahmati, S. Naderi, Bioorg. Med. Chem. Lett. 15, 5553 (2005)
A. Shaabani, E. Farhangi, A. Rahmati, Comb. Chem. High Throughput Screen. 9, 771 (2006)
J. Liu, M. Lei, L. Hu, Green Chem. 14, 840 (2012)
N. Kaur, K. Kaur, T. Raj, G. Kaur, A. Singh, T. Aree, S.-J. Park, T.-J. Kim, N. Singh, D.O. Jang, Tetrahedron 71, 332 (2015)
C.S. Yao, S. Lei, C.H. Wang, T. Li, C. Yu, X. Wang, S. Tu, J. Heterocycl. Chem. 47, 26 (2010)
C.S. Yao, S. Lei, C.H. Wang, C.X. Yu, Q.Q. Shao, S.J. Tu, Chin. J. Chem. 26, 2107 (2008)
L. Seyedakbari, G.M. Ziarani, A. Badiei, M. Yadavi, P. Hajiabbasi, A. Abolhasani Soorkic, Rev. Chim. (Bucharest) 64, 832 (2013)
R. Ghorbani-Vaghei, Z. Toghraei-Semiromi, R. Karimi-Nami, Z. Salimi, Helv. Chim. Acta 97, 979 (2014)
M.N. Reddy, J. Oh, Y.T. Jeong, C. R. Chimie 17, 484 (2014)
A. Shaabani, A. Rahmati, A.H. Rezayan, M. Darvishi, Z. Badri, A. Sarvari, QSAR Comb. Sci. 26, 973 (2007)
M. Abedini, F. Shirini, M. Mousapour, Res. Chem. Intermed. (2015). doi:10.1007/s11164-015-2150-y
H.R. Shaterian, N. Fahimi, K. Azizi, Res. Chem. Intermed. 40, 1879 (2014)
L. Wu, F. Yan, Ch. Yang, Bull. Chem. Soc. Ethiop. 24, 417 (2010)
Acknowledgments
We are thankful to the University of Guilan Research Council for the partial support of this work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Abedini, M., Shirini, F., Mousapour, M. et al. Poly(vinylpyrrolidonium) perchlorate catalyzed one-pot synthesis of tricyclic dihydropyrimidine derivatives. Res Chem Intermed 42, 6221–6229 (2016). https://doi.org/10.1007/s11164-016-2456-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2456-4