Abstract
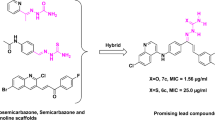
A series of 2-(substituted-phenyl)-3-(((3-(pyridin-4-yl)-1-(p-tolyl)-1H-pyrazol-4-yl)methylene)amino)-quinazolin-4(3H)-ones have been synthesized. The structures of the synthesized compounds were assigned on the basis of IR, 1H NMR, 13C NMR, and mass spectral data, while their abilities to inhibit growth of Mycobacterium tuberculosis in vitro have been determined. The results show that compounds 5a, 5c, 5d, 5g, and 5k exhibited excellent antitubercular activity with percentage inhibition of 96, 90, 94, 93, and 92, respectively at a minimum inhibitory concentration (MIC) of <6.25 μg/mL, whereas compounds 5b, 5e, 5f, 5h, 5i, 5j, and 5l exhibited moderate- to- good antitubercular activity with percentage inhibition of 68, 70, 67, 64, 59, 73, and 67, respectively, at a MIC of >6.25 μg/mL. From the secondary screening, the actual MIC of compounds 5a, 5c, 5d, 5g, and 5k are <3.125.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis is one of the serious health problems with a wide variety of manifestations caused by Mycobacterium tuberculosis, and as per the recent report, it has been estimated that approximately onethird of the world’s population is infected with this microorganism. The treatment of mycobacterial infections, especially the tuberculosis, has become an important problem due to the emergence of monodrug and multidrug-resistant strains of M. tuberculosis (Rattan et al., 1998; Singh et al., 2006). Therefore, there is a need for developing new drugs of new structural classes and with a novel mechanism of action other than isoniazid (INH), rifampicin (RIF), and pyrazinamide (PZA). In this regard, since the last decade, the search for new antitubercular substances has been ranked among the priority areas of chemotherapeutic research.
Quinazoline derivatives represent one of the most active classes of compounds possessing a wide spectrum of biological activity (Apfel et al., 2001). They are widely used in pharmaceuticals and agrochemicals (Tobe et al., 2003); for example, fluquinconazole fungicide for the control of agriculture diseases (Guang-Fang et al., 2007). Several reports have been published on the biological activities of quinazoline derivatives, including their bactericidal, herbal, and antitumor activity (Raffa et al., 1999; Chenard et al., 2001). Thus, their synthesis has been of great interest in the elaboration of biologically active heterocyclic compounds. Recently, it was reported that some iodoquinazolines exhibited moderate antibacterial activity (Alafeefy, 2008). Prompted by these findings, this article reports the design and synthesis of an extension series of 3-substituted 2-phenylquinazolin-4(3H)-one derivatives and tested their antibacterial activities.
Pyrazole derivatives are known to possess wide spectrum of pharmacological properties such as antibacterial (Aggarwal et al., 2006), antifungal (Deohate et al., 2004), antidiabetic (Kees et al., 1996), herbicidal (Meazza et al., 2004), antitumor (Park et al., 2005) antianxiety (Wustrow et al., 1998), and as active pharmacophore in celecoxib (as COX-2 inhibitor) (Habeeb et al., 2001) and sildenafil citrate (Martel et al., 1997) (as cGMP-specific phosphodiesterase-type 5 inhibitor), etc. Pyrazoles play an essential role in biological active compounds and therefore represent an interesting template for medicinal chemistry.
Celecoxib contains pyrazole nucleus and is a sulfa nonsteroidal anti-inflammatory drug (NSAID) and selective COX-2 inhibitor used in the treatment of osteoarthritis, rheumatoid arthritis, acute pain, painful menstruation, and menstrual symptoms, and to reduce numbers of colon and rectum polyps in patients with familial adenomatous polyposis. While Methaqualone contains quinazolinone nucleus and is a sedative–hypnotic drug that produces effect similar to barbiturates, a general central nervous system depressant. The sedative–hypnotic activity was first noted by Indian researchers in the 1950s, and in 1962, methaqualone itself was patented in the U.S. by Wallace and Tiernan.
Looking to the medicinal importance of 4(3H)-quinazolinone and pyrazole, we report in this article the synthesis of new class of heterocyclic molecules in which all of these moieties are present and try to develop potential bioactive molecules. The structures of the compounds synthesized were assigned on the basis of elemental analysis, IR, 1H NMR, 13C NMR , and Mass spectral data. These compounds were evaluated for their antitubercular activity.
Results and discussion
Synthesis
The synthetic pathway to obtain the intermediate and target compounds in this study is depicted in Scheme 1. p-Methyl phenyl hydrazine (starting compound-1) reacted with 1-(Pyridin-4-yl)ethanone by using methanol as solvent at RT for 10 h to produce intermediate compound-2, which on further treatment with DMF and POCl3 with constant stirring at 100 °C produced intermediate-(3) compound. Compound-(3) is reacted with different (4a–l) by ZnCl2 as a catalyst and 1,4-dioxan as a solvent to produce final compounds (5a–l) having good- to- excellent yields. The final compound 5a was characterized using IR and 1H-NMR, 13C-NMR, and Mass spectra. IR spectra showed that absorption bands at 3,065, 3,052 cm−1 ranges showed multiple weak absorption peaks corresponding to Qu–H- and Ar–H - stretching vibrations. The absorption peak at 2,916 cm−1 is due to the stretching vibration of active methylene group and the absorption peak at 2,860 cm−1 is due to the stretching vibration of –CH3 group. The strong absorption at 1,695 cm−1 is due to the >C=O stretching vibration. The moderate intensity absorption at 1,601 and 1,590 cm−1 corresponds to a >C=N– stretching vibration. The 1,533, 1,449 cm−1 absorptions are due to the skeleton vibration of the aryl and heterocyclic rings. The vibrations at 1,460 and 1,452 cm−1 are due to the bending vibration of methyl and active methylene groups, respectively. The absorption peak at 845 cm−1 is due to the chlorine atom, the proton of which is present in pyrazole nucleus appeared as a singlet at δ = 8.43 ppm. The proton of the methyl group appeared as a singlet at δ = 2.43 ppm . The proton attached at the nearest position of nitrogen atom in pyridine ring appeared as a doublet at δ = 8.75 ppm. The proton of the =CH group appeared as a single at δ = 8.20 ppm. The chemical shifts of the final compound carbons vary from δ = 166.7 to 21.3 ppm. The carbon nuclei under the influence of a strong electronegative environment appeared downfield. The carbons of the pyrazole ring appeared between δ = 114.0 and 133.3 ppm. The carbon attached with electronegative chloro atom appeared at δ = 132.1 ppm. The carbon of the methylene group appear at δ = 143.3 ppm. The carbon of the carbonyl group which is present in quinazolinone ring appeared as δ = 166.7 ppm. Moreover, the mass spectrum of (5a) showed a molecular ion peak at m/z = 516.98 (M+), corresponding to a molecular formula C30H21ClN6O.
Structure activity relationship
The structure–activity relationships (SARs) of compounds (5a–l) were determined on the basis of results presented in Table 1. SAR studies revealed that the presence of pyrazole and quinazolinone was essential for a broad range of antitubercular activity. From activity data, it is clear that final compounds containing ortho-directing substitutions of chloro, methyl, and methoxy in basic skeleton led to increase in antitubercular activity. Compound 5a containing 2-Cl group showed excellent inhibition of 96 at MIC = <6.25 μg/mL; compound 5d containing 2-CH3 group showed excellent inhibition of 94 at MIC = <6.25 μg/mL and compound 5g containing 2-OCH3 group showed excellent inhibition of 93 at MIC = <6.25 μg/mL as compared with the standard isoniazide having excellent inhibition of 99 at MIC = <6.25 μg/mL. The compound 5c containing 4-Cl group showed excellent inhibition of 93 at MIC = <6.25 and compound 5k containing 3-NO2 group showed excellent inhibition of 92 at MIC = <6.25 μg/mL. In general, compounds containing electron- releasing groups, such as –CH3 and –OCH3 groups at ortho position and electro negative group –Cl at ortho and para positions led to increase in activity, while electro negative group at meta position led to increased activity. owing to bulky group, ring strain increased and made compound less stable and active. In general, compounds come out as compounds exhibiting more noteworthy antitubercular activity. Compounds demonstrating at least 90 % inhibition in the primary screen (MIC < 6.25 ug/mL) are re-tested against M. tuberculosis H37Rv at lower concentrations to determine the actual minimum inhibitory concentration (MIC).The actual MIC of compounds 5a, 5c, 5d, 5g, and 5k are <3.125.
Experimental part
Materials and methods
All reactions except those in aqueous media were carried out by standard techniques for the exclusion of moisture. Melting points were determined on an electrothermal melting point apparatus and were reported uncorrected. TLC on silica gel plates (Merck, 60, F254) was used for purity checking and reaction monitoring. Column chromatography on silica gel (Merck, 70–230 and 230–400 mesh ASTH for flash chromatography) was applied when necessary to isolate and purify the reaction products. Elemental analysis (% C, H, N) was carried out by a Perkin-Elmer 2400 CHN analyzer. IR spectra of all compounds were recorded on a Perkin-Elmer FT-IR spectrophotometer in KBr. 1H NMR spectra were recorded on Varian Gemini 300 MHz and 13C NMR spectra on Varian Mercury-400, 100 MHz in DMSO-d 6 as a solvent and tetramethylsilane (TMS) as an internal standard. Mass spectra were scanned on a Shimadzu LCMS 2010 spectrometer. Anhydrous reactions were carried out in an oven-dried glassware in nitrogen atmosphere.
Methods of preparation and physical data of synthesized compounds (2 to 5a–l)
4-(1-(2-(p-Tolyl)hydrazono)ethyl)pyridine (2)
To a solution of compound-(I) (0.01 mol) in methanol (50 mL) was added the same amount of 1-(pyridin-4-yl)ethanone (0.01 mol) with stirring and refluxed for 10 h; after the completion of reaction, it was cooled and distilled the excess solvent was distilled and it was poured into iced-cold water. The resulting light brown color product was obtained. The completion of the reaction was checked by TLC [n-hexane/ethyl acetate (V/V = 1:3)]. The crude product was recrystallized from 95 % ethanol to give the intermediate compound-(II).
Yield: 89 %; m.p.: 170–172 °C; IR (KBr) νmax/cm−1: 3412 (–NH stretching), 3060, 3053 (C–H, aromatic), 2860, 2854 (–CH3 stretching), 1605, 1592 (C=N), 1537, 1444 (C=C), 1458, 1452 (–CH3 bending); 1H NMR (DMSO) δ (ppm): 2.34 (s, 3H, –CH3 group), 2.95 (s, 3H, C–CH 3 group), 6.51–6.98 (m, 4H, Ar–H), 7.1 (s, 1H, –NH), 7.74 (d, 2H, J = 7.5 Hz, –N = CH–CH), 8.66 (d, 2H, N=CH–); 13C NMR (DMSO) δ (ppm): 17.0, 21.3, 116.2, 124.1, 129.8, 131.2, 138.4, 149.4, 168.8. MS : m/z 225.29 (M+). Anal. calcd for C14H15N3: C 74.64, H 6.71, N 18.64. Found C 74.72, H 6.80, N 18.72.
3-(Pyridin-4-yl)-1-(p-tolyl)-1H-pyrazole-4-carbaldehyde (3)
Dry N,N′-dimethylformamide (10.16 mol, 0.742 g) was cooled to 0 °C in an inert atmosphere round- bottom flask, and POCl3 (50.84 mol, 7.77 g) was added slowly under stirring over 15–20 min, and stirring was continued for another 15 min at the same temperature. To this mixture, intermediate compound (2) (4-(1-(2-(p-tolyl)hydrazono)ethyl)pyridine) (3.38 mol, 1 g) was added as solid directly. The resulting reaction mixture was refluxed for 2 h at 100 °C in an oil-bath. After completion of the reaction by TLC, the reaction mixture was poured onto the crushed ice, neutralized with saturated NaHCO3; the product obtained was filtered, washed with water and dried. Purification of the crude product by column chromatography on silica gel eluting with ethyl acetate/chloroform (3:7) gave rise to pyrazole aldehyde (3) as a white solid.
Yield: 80 %; m.p.: 186–188 °C; IR (KBr) νmax/cm−1: 3078, 3062 (C–H, aromatic), 2941 (–CH stretching, –CHO group), 1680 (C=O stretching), 1609, 1588 (C=N stretching), 1562, 1440 (C=C, benzene ring), 1459 (–CH bendin–CH3 group) 696 (mono substituted benzene ring). 1H NMR (DMSO) δ (ppm): 2.34 (s, 3H, –CH 3 group), 7.42–7.50 (m, 4H, Ar–H), 7.99 (d, 2H, J = 7.7 Hz, –N=CH–CH), 8.43 (s, 1H, pyra-H), 8.75 (d, 2H, N=CH–). 9.73 (s, 1H, –CH=O), 13C NMR (DMSO) δ (ppm): 21.5, 114.0, 119.3, 121.5, 129.3, 129.7, 133.2, 135.6, 136.3, 140.2, 149.8, 185.3. MS : m/z 263.29 (M+). Anal. calcd for C16H13N3O: C 72.99, H 4.98, N 15.96. Found C 73.08, H 4.92, N 16.03.
3-Amino-2-(substituted-phenyl)quinazolin-4(3H)-one (4a–l)
The compounds (4a–l) were synthesized by method described in literature (Siddappa et al., 2008).
General procedure for the synthesis of 2-(substituted-phenyl)-3-(((3-(pyridin-4-yl)-1-(p-tolyl)-1H-pyrazol-4-yl)methylene)amino)-quinazolin-4(3H)-ones (5a–l)
A solution of compound-3-(pyridin-4-yl)-1-(p-tolyl)-1H-pyrazole-4-carbaldehyde (3) (0.01 mol) in 1,4-dioxan (30 mL) was mixed with 3-amino-2-(substituted-phenyl)quinazolin-4(3H)-one (4a–l). The reaction mixture was stirred vigorously at 90 °C temperature for 1 h and then add catalytic amount of fused ZnCl2 as a catalyst in it. Then the mixture was refluxed for 4–5 h after the completion of reaction,it was cooled, excess solvent was distilled and then poured it into iced-cold water. The resulting light brown color product was obtained. The completion of the reaction was checked by TLC [n-hexane/ethyl acetate (V/V = 1:3)]. The crude product was recrystallized from 95 % ethanol to give the final compounds-(5a–l).
2-(2-Chlorophenyl)-3-(((3-(pyridin-4-yl)-1-(p-tolyl)-1H-pyrazol-4-yl)methylene)amino)-quinazolin-4(3H)-one (5a)
Yield: 68 %; m.p.: 198–200 °C; IR (KBr) νmax/cm−1: 3065, 3052 (C–H, aromatic), 2916 (=CH stretching), 2860 (–CH3 stretching), 1695 (C=O), 1601, 1590 (C=N), 1533, 1449 (C=C), 1460 (=CH bending), 1452 (–CH3 bending), 845 (C–Cl stretching); 1H NMR (300 MHz, DMSO-d 6, δ, ppm): 2.34 (s, 3H, CH3), 8.20 (s, 1H, N=CH), 8.43 (s, 1H, =CH–N), 7.99–8.75 (m, 4H, Pyridine-H), 7.40-8.03 (m, 12H, Ar–H); 13C NMR (100 MHz, DMSO-d 6, δ, ppm): 21.3, 114.0, 119.2, 119.4, 121.3, 121.9, 126.5, 121.8, 123.0, 126.6, 126.9, 127.3, 129.2, 129.6, 130.1, 131.5, 132.1, 132.3, 133.3, 133.5, 135.9, 136.7, 140.3, 143.3, 148.7, 149.6, 149.9, 153.6, 166.7. LCMS (m/z): 516.5 (M+); Anal. Calcd. For C30H21ClN6O: C-69.70, H-4.09, N-16.26; Found: C-69.77, H-4.15, N-16.32 %.
2-(3-Chlorophenyl)-3-(((3-(pyridin-4-yl)-1-(p-tolyl)-1H-pyrazol-4-yl)methylene)amino)-quinazolin-4(3H)-one (5b)
Yield: 72 %; m.p.: 194–196 °C; IR (KBr) νmax/cm−1: 3063, 3056 (C–H, aromatic), 2912 (=CH stretching), 2866 (–CH3 stretching), 1691 (C=O), 1576, 1593 (C=N), 1535, 1446 (C=C), 1463 (=CH bending), 1450 (–CH3 bending), 849 (C–Cl stretching); 1H NMR (300 MHz, DMSO-d 6, δ, ppm): 2.36 (s, 3H, CH3), 8.23 (s, 1H, N=CH), 8.47 (s, 1H, =CH–N), 7.97–8.73 (m, 4H, Pyridine-H), 7.46–8.06 (m, 12H, Ar–H); 13C NMR (100 MHz, DMSO-d 6, δ, ppm): 21.1, 114.2, 119.3, 119.5, 121.3, 121.6, 126.1, 126.6, 121.7, 126.8, 127.3, 129.3, 129.6, 130.0, 130.3, 130.6, 133.5, 133.6, 134.4, 135.8, 136.7, 140.3, 143.3, 148.6, 149.7, 149.9, 153.6, 166.9.LCMS (m/z): 516.5 (M+); Anal. Calcd. For C30H21ClN6O: C-69.70, H-4.09, N-16.26; Found: C-69.78, H-4.16, N-16.34 %.
2-(4-Chlorophenyl)-3-(((3-(pyridin-4-yl)-1-(p-tolyl)-1H-pyrazol-4-yl)methylene)amino)-quinazolin-4(3H)-one (5c)
Yield: 68 %; m.p.: 202–203 °C; IR (KBr) νmax/cm−1: 3069, 3058 (C–H, aromatic), 2920 (=CH stretching), 2858 (–CH3 stretching), 1698 (C=O), 1572, 1596 (C=N), 1531, 1443 (C=C), 1460 (=CH bending), 1448 (–CH3 bending), 843 (C–Cl stretching); 1H NMR (300 MHz, DMSO-d 6, δ, ppm): 2.37 (s, 3H, CH3), 8.26 (s, 1H, N=CH), 8.47 (s, 1H, =CH–N), 7.94–8.71 (m, 4H, Pyridine-H), 7.37–8.03 (m, 12H, Ar–H); 13C NMR (100 MHz, DMSO-d 6, δ, ppm): 21.5, 114.1, 119.4, 119.6, 121.6, 121.9, 126.6, 121.8, 126.6, 127.3, 128.9, 129.1, 129.4, 129.6, 133.3, 133.5, 135.6, 135.9, 136.7, 140.4, 143.4, 148.7, 149.4, 149.8, 153.6, 165.1, 166.6; LCMS (m/z): 516.5 (M+); Anal. Calcd. For C30H21ClN6O: C-69.70, H-4.09, N-16.26; Found: C-69.76, H-4.17, N-16.34 %.
3-(((3-(Pyridin-4-yl)-1-(p-tolyl)-1H-pyrazol-4-yl)methylene)amino)-2-(o-tolyl)quinazolin-4(3H)-one (5d)
Yield: 68 %; m.p.: 198–200 °C; IR (KBr) νmax/cm−1: 3064, 3053 (C–H, aromatic), 2910 (=CH stretching), 2860, 2868 (–CH3 stretching), 1689 (C=O), 1578, 1593 (C=N), 1536, 1440 (C=C), 1464 (=CH bending),1458, 1450 (–CH3 bending), 851 (C–Cl stretching); 1H NMR (300 MHz, DMSO-d 6, δ, ppm): 2.34 (s, 3H, p-CH3), 2.48 (s, 3H, o-CH3), 8.20 (s, 1H, N=CH), 8.43 (s, 1H, =CH–N), 7.99–8.75 (m, 4H, Pyridine-H), 7.40–8.03 (m, 12H, Ar–H); 13C NMR (100 MHz, DMSO-d 6, δ, ppm): 18.9, 21.7, 114.4, 119.6, 121.6, 121.9, 126.7, 121.8, 125.8, 126.6, 127.3, 127.7, 129.2, 129.8, 130.0, 130.2, 131.5, 133.3, 133.5, 135.9, 136.7, 138.1, 140.3, 143.5, 148.7, 149.3, 149.7, 153.6, 166.3; LCMS (m/z): 496.56 (M+); Anal. Calcd. For C31H24N6O: C-74.98, H-4.87, N-16.92; Found: C-74.92, H-4.94, N-16.98 %.
3-(((3-(Pyridin-4-yl)-1-(p-tolyl)-1H-pyrazol-4-yl)methylene)amino)-2-(m-tolyl)quinazolin-4(3H)-one (5e)
Yield: 67 %; m.p.: 221–222 °C; IR (KBr) νmax/cm−1: 3064, 3052 (C–H, aromatic), 2919 (=CH stretching), 2864, 2873 (–CH3 stretching), 1694 (C=O), 1573, 1592 (C=N), 1533 (C=C), 1452, 1458 (–CH3 bending), 856 (C–Cl stretching); 1H NMR (300 MHz, DMSO-d 6, δ, ppm): 2.35 (s, 3H, p-CH3), 2.41 (s, 3H, m-CH3), 8.22 (s, 1H, N=CH), 8.45 (s, 1H, =CH–N), 7.96–8.79 (m, 4H, Pyridine-H), 7.41-8.03 (m, 12H, Ar–H); 13C NMR (100 MHz, DMSO-d 6, δ, ppm): 18.9, 21.3, 114.5, 119.3, 119.7, 121.4, 121.7, 126.5, 121.8, 125.2, 126.3, 126.6, 127.3, 128.5, 128.7, 129.2, 129.6, 130.4, 133.3, 133.5, 135.9, 136.7, 138.5, 140.0, 143.2, 148.7, 149.7, 149.9, 153.5, 166.7.; LCMS (m/z): 496.56 (M+); Anal. Calcd. For C31H24N6O: C-74.98, H-4.87, N-16.92; Found: C-74.93, H-4.96, N-16.97 %.
3-(((3-(Pyridin-4-yl)-1-(p-tolyl)-1H-pyrazol-4-yl)methylene)amino)-2-(p-tolyl)quinazolin-4(3H)-one (5f)
Yield: 74 %; m.p.: 216–217 °C; IR (KBr) νmax/cm−1: 3062, 3054 (C–H, aromatic), 2915 (=CH stretching), 2855, 2869 (–CH3 stretching), 1696 (C=O), 1570, 1591 (C=N), 1532, 1449 (C=C), 1458, 1449 (–CH3 bending), 847 (C–Cl stretching); 1H NMR (300 MHz, DMSO-d 6, δ, ppm): 2.34, 2.39 (s, 6H, p-CH3), 8.24 (s, 1H, N=CH), 8.41 (s, 1H, =CH–N), 7.95–8.72 (m, 4H, Pyridine-H), 7.36-8.09 (m, 12H, Ar–H); 13C NMR (100 MHz, DMSO-d 6, δ, ppm): 21.1, 21.5, 114.2, 119.1, 119.5, 121.3, 121.7, 126.5, 121.9, 126.6, 127.3, 129.2, 129.4, 129.6, 130.3, 133.3, 133.5, 135.7, 136.8, 139.8, 140.6, 143.3, 148.4, 149.2, 149.7, 153.2, 166.4; LCMS (m/z): 496.56 (M+); Anal. Calcd. For C31H24N6O: C-74.98, H-4.87, N-16.92; Found: C-74.90, H-4.95, N-16.99 %.
2-(2-Methoxyphenyl)-3-(((3-(pyridin-4-yl)-1-(p-tolyl)-1H-pyrazol-4-yl)methylene)amino)-quinazolin-4(3H)-one (5g)
Yield: 70 %; m.p.: 243–245 °C; IR (KBr) νmax/cm−1: 3071, 3064 (C–H, aromatic), 2923 (=CH stretching), 2882 (–OCH3 stretching), 2861 (–CH3 stretching), 1692 (C=O), 1579, 1592 (C=N), 1530 (C=C), 1463, 1449 (–OCH3 bending), 1460 (=CH bending), 1454 (–CH3 bending), 854 (C–Cl stretching); 1H NMR (300 MHz, DMSO-d 6, δ, ppm): 3.83 (s, 3H, –OCH3), 2.34 (s, 3H, CH3), 8.20 (s, 1H, N=CH), 8.47 (s, 1H, =CH–N), 7.99–8.77 (m, 4H, Pyridine-H), 7.26–8.05 (m, 12H, Ar–H); 13C NMR (100 MHz, DMSO-d 6, δ, ppm): 21.4, 55.8, 106.2, 111.4, 114.3, 119.2, 119.4, 121.0, 121.2, 121.8, 125.1, 126.5, 121.5, 126.6, 127.4, 129.2, 129.6, 131.1, 133.7, 133.5, 135.9, 136.7, 140.3, 143.3, 148.6, 149.5, 149.7, 153.2, 157.4, 166.9; LCMS (m/z): 512.5 (M+); Anal. Calcd. For C31H24N6O2: C-72.64, H-4.72, N-16.40; Found: C-72.72, H-4.79, N-16.49 %.
2-(3-Methoxyphenyl)-3-(((3-(pyridin-4-yl)-1-(p-tolyl)-1H-pyrazol-4-yl)methylene)amino)-quinazolin-4(3H)-one (5h)
Yield: 73 %; m.p.: 247–249 °C; IR (KBr) νmax/cm−1: 3077, 3059 (C–H, aromatic), 2926 (=CH stretching), 2885 (–OCH3 stretching), 2863 (–CH3 stretching), 1694 (C=O), 1573, 1596 (C=N), 1535 (C=C), 1464 (–OCH3 bending), 1458 (=CH bending), 1452 (–CH3 bending), 855 (C–Cl stretching); 1H NMR (300 MHz, DMSO-d 6, δ, ppm): 3.85 (s, 3H, –OCH3), 2.32 (s, 3H, CH3), 8.25 (s, 1H, N=CH), 8.43 (s, 1H, =CH–N), 7.95–8.75 (m, 4H, Pyridine-H), 7.05–8.1 (m, 12H, Ar–H); 13C NMR (100 MHz, DMSO-d 6, δ, ppm): 21.2, 55.2, 110.2, 114.3, 115.7, 119.0, 119.2, 120.5, 121.2, 121.9, 126.5, 121.8, 126.6, 127.3, 129.2, 129.5, 129.7, 129.9, 133.3, 133.5, 135.9, 136.7, 140.3, 143.3, 148.5, 149.3, 149.7, 153.0, 160.8, 166.6.; LCMS (m/z): 512.5 (M+); Anal. Calcd. For C31H24N6O2: C-72.64, H-4.72, N-16.40; Found: C-72.71, H-4.80, N-16.48 %.
2-(4-Methoxyphenyl)-3-(((3-(pyridin-4-yl)-1-(p-tolyl)-1H-pyrazol-4-yl)methylene)amino)-quinazolin-4(3H)-one (5i)
Yield: 68 %; m.p.: 237–239 °C; IR (KBr) νmax/cm−1: 3074, 3063 (C–H, aromatic), 2929 (=CH stretching), 2877 (–OCH3 stretching), 2866 (–CH3 stretching), 1697 (C=O), 1574, 1597 (C=N), 1534, 1442 (C=C), 1466 (–OCH3 bending), 1460 (=CH bending), 1457 (–CH3 bending), 858 (C–Cl stretching); 1H NMR (300 MHz, DMSO-d 6, δ, ppm): 3.87 (s, 3H, –OCH3), 2.33 (s, 3H, CH3), 8.22 (s, 1H, N=CH), 8.47 (s, 1H, =CH–N), 7.92–8.79 (m, 4H, Pyridine-H), 7.20–8.12 (m, 12H, Ar–H); 13C NMR (100 MHz, DMSO-d 6, δ, ppm): 21.4, 55.8, 114.2, 114.5, 119.2, 119.4, 121.2, 121.7, 126.6, 121.6, 126.5, 127.3, 129.2, 129.5, 131.1, 133.3, 133.5, 135.7, 136.6, 140.1, 143.3, 148.7, 149.4, 149.7, 153.4, 159.4, 166.7; LCMS (m/z): 512.5 (M+); Anal. Calcd. For C31H24N6O2: C-72.64, H-4.72, N-16.40; Found: C-72.73, H-4.81, N-16.47 %.
2-(2-Nitrophenyl)-3-(((3-(pyridin-4-yl)-1-(p-tolyl)-1H-pyrazol-4-yl)methylene)amino)-quinazolin-4(3H)-one (5j)
Yield: 63 %; m.p.: 134–136 °C; IR (KBr) νmax/cm−1: 3068, 3054 (C–H, aromatic), 2924 (=CH stretching), 2867 (–CH3 stretching), 1690 (C=O), 1577, 1593 (C=N), 1550 (–NO2 gp, asymmetric stretching), 1536, 1440 (C=C), 1465 (=CH bending), 1453 (–CH3 bending), 1328 (–NO2 gp, symmetric stretching), 847 (C–Cl stretching); 1H NMR (300 MHz, DMSO-d 6, δ, ppm): 2.39 (s, 3H, CH3), 8.23 (s, 1H, N=CH), 8.48 (s, 1H, =CH–N), 7.97–8.79 (m, 4H, Pyridine-H), 7.59–8.09 (m, 12H, Ar–H); 13C NMR (100 MHz, DMSO-d 6, δ, ppm): 21.2, 114.7, 119.1, 119.5, 120.3, 121.0, 121.5, 124.1, 126.5, 121.8, 126.6, 127.3, 129.2, 129.6, 131.0, 131.9, 133.3, 133.5, 134.9, 135.9, 136.7, 140.3, 143.3, 147.0, 148.7, 149.4, 149.6, 153.7, 166.3; LCMS (m/z): 527.53 (M+); Anal. Calcd. For C30H21N7O3: C-68.30, H-4.01, N-18.59; Found: C-68.37, H-4.08, N-18.66 %.
2-(3-Nitrophenyl)-3-(((3-(pyridin-4-yl)-1-(p-tolyl)-1H-pyrazol-4-yl)methylene)amino)-quinazolin-4(3H)-one (5k)
Yield: 67 %; m.p.: 141–143 °C; IR (KBr) νmax/cm−1: 3067, 3058 (C–H, aromatic), 2932 (=CH stretching), 2869 (–CH3 stretching), 1696 (C=O), 1574, 1598 (C=N), 1555 (–NO2 gp, asymmetric stretching), 1533, 1444 (C=C), 1461 (=CH bending), 1457 (–CH3 bending), 1324 (–NO2 gp, symmetric stretching), 844 (C–Cl stretching); 1H NMR (300 MHz, DMSO-d 6, δ, ppm): 2.37 (s, 3H, CH3), 8.26 (s, 1H, N=CH), 8.46 (s, 1H, =CH–N), 7.94–8.82 (m, 4H, Pyridine-H), 7.42–8.12 (m, 12H, Ar–H); 13C NMR (100 MHz, DMSO-d 6, δ, ppm): 21.1, 114.3, 119.2, 119.5, 119.7, 121.4, 121.9, 125.4, 126.5, 121.6, 126.6, 127.3, 129.0, 129.3, 129.5, 129.9, 133.3, 133.5, 134.3, 135.7, 136.4, 140.3, 143.3, 148.0, 148.4, 149.3, 149.6, 153.9, 166.4; LCMS (m/z): 527.53 (M+); Anal. Calcd. For C30H21N7O3: C-68.30, H-4.01, N-18.59; Found: C-68.38, H-4.09, N-18.68 %.
2-(4-Nitrophenyl)-3-(((3-(pyridin-4-yl)-1-(p-tolyl)-1H-pyrazol-4-yl)methylene)amino)-quinazolin-4(3H)-one (5l)
Yield: 69 %; m.p.: 156–157 °C; IR (KBr) νmax/cm−1: 3061, 3053 (C–H, aromatic), 2932 (=CH stretching), 2864 (–CH3 stretching), 1698 (C=O), 1576, 1598 (C=N), 1558 (–NO2 gp, asymmetric stretching), 1539, 1445 (C=C), 1460 (=CH bending), 1452 (–CH3 bending), 1326 (–NO2 gp, symmetric stretching), 853 (C–Cl stretching); 1H NMR (300 MHz, DMSO-d 6, δ, ppm): 2.32 (s, 3H, CH3), 8.27 (s, 1H, N=CH), 8.44 (s, 1H, =CH–N), 7.93–8.75 (m, 4H, Pyridine-H), 7.63–8.13 (m, 12H, Ar–H); 13C NMR (100 MHz, DMSO-d 6, δ, ppm): 21.7, 55.9, 114.1, 114.4, 119.0, 119.4, 121.3, 121.7, 126.5, 121.6, 126.6, 127.1, 129.2, 129.4, 131.1, 133.3, 133.4, 135.6, 136.7, 140.0, 143.3, 148.8, 149.2, 149.6, 153.5, 159.4, 162.0, 166.2.; LCMS (m/z): 527.53 (M+); Anal. Calcd. For C30H21N7O3: C-68.30, H-4.01, N-18.59; Found: C-68.39, H-4.07, N-18.67 %.
Biological activity
In vitro evaluation of the antitubercular activity was carried out at the Tuberculosis Acquisition Antimicrobial Coordinating Facility (TAACF) screening program, Alabama, USA. Minimum inhibitory concentration (MIC) was determined against M. tuberculosis H37Rv using the radiometric BACTEC (Collins and Franzblau, 1997) and broth dilution (Suling et al., 2000) assay methods. The result of antitubercular activity is presented in Table 1. The activity is considerably affected by substitutions at the phenyl ring of the pyrazole–quinazolinone nucleus. For the antitubercular activity, isoniazide is taken as a standard, which showed a percentage inhibition of 99 at an MIC range of below 6.25 μg/mL. It has been observed that compounds 5a, 5d, and 5g having chloro, methyl, and methoxy group at second position, showed excellent antitubercular activity with percentage inhibition of 96, 94, and 93, respectively at an MIC range below 6.25 μg/mL, whereas compound 5c having chloro group at para position, and compound 5k having nitro group at meta position showed excellent antitubercular activity with percentage inhibitions of 90 and 92, respectively, at the same MIC range. The presence of same substituent at any other position of phenyl ring remarkably reduced the antitubercular activity. Compounds demonstrating at least 90 % inhibition in the primary screen (MIC < 6.25 ug/mL) are re-tested against M. tuberculosis H37Rv at lower concentrations to determine the actual minimum inhibitory concentration (MIC). The actual MIC of compounds 5a, 5c, 5d, 5g, and 5k are <3.125.
Conclusion
In summary, we have developed a new, efficient and environmentally benign methodology toward the synthesis of 2-(substituted-phenyl)-3-(((3-(pyridin-4-yl)-1-(p-tolyl)-1H-pyrazol-4-yl)methylene)amino)-quinazolin-4(3H)-one (5a–l). This synthetic strategy allows for the assimilation of two promising bioactive nuclei in a single scaffold in an easy way. Reviewing the antitubercular activity data, it has been concluded that the presence of chloro, methyl, and methoxy groups at ortho position, chloro at para position and nitro at meta position emerged as active in screening. Compound 5a, 5c, 5d, 5g, and 5k possess excellent antitubercular activity. Hence, there is enough scope for further study in the developing these as good lead compounds. Further studies on these compounds and optimization of their structures leading to novel analogues with superior biological properties are ongoing in our laboratory.
References
Aggarwal R, Singh SP, Kumar V, Tyagi P (2006) Synthesis and antibacterial activity of some new 1-heteroaryl-5-amino-3H/methyl-4-phenylpyrazoles. Bioorg Med Chem 14:1785–1791. doi:10.1016/j.bmc.2005.10.026
Alafeefy AM (2008) Synthesis and antimicrobial activity of some new quinazolin-4(3H)-one derivatives. Pharm Biol 46(10):751–756
Apfel C, Banner DW, Bur D, Dietz M, Hubschwerlen C, Locher H, Marlin F, Masciadri R, Pirson W, Stalder H (2001) 2-(2-Oxo-1,4-dihydro-2H-quinazoli-3-yl)- and 2-(2,2-dioxo-1,4-dihydro-2H-6 benzo[1,2,6]thiadiazin-3-yl)-N-hydroxy-acetamides as potent and selective peptide deformylase inhibitors. J Med Chem 44:1847–1852
Chenard BL, Welch WM, Blake JF, Butler TW, Reinhold A, Ewing FE, Menniti FS, Pagnozzi MJ (2001) Quinazolin-4-one a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonists: structure–activity relationship of the C-2 side chain tether. J Med Chem 44:1710–1717
Collins L, Franzblau SG (1997) Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother 41:1004–1009
Deohate PP, Deohate JP, Berad BN (2004) Synthesis of some novel 1,2,4-dithiazolidines and their antibacterial and antifungal activity. Asian J Chem 16:255–260
Guang-Fang X, Bao-An S, Pinaki SB, Song Y, Pei-Quan Z, Lin-Hong J, Wei X, De-Yu H, Ping L (2007) Synthesis and antifungal activity of novel S-substituted 6-fluoro-4-alkyl(aryl)thioquinazoline derivatives. Bioorg Med Chem 15:3768–3774
Habeeb AG, Rao PNP, Knaus EE (2001) Design and synthesis of celecoxib and rofecoxib analogues as selective cyclooxygenase-2 (COX-2) inhibitors: replacement of sulfonamide and methylsulfonyl pharmacophores by an azido bioisostere. J Med Chem 44:3039–3042. doi:10.1021/jm010153c
Kees KL, Fitzgerald JJ, Steiner KE, Mattes JF, Mihan B, Tosi T, Moondoro D, McCaleb ML (1996) New potent antihyperglycemic in db/db mice: synthesis and structure-activity relationship studies of (4-substituted benzyl)(trifloromethyl)pyrazoles and -pyrazolones. J Med Chem 39:3920–3928. doi:10.1021/jm960444z
Martel AM, Graul A, Rabbaseda X, Castaner R (1997) Slidenafil: treatment of erectile dysfunction, phosphodiesterase V inhibitor. Drugs Future 22:138–143
Meazza G, Bettarini F, Porta PL, Piccardi P, Signorini E, Portoso D, Fornara L (2004) Synthesis and herbicidal activity of novel heterocyclic protoporphyrinogen oxidase inhibitors. Pest Manag Sci 60:1178–1188. doi:10.1002/ps.923
Park H-J, Lee K, Park S-J, Ahn B, Lee J-C, Cho HY, Lee K-I (2005) Identification of antitumor activity of pyrazole oxime ethers. Bioorg Med Chem Lett 15:3307–3312. doi:10.1016/j.bmcl.2005.03.082
Raffa D, Daidone G, Schillaci D, Maggio B, Plescia F (1999) Synthesis of new 3-(3-phenyl-isoxazo-5-yl) or 3-[(3-phenylisoxazol-5-yl)-amino]-substituted 4(3H)quinazolinone derivatives with antineoplastic activity. Pharmazie 54:251–254
Rattan A, Kalia A, Ahmad N (1998) Multidrug-resistant Mycobacterium tuberculosis: molecular perspectives. Emerg Infect Dis 4:195–199
Siddappa K, Reddy T, Reddy CV (2008) Synthesis, characterization and antimicrobial studies of 3-[(2-hydroxy-quinolin-3-ylmethylene)-amino]-2-phenyl-3H-quinazolin-4-one and its metal(II) complexes. E J Chem 5:155–162
Singh P, Mishra AK, Malonia SK, Chauhan DS, Sharma VD, Venkatesan K, Katoch VM (2006) The paradox of pyrazinamide: an update on the molecular mechanisms of pyrazinamide resistance in Mycobacteria. J Commun Dis 38(3):288–298
Suling WJ, Seitz LE, Pathak V, Barrow WW, Reynolds RC (2000) Antimycobacterial activities of 2,4-diamino-5-deazapteridine derivatives and effects on mycobacterial dihydrofolate reductase. Antimicrob Agents Chemother 44(10):2784–2793
Tobe M, Isobe Y, Tomizawa H, Nagasaki T, Obara F, Hayashi H (2003) Structure–activity relationships of 6-fluoroquinazolines: dual-acting compounds with inhibitory activities toward both TNF alpha production and T cell proliferation. Bioorg Med Chem 11:609–616
Wustrow DJ, Rubin CR, Knobelsdorf JA, Akunne H, MacKenzie DR, Pugsley TA, Zoski KT, Heffner TG, Wise LD (1998) Pyrazolo[1,5-a]pyrimidine CRF-1 receptor antagonists. Bioorg Med Chem Lett 8:2067–2070. doi:10.1016/S0960-894X(98)00372-2
Acknowledgment
The authors are thankful to Cadila Pharmaceuticals Limited, Dholka, Ahmedabad for providing research and library facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandit, U., Dodiya, A. Synthesis and antitubercular activity of novel pyrazole–quinazolinone hybrid analogs. Med Chem Res 22, 3364–3371 (2013). https://doi.org/10.1007/s00044-012-0351-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0351-0