Abstract
In the Mediterranean Sea, pelagic longline fisheries, targeting tuna and swordfish, have contributed significantly to the bycatch of threatened chondrichthyan species, such as blue shark (Prionace glauca). The Mediterranean blue shark population is assessed as critically endangered, making a timely implementation of mitigation measures crucial. A comprehensive understanding of blue shark habitat use dynamics is essential for deriving appropriate mitigation measures. This study aimed at evaluating vertical movement behaviour and investigating factors potentially influencing the movements of blue sharks in the Mediterranean Sea. Twenty-six blue sharks, bycaught in a longline fishery in the southern Adriatic, were tagged with pop-up satellite archival tags. Analysis of data from thirteen recovered tags revealed a distinctive diel movement pattern. Blue sharks used shallower waters during the night and deeper waters during the day, characterised by steep ascents and descents during sunset and sunrise, respectively. In addition, lunar phases were also influencing the depth of blue shark movements, with sharks using deeper waters right before and during full-moon. Shark size, salinity, currents, spatial location and time of the year were additional factors influencing blue shark depth use. The observed tendency of blue sharks to use deeper areas at daytime and prior and during the full moon period offers possibilities to develop and test bycatch mitigation strategies. Aligning longline fishing schedules and fishing depths with blue shark behaviour during the fishing seasons could hold promise to effectively reduce spatio-temporal overlap between fishing and blue shark distribution and may ultimately decrease the bycatch impact of the fishery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past half-century, global chondrichthyan populations have declined alarmingly worldwide (Dulvy et al. 2014; Williamson et al. 2019). Due to their specific life history traits (i.e., slow growth rate, late maturation, low fecundity and low egg production), chondrichthyans are highly susceptible to overfishing (Musick et al. 2000; Dulvy et al. 2014; Williamson et al. 2019). In the Mediterranean Sea, pelagic longline fisheries targeting tuna and swordfish contribute significantly to unwanted bycatches, with at least 15 shark and ray species affected (Bradai et al. 2012; FAO 2016). This issue is particularly pronounced in the Alboran Sea (34.3%) and the Adriatic Sea (15.1%) (Bradai et al. 2012), where a substantial fleet of 30 commercial fishing vessels operates in the Southern Adriatic Sea (European Union [EU] Fleet Register). More than half of Mediterranean shark species are at a heightened risk of extinction (Dulvy et al. 2016), prompting the General Fisheries Commission for the Mediterranean (GFCM) to establish the "International Plan of Action" for sharks in 2000 (FAO 2000). This plan encompasses mitigation measures, including ad-hoc actions to reduce unwanted bycatch of pelagic sharks, the prohibition of finning practices, and the restriction of the capture and sale of sharks and ray species listed in Annex II of the Specially Protected Areas and Biological Diversity in the Mediterranean (SPA/BD) Protocol of the Barcelona Convention (GFCM 2012; FAO 2022).
The management and protection of elasmobranchs in the Mediterranean Sea are, however, more complicated than in other regions since the approaches used are generic and have an “one size solution”, which does not adequately account for the wide range of diversity within the group (Dulvy et al. 2017). There are also additional complex elements in the Mediterranean Sea that increase the difficulty of conservation, such as the variety of legal frameworks, including those pertaining to fisheries, the environment, and social and economic concerns (Cavanagh and Gibson 2007; Bradai et al. 2018). In response, scientists and environmental managers are compelled to investigate other approaches that tackle this conservation issue from the ground up, based on more selective fishing gears and fisher engagement.
The blue shark, Prionace glauca, L. 1758, is strongly affected by bycatch in pelagic longline fisheries. It is a relatively abundant and widely distributed pelagic shark species (Compagno 1984; Nakano and Stevens 2008; Druon et al. 2022), inhabiting tropical to temperate waters of the Atlantic and Indo-Pacific Oceans, as well as their adjacent seas, including the Mediterranean Sea. It ranks among the most frequently caught and landed pelagic shark species worldwide (Gilman et al. 2007; Córdova-Zavaleta et al. 2018) and is a primary species in the international shark meat and fin trade (Dent and Clarke 2015; Eriksson and Clarke 2015; Niedermüller et al. 2021). The global population of blue shark has been classified as “near threatened” by the International Union for Conservation of Nature (IUCN) (Rigby et al. 2019), while the Mediterranean population has been classified as “critically endangered” (Sims et al. 2016), highlighting the urgency of monitoring this particular population and the need for effective bycatch mitigation measures. The main cause of the decline in the blue shark population is fishing mortality, both in targeted fisheries and as unwanted bycatch in fisheries targeting other fish species (Sims et al. 2016). In addition, blue sharks have recently been listed in the CITES Appendix II. This measure requires the EU and countries to control the trade and the landing from international waters (Commission Regulation (EU) 2023/966). The EU Scientific Review Group for CITES have recently decided on a negative opinion for Mediterranean blue sharks’ landings from international waters (https://www.speciesplus.net/api/v1/documents/16334) and a zero quota for exports of blue shark from the Mediterranean stock for the year 2024, calling for urgent mitigation measures to be identified to support fishers to avoid blue shark bycatch.
Concrete initiative to reduce the incidental captures of blue shark, which may still be landed despite lower commercial value (FAO 2016) and are historically fished with longlines, typically involve modifications to the gear and/or the fishing strategies (Gilman et al. 2016). Changes to longline gear include adjustments to the distance between floats (to control the depth at which hooks fish), the selection of leader or branch line material (e.g., wire or nylon), bait type, and hook shape and size (Gilman et al. 2016). Consideration of the consequences on catch rates of target species is paramount in bycatch mitigation research, as fishers are more likely to adopt mitigation strategies that do not result in a reduction in the target species catch (Hall et al. 2007; Ward and Hindmarsh 2007; Campbell and Cornwell 2008; Carbonara et al. 2023). However, numerous factors, such as environmental and biotic factors (e.g., Schlaff et al. 2014; Andrzejaczek et al. 2022; Lubitz et al. 2022), are involved in elasmobranch movements and therefore in the interactions between fishing activities and elasmobranchs, and they must be taken into account to develop concrete mitigation measures.
The blue shark is in fact an opportunistic predator (Henderson et al. 2001; Loor-Andrade et al. 2017) and it exhibits extensive vertical movements, spanning from the ocean surface to water depths exceeding 1500 m (Strasburg 1958; Queiroz et al. 2012; Vedor et al. 2021; Druon et al. 2022), with regular upward and downward dives (Druon et al. 2022). These vertical movements are thought to enhance foraging success by increasing spatial overlap with their prey or actively pursuing the vertical movements of key prey species (Hays 2003; Campana et al. 2011; Bandara et al. 2021). Temperature changes experienced during the vertical movements may also be linked to important physiological and metabolic processes in the sharks (Campana et al. 2011; Wanatabe et al. 2021). However, these extensive vertical movements also increase the likelihood of encounters with fishing gears, operating both within the euphotic zone and in deeper layers. Therefore, a comprehensive understanding of elasmobranch habitat use dynamics is essential to explore potential spatio-temporal mitigation measures to effectively reduce by-catch of these top predators (GFCM 2012; Williamson et al. 2019).
While the vertical movement behaviour of blue sharks is well-documented globally (e.g., Stevens et al. 2010; Campana et al. 2011; Musyl et al. 2011; Queiroz et al. 2010; 2012; Vedor et al. 2021; Watanabe et al. 2021), observations in the Mediterranean Sea, characterized by deep, hypersaline and non-tidal basins adjacent land masses, are lacking (but see spatial movements studied in Poisson et al. 2024). To address this gap and tailor mitigation measures to the regional movement behaviour of blue sharks in the Mediterranean Sea, 26 blue sharks, bycaught in the southern Adriatic longline fishery targeting swordfish, were equipped with pop-up satellite archival tags. The objectives were two-fold: 1) evaluating patterns in vertical movement behaviour and 2) investigating possible biological and environmental factors influencing these movements. The results could be instrumental in formulating mitigation measures to reduce interactions with longlines, consequently decreasing the amount of unwanted blue shark bycatch and improving recovery conditions for blue shark while improving the sustainability of the swordfish fishery in the Adriatic Sea.
Material and methods
Studied area
The Adriatic Sea is a semi-enclosed basin in the north-central Mediterranean Sea, enclosed between the Italian peninsula and the Balkans. The Adriatic is divided into two Geographical Sub Areas (GSA sensu GFCM-FAO), a shallower northern area (GSA17) and a deeper southern area (GSA18). In general, the western coast of the Adriatic Sea is flat and mostly sandy and muddy, whereas the eastern coast is mostly steep and rocky. In particular, the GSA18 area includes the Italian coasts of the Apulia region, on the western side, and those of Montenegro and Albania on the eastern side. The Southern Adriatic Sea extends from the line between Gargano and Montenegro coast to the boundary with the Ionian Sea at the latitude of Otranto (Artegiani et al. 1997). A deep central depression, known as the “South Adriatic Pit” (or “Bari Pit”) which reaches a depth of 1233 m, characterizes the southern part of the Adriatic Sea. The northern and southern portions of the GSA18 are different; the first is characterized by a wide continental shelf (about 45 nautical miles) and a very steady slope; in the second, the bottom is steeper, with the shelf edge much closer to the coast (about eight miles from the Cape of Otranto).
The prevailing currents in Southern Adriatic Sea flow counter clockwise from the Strait of Otranto along the eastern coast and then back to the Strait along the western coast. As a consequence, the inflowing water masses from the Ionian Sea are warmer and saltier than cold waters of the North Adriatic Sea outflowing along the western shore (Vilibic and Orlic 2002).
Tagging procedures
In the context of the SafeShark (WWF 2022) and MedByCatch (https://medasset.org/portfolio-item/medbycatch-project/) projects (2019–2021), a total of 26 blue sharks were tagged with pop-up satellite archival tags (Wildlife Computers™ pop-up tag) during the monitoring of seven fishing trips (28 fishing days) of pelagic longliners targeting swordfish in the southern Adriatic Sea in Italian and international waters (2019–2021) (Table 1).
In more details, the total mainline length was between 30 and 40 km, and a hook was attached to a dropline with a length of about 13 m, and each dropline was attached to the main line every ~ 58 m (Benoît et al. 2010; Dapp et al. 2016; Carbonara et al. 2023). Hooks used during the fishing season were J-type hooks (76 mm long) and were at about 30 m deep. The longline was set in the early afternoon (15:00–16:00) and the operation was completed in about three hours. The longline haul back began at night and finished in the morning around 7:00–8:00. The longline hauling started with the last hooks. Therefore, the hooks remained at sea (soaking time) for between 10 and 20 h (the time between the last hook set at sea and the first hook recovered). The bait used in the study was frozen mackerel (Scombridae), and an artificial light was attached to the middle of each dropline.
Upon retrieval, hooked sharks were subject to a procedural sequence for tagging. Only healthy sharks were tagged in this study, i.e., sharks in overall good condition with vigorous movements (Carbonara et al. 2023). Sharks were blindfolded with a cool, wet cotton cloth, and a tube, inserted into their mouths, gently pumped seawater to simulate normal swimming behaviour and maintain adequate oxygen flow over their gills (Poisson et al. 2012). The blindfold induced a slight sedative effect, keeping the shark calm (Bruce and Bradford 2013). Immediately before inserting the tag, the shark was gently rolled into a left or right lateral recumbency ensuring a cataleptic state for ease of access and tagging (Otway 2020). These calming measures, crucial for human safety and to minimize stress of the captured sharks, facilitated a controlled tagging environment (Otway 2020). The tags were attached on the exposed side, close to the first dorsal fin, by inserting the dart through a 2 cm incision in the skin covering the epaxial muscle mass using a stainless-steel applicator (Fig. 1). Concurrently, the total length (TL) and sex, identified by the presence of pterygopods, were recorded. To conclude the process, blue sharks were released by one or two individuals supporting the pectoral and caudal fins, gently lowering the animal over the side of the vessel (AFMA 2014; Poisson et al. 2012; FAO and ACCOBAMS 2018). The entire procedure, encompassing unhooking, taking measurements, and tagging adhered to a time frame ranging from four to eight minutes.
Tagging procedure of the blue sharks bycaught in the swordfish fishery in the southern Adriatic Seat. Top: Calming the shark by covering the eyes with a wet cloth, providing continuous oxygenation of the gills through a seawater hose and placing it in a rolled lateral recumbency position. The sPAT anchor was inserted using a stainless-steel applicator at the tagging site. Bottom-left: Releasing the tagged blue shark by carefully lowering it by the pectoral and caudal fins over the side of the vessel by one or two individuals. Bottom-right: A blue shark swimming shortly after release. Total procedure time: 4–8 min
In this experiment, two types of pop-up tags were used: survivorship tags (sPAT) and archival tag (miniPAT). Each was deployed with a respective pre-set release time (D-time) of one month (sPAT) and one year (miniPAT). Depth (resolution of 0.5 m) and ambient temperature (resolution of 0.05 °C) data were recorded at 10-min intervals by sPATs for the last five days of D-time and by miniPATs for the entire D-time.
Data processing
Data processing described below required the use of the R software version 4.1.1 (R Core Team 2022).
For sharks tagged with miniPATs, coordinate positions were reconstructed using GPE3 State-space Wildlife ComputerTM software, incorporating tag data and environmental variables (current and salinity) from Copernicus products (CMEMS). Twilight times (HH:MM), sea surface temperature (°C), and dive depth (m) were used to estimate the positions of the specimens via a diffusion-based movement model, generating time-discrete, gridded probability surfaces (of 0.25 degrees in latitude and longitude) throughout the deployment. From these grid surfaces, the most likely animal location at a given time was derived (Wildlife Computer 2007). For sharks tagged with sPATs, tag release position served as a proxy for shark position.
Diel variations in the behaviour of blue sharks, were categorised into day and night periods based on the depth data using the R-package “NightDay” (Hughes-Brandl 2018). Moreover, in line with previous studies on diel vertical migration (DVM) of blue sharks, three behavioral classes were defined based on individual depth distribution (Campana et al. 2011; Queiroz et al. 2012; Vedor et al. 2021): (i) depth-oriented if a shark spent more than 50% of the night-time in water depths above 60 m, (ii) regular (i.e., normal DVM; nDVM) was characterised by spending more than 50% of the night in water depths above 60 m and more than 50% of the day in water depths below 60 m, and (iii) surface-oriented behaviour was characterised by spending over 50% of both day and night-time in water depths above 60 m. This depth threshold was used because it corresponded to the 75th percentile of the depth data. The moon phase, categorized into eight steps (New moon, Waxing crescent, First quarter, Waxing gibbous, Full, Waning gibbous, Last quarter, Waning crescent), was determined using the “lunar” package (Lazaridis 2022).
Statistical analyses
All statistical analyses were performed using the R software version 4.1.1 (R Core Team 2022) and carried out at the 95% level of significance. Data are presented as mean ± standard error.
The effect of the day/light cycle on the distribution frequency of shark depth was assessed using the Kolmogorov–Smirnov test. The vertical movement pattern of blue shark based on raw depth data was analyzed using a generalised additive model (GAM) (Wood 2011). Explanatory variables were evaluated for autocorrelation using the variance inflation factor (VIF) and Pearson correlation matrix. The final model, using the Gaussian family distribution and link function identity, included continous explanatory variables: 1) longitude (X), 2) latitude (Y), 3) sea surface temperature (temp), 4) salinity, 5) current as vector of combination northing (cury) and easting (curx) components (cur), 6) total length (TL), 7) temporal combination by tensor (ti) of hours and months. Thin plate smoothers were used as default for each spline except for the hours variables where the cyclic “cc” smoother was used. Month was used as a factorial explanatory variable. Interactions between variables were included in the model for the geographycal coordinates using bidimentional thin plate smoothers, while the interaction between hours and month variables was included as a tensor ti product of cyclic smoother (for hours) and thin plate smoother (for month). Model overfitting was controlled by using the gamma = 1.4 parameter in the GAM parametrization. The residuals of the models were tested with the Shapiro–Wilk normality test. The models were estimated using the “mgcv” library (Wood 2006, 2017). The resulting formula of the final model was:
Finally, the effect of moon phase on average blue shark depth use was assessed using a linear model with the moon phase as a fixed factor and the individual as a random factor. Depth was log-transformed to respect assumptions of model application (normality and homescadasticity). A pairwise post-hoc test with a Bonferroni correction was used to assess differentiation between mon phases.
Results
Tagged blue sharks
The shark tagging was performed in summer (August–September; Table 1) during the swordfish fishing season. The sPAT tags recorded data during September–October (last 5 days of D-time), while miniPAT tags covered a wider time window between August and the following January (93–126 days) (Table 1).
Overall, data analysed in this study were obtained from a subset of 13 individuals, out of the 26 tagged blue sharks (50%). The duration of data availability for individuals ranged from 5 to 126 days (Table 1), resulting in a total of 503 days monitored, yielding approximately 72,400 temperature and depth data points. Out of the 13 tagged blue sharks without valid data: two individuals were deceased (in accordance with the tag features, the tag releases from the fish before the D-time if the depth registered is the same for two consecutive days); five individuals did not report (no signal was registered from the tag); two individuals’ transmission of data failed (only the tag position was transmitted); two individuals’ data was incomplete (< 10% of the full data recorded); and two individuals’ data was excluded because of potential fishing of the shark or likely data transmission failure (Table 1).
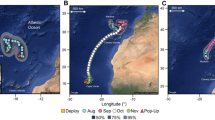
The 13 blue sharks with functional tags exhibited a total length range of 128 cm to 210 cm and consisted of nine females and four males (Table 1). Most sharks captured and released in the southern Adriatic Sea remained in this area until the tag release, corresponding to the end of the monitoring period (Fig. 2). Seven of these sharks swam into deeper Ionian waters, one remained in shallower waters (< 200 m) in the northern part of the Southern Adriatic, and the remaining sharks stayed around the deeper waters of the Bari Pit (Fig. 2).
Depth distribution
Dive behaviour was recorded in detail (10-min interval) during the last five days of the tagging period for nine of the 13 blue sharks (sPAT id tag 67857, id tag 67858, id tag 201522, id tag 201523, id tag 201524, id tag 201527, id tag 201528, id tag 205162, id tag 205163) (Fig. 3). For the remaining four sharks (id tag 205127, id tag 205128, id tag 205129, id tag 215758), diving data were successfully transmitted in high detail for a period ranging from 93 (id tag 205129) to 126 days (id tag 205128) (Table 1, Fig. 4). During these recorded periods, the sharks overall performed diel vertical movements—surfacing or remaining in shallower waters at night and diving to depths of up to 1188 m during the day (Fig. 3). These diel vertical movements are clearly visible in Fig. 3 for fish with five days of monitoring (e.g., id 201522, id 201523, id 201528; Fig. 3).
The depth frequency distribution by time period (night and day) revealed significantly different distribution patterns (K-S p < 0.05; Fig. 5). Blue sharks used greater depths during the daytime and stayed in shallower waters during the night time, with an average day-time depth of 268.4 ± 2.1 m and an average night-time depth of 71.6 ± 1.0 m. The 75th percentile of depth frequency distribution at night was 60 m, while during the day, it was 546 m.
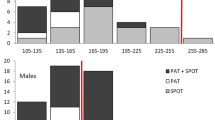
“Regular” (nDVN) was the most abundant behavioural class (eight blue sharks; Fig. 6), followed by surface-oriented (four blue sharks; Fig. 6) and depth-oriented (one blue shark; Fig. 6).
Time proportion spent in different depth classes (m) for each tagged blue shark (n = 13). Depth classes correspond to the following: 0 (surface)—19.9 m, 20–39.9 m, 40–59.9 m, 60–79.9 m, 80–99.9 m, 100–119.9 m, 120–139.9 m, 140–159.9 m, 160–179.9 m, 180–199.9 m, 200 m and lower depths. Depth class bars are represented at the upper value of each range, depth being negative values. Tags coloured in red indicate the sharks with “regular” behavioural class (n = 8), in green indicate the “surface-oriented” class (n = 4) and in blue indicate the “deep-oriented” class (n = 1)
GAM analysis
The covariates tested for collinearity showed no VIF values greater than 3 and none of the Pearson correlation coefficients was greater than 0.5 (Fig. S1), indicating no collinearity. Consequently, none of the covariates were excluded from the model analysis. In the Gaussian GAM model, the splines of both continuous and categorical variables were significant (p < 0.05) (Fig. 7, see also Fig. S2), explaining 94.7% of deviance. Among the current explanatory variables, month showed the best results in terms of Aikake information criterion (dropping month covariate from the final model increased AIC of 12770.04). Although the residuals of all models were not normally distributed (Fig. S2), they were considered sufficiently symmetric with skewness indices falling within the ± 1 range (Hair et al. 2010).
Splines of the covariates estimated by the GAM models affecting diving depth (m) in blue shark (n = 13). A time (hours); B ambient temperature (temp); C current (cur); D salinity; E total individual length of shark (TL); F temporal combination by tensor (ti) of hours (cyclic spline) and month; G combination of longitude (X), latitude (Y); H months as factorial explanatory variables (8: August; 9: September; 10: October; 11: November; 12: December; 1: January)
Dive depth of the sharks varied with time of day, temperature, salinity, month, and size of sharks (TL) (Fig. 7). The shallowest depths were reached between approximately 18:00–4:00, with minimum depths occurring right before the descent in the morning and right after the ascent around sunset, each lasting approximately four hours. Consequently, the highest depths were usually explored between approximately 7:00 and 15:00, peaking at 13:00 (Fig. 7A). The highest ambient temperatures (about 28 °C, Fig. 7B) were observed in surface waters. Depth distribution of blue sharks appeared to be inversely correlated with current (i.e., stronger currents in shallow waters; Fig. 7C). The salinity showed a general positive correlation with water depth of blue shark, with a slight decrease occurring between 38.75 and 39 ‰ (Fig. 7D). The smaller sharks (< 140 cm TL) used to occupy shallower waters than larger sharks (> 180 cm TL) (Fig. 7E). Moreover, the tensor time/month (Fig. 7F) and geographical coordinates (Fig. 7G) significantly influenced the blue shark depth distribution. Based on months, diving behaviour varied between the summer/autumn (August, September, October and November) and winter (December and January), with the shallowest dives occurring during winter (Fig. 7H).
Moon phase effect
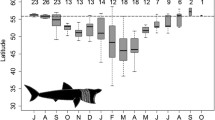
The blue sharks tended to swim at significantly greater depths during or right before the full moon period (Waning gibbous) (p < 0.05; Fig. 8). Blue sharks were at a median depth of 44.92 m during Waning gibbous and 47.33 m during full moon while they were at a median depth comprised between 21.58 and 29.50 m during the rest of the moon cycle.
Boxplot representing the depth (m) of tagged blue sharks (n = 13) during the night according to the moon phase (new, waxing crescent, first quarter, waxing gibbous, full, waning gibbous, last quarter, waning crescent). Different letters indicate significant differences for depth (m) between given moon phases (linear model followed by a post hoc test, p < 0.05)
Discussion
Tag performance
One of the most probable causes of failed or incomplete data transmission affecting four tagged sharks, as well as the failure to report of some tags (five sharks), is low battery charge and satellite coverage. Indeed, if a tag has a low battery charge, it is unable to transmit all the data and/or position during satellite passages, which in the case of low coverage occurs at intervals of several hours or days, or it transmits data only partially before completely running out of battery charge (Musyl et al. 2011). Failure to report may also be attributed to other factors such as recapture, tag destruction by fishers, or malfunction of the tag, all of which could result in a failed or incomplete data transmission. Data obtained for two specific sharks, namely 201525 and 201530, were also excluded from the study. This decision was based on the likelihood of the first shark (201525) being fished, as indicated by a very low depth range corresponding to longline depths before surface records during the last two recording days (Fig. S3). In addiction the tag got released 5 days before D-time. Moreover, the exclusion of data for the second shark (201530) was due to the probable failure of transmission, potentially caused by temporal transmission misalignment, according to the manufacturer's experience. This is supported by almost the same depth being recorded for four out of the five days (Fig. S3). For these two sharks, it was not possible to distinguish between potential unexpected natural behaviour and technical issues that could result in artifact data. Therefore, this data was excluded from the analyses. Despite the loss of data from half of the tagged blue sharks, the final number of individual sharks studied falls within the typical range of individuals examined in the scientific literature (e.g., Campana et al. 2011; Musyl et al. 2011; Queiroz et al. 2012; Vedor et al. 2021).
Vertical movements
Our data indicate that blue shark movements in the Central Mediterranean cover a wide vertical range, reaching as shallow as the surface and diving to depth up of 1188.5 m (e.g., shark 205162, dated 10/10/2020). Similar patterns of depth range have been observed in other ocean regions. In the North Atlantic, for instance, a maximum depth of 1704 m was recorded (Vedor et al. 2021). This is consistent with data from the North-East Atlantic, where depths of 1160 m and 1706 m were reported (Queiroz et al. 2012; 2016), as well as in the North-West Atlantic, where depths of 1000 m were documented (Campana et al. 2011). However, narrower depth ranges were recorded in other regions, such as in South-Eastern Taiwan, Pacific Ocean (up to 422 m; Watanabe et al. 2021), or in the Central Pacific (up to 581 m; Musyl et al. 2011) and the Indian Ocean (up to 445.5 m; Rochman et al. 2021).
The 13 blue sharks with transmitted data exhibited a clear diel pattern in vertical habitat use, staying closer to the surface during the night and in deeper waters during the day. Diel cyclical vertical movements have often been described for sharks associated with coastal and shelf areas, where the diurnal movements of zooplankton trigger a cascade of vertical movements among of predators at higher trophic levels (Shepard et al. 2006; Rodríguez-Cabello et al. 2016). However, to the best of our knowledge, this behaviour has not been previously described in the Mediterranean Sea for a pelagic shark like the blue shark, neither in shelf nor open sea areas. The day/night pattern in movements of Mediterranean blue sharks, described here for the first time, appears to be remarkably consistent and repetitive in both time and location.
The pronounced day/night vertical movement patterns of blue sharks may be related to several factors, among which there is foraging. Blue sharks are opportunistic predators with a wide-ranging diet, including teleosts, cephalopods and pelagic crustaceans, which they target within the water column (Kubodera et al. 2007; Lopez et al. 2010; Markaida and Sosa-Nishizaki 2010). This contrasts with other pelagic fish species, such as bigeye tunas (Thunnus obesus) and ocean sunfish (Mola mola), which exhibit more specialised diets and primarily forage in deeper waters (Nakamura and Sato 2014; Lin et al. 2020).
Interestingly, in our study, the nDVM and surface-oriented behaviour of sharks observed were frequently associated with areas where preys tend to aggregate near the surface, such as upwelling zones (Sims et al. 2006; Campana et al. 2011). Indeed, bathymetric characteristics (Genin 2004; Cotté and Simard 2005) may facilitate the ascent of plankton from the bathyal zone to shallower waters, creating upwelling regions that attract pelagic predators (Rykaczewski and Checkley 2008; Eisele et al. 2021), including sharks (Sims 2003; Ryan et al. 2017). In the Southern Adriatic, a vortex generates water upwelling from its centre, and the water masses in the Southern Adriatic pit contribute to the circulation of deep and nutrient-rich waters throughout the Mediterranean (Vilibić and Orlić 2002). In these areas, sharks optimize their time in shallow layers with high chlorophyll a concentration, where prey densities are expected to be higher (Ainley et al. 2005). Vertical movements, driven by the pursuit of prey, could explain the observed depth-oriented behaviour. Prey aggregations (e.g., squid) at depth have been documented (Clarke et al. 1996; Bianchi et al. 2013; Galván-Magaña et al. 2013), and those can vary with environmental conditions (Cones et al. 2022). Other top predators that feed on similar vertically migrating prey, such as tuna, fin whales and swordfish, also displayed consistent cyclical patterns of tracking deep water, likely to maximise foraging success (Schaefer et al. 2009; Dewar et al. 2011; Sepúlveda et al. 2018). Shifts in diel behaviours (e.g., transitioning from regular nDVM to a depth-oriented behaviour) in response to increases in the abundance of deep-water prey, like cephalopods or mesopelagic fish, have been previously observed in blue sharks in the North Atlantic (Campana et al. 2011; Queiroz et al. 2012; Braun et al. 2023) but remain to be investigated for the Mediterranean population.
Another factor that could explain daily vertical migration is the sharks' need for thermoregulation. Despite their ectothermic physiology, blue sharks can dive to great depths, occasionally exceeding 1000 m (Queiroz et al. 2017; 2012; present data), and venture through a wide range of water temperatures (Stevens et al. 2010; Campana et al. 2011; Queiroz et al. 2012; Watanabe et al. 2021), spanning from 13.3 to 28.7 °C in our case (Fig. 8). Watanabe et al. (2021) argued that blue sharks exhibit a distinct thermoregulatory behaviour compared to other pelagic fish (e.g., bigeye tunas, ocean sunfish). Indeed, blue sharks appear to avoid both excessive decreases and increases in muscle temperature by engaging in semi-diel alternating descents and ascents, whereas other species studied to date avoid overcooling by rewarming at shallow depths after the excursions to deep cold waters, with a different pattern than day/night (Watanabe et al. 2021). Therefore, the thermoregulation behaviour of blue sharks seems to be linked to foraging behaviour. The main prey of blue shark are squids and teleost (Markaida and Sosa-Nishizaki 2010) and the predation/feeding activity corresponds to a burst swimming event (Watanabe et al. 2021) that is supported by warm/superficial water. Moreover, the study of Watanabe et al. (2021) reported that the feeding burst-swimming occurred over a wide depth range (5 − 293 m) in superficial water, suggesting that blue sharks maximise prey encounter rates by moving vertically. A semi-diel/diel migration pattern can be observed at a global scale, from plankton to top predators (Longhurst and Harrison 1989; Zhang and Dam 1997), being possibly associated with thermoregulatory movements and/or as an optimisation of foraging (e.g., Sims et al. 2006; Last et al. 2016; Hafker et al. 2017).
The diel movement pattern in blue sharks may additionally create an energetic advantage. Firstly, conserving metabolic expenditure, by spending daytime in deep, cold waters and foraging at night in shallow, warm waters as found in catsharks, may enhance their energetic efficiency (Sims et al. 2006). Secondly, passive gliding behaviour during the descending phases of dives followed by active ascents with negative buoyancy has been found to provide substantial energy savings when compared to continuous horizontal swimming (Watanabe et al. 2019; 2021). Extended gliding behaviour during descents has been reported in white sharks and whale sharks (Gleiss et al. 2011a, 2011b), suggesting a widespread strategy among large-bodied sharks. Furthermore, large sharks, moving near the surface, inevitably generate waves and incur increased drag, which may lead to increased body movements and higher energy consumption, even while fully submerged (Alexander 2003). Additional studies need to be performed to assess if these behaviours apply to Mediterranean blue sharks. Overall, vertical movement patterns are likely linked to a complex strategy of thermoregulation, feeding and energetic expenditure (Watanabe et al. 2019; 2021). After making deep dives, blue sharks exhibit a behaviour where they spend time at the surface until their bodies have time to fully rewarm (Watanabe et al. 2021; Vedor et al. 2021), hunting and feeding at the same time (Watanabe et al. 2021).
Lunar phase influence
The vertical movements of blue sharks were closely linked with the light cycle, with ascents around sunset and descents during sunrise. Similar twilight-induced movements patterns also characterised vertical changes in habitat use of blue sharks in other areas (e.g., Campana et al. 2011; Musyl et al. 2011; Queiroz et al. 2012; Rochman et al. 2021; Vedor et al. 2021; Watanabe et al. 2021). The role of illumination is also highlighted by the significant influence of the lunar cycle on blue shark depth use. Blue sharks exhibited a preference for deeper waters immediately preceding the full moon phase (waning gibbous) and during the full moon, both during the day and night, compared to the rest of the lunar cycle. This finding may contradict initial expectations, as highly mobile pelagic shark species and other apex predators are assumed to benefit from increased light intensity and duration (Midway et al. 2019). However, blue sharks are thought to be adapted to low light, their visual abilities enabling them to hunt at night (Sciarrotta and Nelson 1977). Indeed, due to its diel vertical migration patterns, blue shark is able to navigate in the water column from the surface to more than 1000 m in a short time, which exposes the eye to big changes in light intensities (Cohen 1990; Collin 2018).
In fact, our data align with previous findings from the open ocean (North Atlantic, Pacific), indicating a deeper distribution of blue sharks associated with the full moon (Campana et al. 2011; Vedor et al. 2021; Elliott et al. 2022). The influence of lunar phases on vertical movement has been documented in several oceanic fish species (e.g., Poisson et al. 2010; Afonso et al. 2014), including target species of fisheries in which blue sharks are incidentally caught, such as swordfish and bluefin tuna (Campana et al. 2005; Abascal et al. 2010; Eveson et al. 2018; Carbonara et al. 2023). This lunar effect extends to other elasmobranchs, such as whale sharks (Graham et al. 2006), basking sharks (Shepard et al. 2006), and tiger sharks (Lowry et al. 2007). Beyond an internal light-intensity-induced control of depth use, the presence of blue sharks in deeper waters before and during nightly full moon periods may be related to a prey-predator relationship pattern (Prihartato et al. 2016): planktivorous organisms exhibit a phototactic behaviour during the full moon, avoiding the surface layer due to higher luminosity at night, as a predator avoidance mechanism (Prihartato et al. 2016). As a result, higher trophic level taxa (i.e., zooplankton, fishes like cod (Giske et al. 1990) and cephalopods) are also found in deeper waters during the full moon period, and likely also during waning gibbous, in contrast to the dark new moon period night (Lerner et al. 2013). Consequently, apex predators like blue sharks or dolphins exploit these deeper waters (Benoit-Bird et al. 2009).
Generalised additive model
The GAM analysis here carried out showed that time of day is a fundamental factor in the vertical migrations of blue sharks (Campana et al. 2011; Musyl et al. 2011; Queiroz et al. 2012; Rochman et al. 2021; Vedor et al. 2021; Watanabe et al. 2021). The diel pattern observed is associated with the temperature structure of the water column in the Mediterranean. Generally, temperatures at depth are relatively stable, varying ± 2 °C from 13 °C throughout the year. However, surface waters are more season-dependent and may heat up to 28 °C. While the monitoring period covered only six months, an avoidance of overheating may explain GAM results showing blue sharks tend to use greater depths in warmer months (i.e., August, September), than colder months (e.g., December, January) (Musyl et al. 2011; Watanabe et al. 2021). In contrast, during winter, when surface waters are cooler, there might be less need to dive to great depths to avoid overheating. As previously mentioned, diel vertical movements are linked to different factors (e.g., Sims et al. 2006; Last et al. 2016; Hafker et al. 2017; Braun et al. 2023) and seen across trophic levels (Longhurst and Harrison 1989; Zhang and Dam 1997; Braun et al. 2023), including deep-sea communities (Aguzzi et al. 2010; 2018). Such movements have been observed in deep-sea communities at depths exceeding 1000 m, influenced by the strength and direction of deep-water currents (Uiblein et al. 2002; Trenkel et al. 2004; Lorance and Trenkel 2006), temperature fluctuations, and salinity changes (Ratsimandresy et al. 2017). Similarly, the results here reported indicated a general positive correlation between salinity and the depth of blue sharks in the Mediterranean. In the Southern Atlantic, salinity appears to be one of the parameters influencing the distribution of blue sharks (Rondon-Medicci et al. 2023), similarly to what was found in other regions for other shark species (Heupel and Simpfendorfer 2008; Ubeda et al. 2009). This correlation could be explained by the fact that most sharks are stenohaline and that individuals may move across depths to seek for optimal salinity ranges in order to lower energetic costs of osmoregulation (Schlaff et al. 2014). This effect may also be indirectly linked to the productivity of areas with higher salinity (Carvalho et al. 2011). The results of the GAM analysis also revealed that blue sharks encounter weaker water currents at greater depths, while in shallower waters, they experience stronger currents. This behaviour could be linked to more intense swimming activity at the surface (Watanabe et al. 2011), potentially linked to predation (see the previous section).
The spline analysis between size and depth revealed a positive trend in blue sharks up to approximately 170 cm in size, after which the trend became relatively flat. Blue sharks, lacking a swim bladder and being negatively buoyant, expend swimming energy to descend/ascend from a particular depth. Due to the limited hydraulic lift relative to their body size, smaller blue sharks may face challenges in reaching greater depths (Rochman et al. 2021). In various regions, including the Atlantic (Fitzmaurice et al. 2005; Carvalho et al. 2011; Coelho et al. 2018) and the Indian (Coelho et al. 2018) oceans, blue sharks exhibit size-based segregation. In the Atlantic, juveniles tend to aggregate in more coastal areas while adults can frequent both offshore and inshore regions (Coelho et al. 2018). The northernmost part of the study area is characterized by shallow waters and high productivity (Maiorano et al. 2019; STECF 2019), which may influence juveniles to remain in the Southern Adriatic, while adult specimens migrate from this area. Similar cyclic migrations linked to reproduction (Kohler et al. 2002; Hazin et al. 1994) and feeding activities (Carey et al. 1990) have been observed in adult blue sharks in the Atlantic (Campana et al. 2011; Coelho et al. 2018; Queiroz et al. 2012; Carvalho et al. 2011), Indian (Coelho et al. 2018), and Pacific (Kai and Fujinami 2020) oceans. While it is plausible to hypothesize a similar behaviour for blue sharks in the Mediterranean sea, confirming spatial segregation between juveniles and adults and the presence of migration patterns requires additional spatial and temporal data from tagged animals to establish a more extensive space/time database.
Implications for by-catch mitigation
In the Southern Adriatic, blue sharks are frequently captured as bycatch in longline fishing operations targeting swordfish (Carbonara et al. 2023). In general, in fisheries, the full moon period, characterised by brighter nights, is traditionally considered a favourable time to catch swordfish (DeBruyn and Meeuwig 2001; Poisson et al. 2010; Ceyhan et al. 2018). The observed behaviour of blue sharks, staying at deeper depths before and during the full moon period, presents an opportunity as an effective mitigation measure. The surface longline fishing method employed in the southern Adriatic operates at shallower depths (approx. 30 m; Carbonara et al. 2013) than those frequented by blue sharks during the well-lit nights. Successful bycatch mitigation measures generally aim to minimize bycatch while preserving the catch of targeted species, such as swordfish in this case. This finding supports the idea that concentrating fishing efforts immediately before and during the full moon period in near-surface layers could have a substantial impact on reducing blue sharks’ bycatch (Orbesen et al. 2017). However, eventual consequences on other top-predators bycatch (e.g., rays, turtles) should be investigated since blue shark is not the only species a risk in the area investigated (Carbonara et al. 2023).
In addition, the pelagic longline setup typically consists of a mainline, which can extend up to 40 km in length. The mainline is suspended in the water column, with baited hooks attached to droplines at intervals of about 60 m. The longline is lowered/recovered in the sea following a timing that allows fishing, mostly during the night hours (Carbonara et al. 2023). Based on the findings of our study, it is apparent that blue sharks predominantly inhabit near-surface waters of the southern Adriatic during the night time, particularly within the first 60 m. This overlap between the longline fishing effort and the vertical habitat of blue sharks occurs both spatially and temporally, contributing to explain high by-catch rate for this species. In order to minimize the capture of blue sharks, it may be beneficial to consider alternative timing for setting and retrieving the longline. These mitigation measures should, however, not go in detriment of the economic performance of the activity and has to be discussed with fishers. The study highlighted how direct fisher engagement can be fundamental in the experimentation and application of novel fishing techniques, stressing once again how concrete mitigation measure for blue sharks must be based on a concrete fishers’ participation in the management of the marine biodiversity by a bottom-up processes, as recommended by the EU Marine Strategy Framework Directive (Directive 2008/56/EC) and by the General Fishery Commission for the Mediterranean Sea (Recommendation GFCM/42/2018/2, Regulation (EU) 2015/2102).
Overall, the introduction of novel management measures, such as the adjustment of the fishing schedule to periods when blue sharks show a lower catchability, could be effective to reduce blue sharks unintended capture and prevent their decline in the Mediterranean Sea (Tolotti et al. 2015). This approach, focusing on conservation-oriented habitat targets for blue sharks, may also be applicable to other pelagic shark species (Vedor et al. 2021; Queiroz et al. 2012), especially in a controversial maritime scenario like the Mediterranean Sea (Katsanevakis et al. 2015).
Data availability
Datasets are available from the corresponding author on reasonable request.
Change history
22 August 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11160-024-09886-8
References
Abascal FJ, Mejuto J, Quintans M, Ramos-Cartelle A (2010) Horizontal and vertical movements of swordfish in the Southeast Pacific. ICES J Mar Sci 67:466–474. https://doi.org/10.1093/icesjms/fsp252
AFMA (2014) Australian Fisheries Management Authority submission for reassessment of the Eastern Tuna and Billfish Fishery 2014. Australian Government, Canberra
Afonso P, McGinty N, Graça G, Fontes J, Inácio M, Totland A, Menezes G (2014) Vertical migrations of a deep-sea fish and its prey. PLoS ONE 9:e97884. https://doi.org/10.1371/journal.pone.0097884
Aguzzi J, Costa C, Furushima Y, Chiesa JJ, Menesatti P, Iwase R, Fujiwara Y (2010) Behavioral rhythms of hydrocarbon seep fauna in relation to internal tides. Mar Ecol Prog Ser 418:47–56. https://doi.org/10.3354/meps08835
Aguzzi J, Fanelli E, Ciuffardi T, Schirone A, De Leo FC, Doya C, Kawato M, Miyazaki M, Furushima Y, Costa C, Fujiwara Y (2018) Faunal activity rhythms influencing early community succession of an implanted whale carcass offshore Sagami Bay. Jpn Sci Rep 8:11163. https://doi.org/10.1038/s41598-018-29431-5
Ainley DG, Spear LB, Tynan CT, Barth JA, Pierce SD, Ford RG, Cowles T (2005) Physical and biological variables affecting seabird distributions during the upwelling season of the northern California Current. Deep Sea Res. II Top Stud Oceanogr 52:123–143. https://doi.org/10.1016/j.dsr2.2004.08.016
Alexander RM (2003) Principles of animal locomotion. Princeton University Press
Andrzejaczek S, Lucas TCD, Goodman MC et al (2022) Diving into the vertical dimension of elasmobranch movement ecology. Sci Adv. https://doi.org/10.1126/sciadv.abo1754
Artegiani A, Bregant D, Paschini E, Pinardi N, Raicich F, Russo A (1997) The Adriatic Sea general circulation. Part I: air-sea interactions and water mass structure. J Phys Oceanogr 27:1492–1514. https://doi.org/10.1175/1520-0485(1997)027%3c1492:TASGCP%3e2.0.CO;2
Bandara K, Varpe Ø, Wijewardene L, Tverberg V, Eiane K (2021) Two hundred years of zooplankton vertical migration research. Biol Rev 96:1547–1589. https://doi.org/10.1111/brv.12715
Benoît HP, Hurlbut T, Chassé J (2010) Assessing the factors influencing discard mortality of demersal fishes using a semi-quantitative indicator of survival potential. Fish Res 106:436–447. https://doi.org/10.1016/j.fishres.2010.09.018
Benoit-Bird KJ, Au WW, Wisdoma DW (2009) Nocturnal light and lunar cycle effects on diel migration of micronekton. Limnol Oceanogr 54:1789–1800. https://doi.org/10.4319/lo.2009.54.5.1789
Bianchi D, Stock C, Galbraith ED, Sarmiento JL (2013) Diel vertical migration: ecological controls and impacts on the biological pump in a one-dimensional ocean model. Glob Biogeochem Cycles 27:478–491. https://doi.org/10.1002/gbc.20031
Bradai MN, Saidi B, Enajjar S (2012) Elasmobranchs of the Mediterranean and Black Sea: status, ecology and biology bibliographic analysis. Studies and Reviews—General Fisheries Commission for the Mediterranean No. 91
Bradai MN, Saidi B, Enajjar S (2018) Chapter 10. Overview on Mediterranean Shark’s fisheries: impact on the biodiversity. In: Türkoglu M, Önal U, Ismen A (eds) Marine ecology: biotic and abiotic interactions, pp 211–230. https://doi.org/10.5772/intechopen.74923
Braun CD, Della Penna A, Arostegui MC, Thorrold SR (2023) Linking vertical movements of large pelagic predators with distribution patterns of biomass in the open ocean. PNAS 120(47):e2306357120. https://doi.org/10.1073/pnas.2306357120
Bruce BD, Bradford RW (2013) The effects of shark cage-diving operations on the behaviour and movements of white sharks, Carcharodon carcharias, at the Neptune Islands, South Australia. Mar Biol 160:889–907. https://doi.org/10.1007/s00227-012-2142-z
Campana SE, Marks L, Joyce W, Kohler N (2005) Catch, by-catch and indices of population status of blue shark (Prionace glauca) in the Canadian Atlantic. Col Vol Sci Pap ICCAT 58:891–934
Campana SE, Dorey A, Fowler M, Joyce W, Wang Z, Wright D, Yashayaev I (2011) Migration pathways, behavioural thermoregulation and overwintering grounds of blue sharks in the Northwest Atlantic. PLoS ONE 6:e16854. https://doi.org/10.1371/journal.pone.0016854
Campbell LM, Cornwell ML (2008) Human dimensions of bycatch reduction technology: current assumptions and directions for future research. Endanger Spec Res 5:325–334. https://doi.org/10.3354/esr00172
Carbonara P, Prato G, Niedermüller S, Alfonso S, Neglia C, Donnaloia M, Lembo G, Spedicato MT (2023) Mitigating effects on target and by-catch species fished by drifting longlines using circle hooks in the South Adriatic Sea (Central Mediterranean). Front Mar Sci 10:1124093. https://doi.org/10.3389/fmars.2023.1124093
Carey FG, Scharold JV, Kalmijn AJ (1990) Movements of blue sharks (Prionace glauca) in depth and course. Mar Biol 106:329–342. https://doi.org/10.1007/BF01344309
Carvalho FC, Murie DJ, Hazin FH, Hazin HG, Leite-Mourato B, Burgess G (2011) Spatial predictions of blue shark (Prionace glauca) catch rate and catch probability of juveniles in the Southwest Atlantic. ICES J Mar Sci 68:890–900. https://doi.org/10.1093/icesjms/fsr047
Cavanagh RD, Gibson C (2007) Overview of the conservation status of cartilaginous fishes (Chondrichthyans) in the Mediterranean Sea. IUCN, Gland, Switzerland and Malaga, Spain
Ceyhan T, Tserpes G, Akyol O, Peristeraki P (2018) The effect of the lunar phase on the catch per unit effort (CPUE) of the Turkish swordfish longline fishery in the eastern Mediterranean Sea. Acta Ichthyol Piscat 48:213–219. https://doi.org/10.3750/AIEP/02431
Clarke MR, Clarke DC, Martins HR, Silva HM (1996) The diet of the blue shark (Prionace glauca L.) in Azorean waters. Arquipel Cienc Biol Mar 14:41–56
Coelho R, Mejuto J, Domingo A, Yokawa K, Liu KM, Cortés E, Romanov EV, da Silva C, Hazin F, Arocha F, Mwilima AM, Bach P, Ortiz de Zárate V, Roche W, Lino PG, García-Cortés B, Ramos-Cartelle AM, Forselledo R, Mas F, Ohshimo S, Courtney D, Sabarros PS, Perez B, Wogerbauer C, Tsai W-P, Carvalho F, Santos MN (2018) Distribution patterns and population structure of the blue shark (Prionace glauca) in the Atlantic and Indian Oceans. Fish Fish 19:90–106. https://doi.org/10.1111/faf.12238
Cohen JL (1990) Vision in elasmobranchs. In: Douglas RH, Djamgoz MBA (eds) The visual system of fish. Chapman and Hall, London, pp 465–490
Collin SP (2018) Scene through the eyes of an apex predator: a comparative analysis of the shark visual system. Clin Exp Ophthalmol 101:624–640. https://doi.org/10.1111/cxo.12823
Compagno LJV (1984) Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Part 1— Hexanchiformes to Lamniformes. FAO Fisheries Synopsis, 125
Cones SF, Jézéquel Y, Ferguson S, Aoki N, Mooney TA (2022) Pile driving noise induces transient gait disruptions in the longfin squid (Doryteuthis pealeii). Front Mar Sci 9:1070290. https://doi.org/10.3389/fmars.2022.1070290
Córdova-Zavaleta F, Mendo J, Briones-Hernández SA, Acuna-Perales N, Gonzalez-Pestana A, Mangel JC (2018) Food habits of the blue shark, Prionace glauca (Linnaeus, 1758), in waters off northern Peru. Fish Bull 116:310–322
Cotté C, Simard Y (2005) Formation of dense krill patches under tidal forcing at whale feeding hot spots in the St. Lawrence. Estuary Mar Ecol Prog Ser 288:199–210. https://doi.org/10.3354/meps288199
Dapp DR, Huveneers C, Walker TI, Drew M, Reina RD (2016) Moving from measuring to predicting bycatch mortality: Predicting the capture condition of a longline-caught pelagic shark. Front Mar Sci 2:126. https://doi.org/10.3389/fmars.2015.00126
DeBruyn AMH, Meeuwig JJ (2001) Detecting lunar cycles in marine ecology: periodic regression versus categorical ANOVA. Mar Ecol Prog Ser 214:307–310
Dent F, Clarke S (2015) State of the global market for shark products. FAO Fisheries and Aquaculture Technical Paper No. 590. FAO, Rome, p 187
Dewar H, Prince ED, Musyl MK, Brill RW, Sepulveda C, Luo J, Foley D, Orbesen ES, Domeier ML, Nasby-Lucas N, Snodgrass D, Laurs RM, Hoolihan JP, Block BA, McNaughton LM (2011) Movements and behaviors of swordfish in the Atlantic and Pacific Oceans examined using pop-up satellite archival tags. Fish Oceanogr 20:219–241. https://doi.org/10.1111/j.1365-2419.2011.00581.x
Druon J-N, Campana S, Vandeperre F, Hazin FHV, Bowlby H, Coelho R, Queiroz N, Serena F, Abascal F, Damalas D, Musyl M, Lopez J, Block B, Afonso P, Dewar H, Sabarros PS, Finucci B, Zanzi A, Bach P, Senina I, Garibaldi F, Sims DW, Navarro J, Cermeño P, Leone A, Diez G, Zapiain MTC, Deflorio M, Romanov EV, Jung A, Lapinski M, Francis MP, Hazin H, Travassos P (2022) Global-scale environmental Niche and Habitat of Blue Shark (Prionace glauca) by size and sex: a pivotal step to improving stock management. Front Mar Sci 9:828412. https://doi.org/10.3389/fmars.2022.828412
Dulvy NK, Fowler SL, Musick JA, Cavanagh RD, Kyne PM, Harrison LR, Carlson JK, Davidson LN, Fordham SV, Francis MP, Pollock CM, Simpfendorfer CA, Burgess GH, Carpenter KE, Compagno LJ, Ebert DA, Gibson C, Heupel MR, Livingstone SR, Sanciangco JC, Stevens JD, Valenti S, White WT (2014) Extinction risk and conservation of the world’s sharks and rays. Elife 3:1–34. https://doi.org/10.7554/eLife.00590
Dulvy NK, Allen DJ, Ralph GM, Walls RHL (2016) The conservation status of sharks, rays and chimaeras in the Mediterranean Sea. Malaga, Spain
Dulvy NK, Simpfendorfer CA, Davidson LN, Fordham SV, Bräutigam A, Sant G, Welch DJ (2017) Challenges and priorities in shark and ray conservation. Curr Biol 27(11):R565–R572. https://doi.org/10.1016/j.cub.2017.04.038
Eisele MH, Madrigal-Mora S, Espinoza M (2021) Drivers of reef fish assemblages in an upwelling region from the Eastern Tropical Pacific Ocean. J Fish Biol 98:1074–1090. https://doi.org/10.1111/jfb.14639
Elliott RG, Montgomery JC, Della Penna A, Radford CA (2022) Satellite tags describe movement and diving behaviour of blue sharks Prionace glauca in the southwest Pacific. Mar Ecol Prog Ser 689:77–94. https://doi.org/10.3354/meps14037
Eriksson H, Clarke S (2015) Chinese market responses to overexploitation of sharks and sea cucumbers. Biol Conserv 184:163–173. https://doi.org/10.1016/j.biocon.2015.01.018
Eveson JP, Patterson TA, Hartog JR, Evans K (2018) Modelling surfacing behaviour of southern bluefin tuna in the Great Australian Bight. Deep-Sea Res II. Top Stud Oceanogr 157:179–189. https://doi.org/10.1016/j.dsr2.2018.03.007
FAO (2000) Fisheries management. 1. Conservation and management of sharks. No. 4, Suppl. 1. Technical Guidelines for Responsible Fisheries. FAO, Rome
FAO (2016) The state of Mediterranean and black Sea fisheries. General Fisheries Commission for the Mediterranean), Rome
FAO (2022) The state of world fisheries and aquaculture 2022. Towards blue transformation. FAO, Rome
FAO and ACCOBAMS (2018) Good practice guide for the handling of sharks and rays caught incidentally in Mediterranean pelagic longline fisheries. FAO, Rome
Fitzmaurice P, Green P, Keirse G, Kenny M, Clarke M (2005) Stock discrimination of the blue shark, based on Irish tagging data. Col Vol Sci Pap ICCAT 58:1171–1178
Galván-Magaña F, Polo-Silva C, Hernández-Aguilar SB, Sandoval-Londoño A, Ochoa-Díaz MR, Aguilar-Castro N, Castañeda-Suárez D, Chavez-Costa AC, Baigorrí-Santacruz Á, Torres-Rojas YE, Abitia-Cárdenas LA (2013) Shark predation on cephalopods in the Mexican and Ecuadorian Pacific Ocean. Deep-Sea Res. II. Top Stud Oceanogr 95:52–62. https://doi.org/10.1016/j.dsr2.2013.04.002
Genin A (2004) Bio-physical coupling in the formation of zooplankton and fish aggregations over abrupt topographies. J Mar Syst 50:3–20. https://doi.org/10.1016/j.jmarsys.2003.10.008
GFCM (2012) Recommendation GFCM/36/2012/3 on fisheries management measures for conservation of sharks and rays in the GFCM area
Gilman E, Clarke S, Brothers N, Alfaro-Shigueto J, Mandelmann J, Mangel J, Petersen S, Piovano S, Thomson N, Dalzell P, Donoso M, Goren M, Werner T (2007) Shark depredation and unwanted bycatch in pelagic longline fisheries: industry practices and attitudes, and shark avoidance strategies. Western Pacific Regional Fishery Management Council, Honolulu
Gilman E, Chaloupka M, Merrifield M, Malsol ND, Cook C (2016) Standardized catch and survival rates, and effect of a ban on shark retention, Palau pelagic longline fishery. Aquat Conserv Mar Freshw 26:1031–1062. https://doi.org/10.1002/aqc.2599
Giske J, Aksnes DL, Baliño BM, Kaartvedt S, Lie U, Nordeide JT, Salvanes AGV, Wakili SM, Aadnesen A (1990) Vertical distribution and trophic interactions of zooplankton and fish in Masfjorden, Norway. Sarsia 75:65–81. https://doi.org/10.1080/00364827.1990.10413442
Gleiss AC, Jorgensen SJ, Liebsch N, Sala JE, Norman B, Hays GC, Quintana F, Grundy E, Campagna C, Trites AW (2011a) Convergent evolution in locomotory patterns of flying and swimming animals. Nat Commun 2:352. https://doi.org/10.1038/ncomms1350
Gleiss AC, Norman B, Wilson RP (2011b) Moved by that sinking feeling: variable diving geometry underlies movement strategies in whale sharks. Funct Ecol 25:595–607. https://doi.org/10.1111/j.1365-2435.2010.01801.x
Graham RT, Roberts CM, Smart JC (2006) Diving behaviour of whale sharks in relation to a predictable food pulse. J R Soc Interface 3:109–116. https://doi.org/10.1098/rsif.2005.0082
Hafker NS, Meyer B, Last KS, Pond DW, Huppe L, Teschke M (2017) Circadian clock involvement in zooplankton diel vertical migration. Curr Biol 27:2194–2201. https://doi.org/10.1016/j.cub.2017.06.025
Hall MA, Nakano H, Clarke S, Thomas S, Molloy J, Peckham SH, Laudino-Santillán J, Nichols WJ, Gilman E, Cook J, Martin S, Croxall JP, Rivera K, Moreno CA, Hall SJ (2007) Working with fishers to reduce by-catches. In: Kennelly SJ (eds) By-catch reduction in the world’s fisheries. Reviews: methods and technologies in fish biology and fisheries, vol 7. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-6078-6_8
Hays GC (2003) A review of the adaptive significance and ecosystem consequences of zooplankton diel vertical migrations. In: Jones MB, Ingólfsson A, Ólafsson E, Helgason GV, Gunnarsson K, Svavarsson J (eds) Migrations and dispersal of marine organisms. Developments in hydrobiology, vol 174. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-2276-6_18
Hazin FH, Kihara K, Otsuka K, Boeckman CE, Leal EC (1994) Reproduction of the blue shark Prionace glauca in the south-western equatorial Atlantic Ocean. Fish Sci 60:487–491. https://doi.org/10.2331/fishsci.60.487
Henderson AC, Flannery K, Dunne J (2001) Observations on the biology and ecology of the blue shark in the North-east Atlantic. J Fish Biol 58:1347–1358. https://doi.org/10.1111/j.1095-8649.2001.tb02291.x
Heupel MR, Simpfendorfer CA (2008) Movement and distribution of young bull sharks Carcharhinus leucas in a variable estuarine environment. AB 1: 277–289. https://doi.org/10.3354/ab00030
Hughes-Brandl M (2018) _NightDay: Night and Day Boundary Plot Function_. R package version 1.0.1.1. https://CRAN.R-project.org/package=NightDay
Kai M, Fujinami Y (2020) Estimation of mean movement rates for blue sharks in the northwestern Pacific Ocean. Anim Biotelem 8:35. https://doi.org/10.1186/s40317-020-00223-x
Katsanevakis S, Levin N, Coll M, Giakoumi S, Shkedi D, Mackelworth P, Levy R, Velegrakis A, Koutsoubas D, Caric H, Brokovich E, Öztürk B, Kark S (2015) Marine conservation challenges in an era of economic crisis and geopolitical instability: the case of the Mediterranean Sea. Mar Policy 51:31–39. https://doi.org/10.1016/j.marpol.2014.07.013
Kohler NE, Turner PA, Hoey JJ, Natanson LJ, Briggs R (2002) Tag and recapture data for three pelagic shark species: blue shark (Prionace glauca), shortfin mako (Isurus oxyrinchus), and porbeagle (Lamna nasus) in the North Atlantic Ocean. Col Vol Sci Pap ICCAT 54:1231–1260
Kubodera T, Watanabe H, Ichii T (2007) Feeding habits of the blue shark, Prionace glauca, and salmon shark, Lamna ditropis, in the transition region of the Western North Pacific. Rev Fish Biol Fish 17:111–124. https://doi.org/10.1007/s11160-006-9020-z
Last KS, Hobbs L, Berge J, Brierley AS, Cottier F (2016) Moonlight drives ocean-scale mass vertical migration of zooplankton during the Arctic winter. Curr Biol 26:244–251. https://doi.org/10.1016/j.cub.2015.11.038
Lazaridis E (2022) lunar: lunar phase & distance, seasons and other environmental factors (version 0.2-01). Available from CRAN
Lerner JD, Kerstetter DW, Prince ED, Talaue-McManus L, Orbesen ES, Mariano A, Snodgrass D, Thomas GL (2013) Swordfish Vertical Distribution and Habitat Use in Relation to Diel and Lunar Cycles in the Western North Atlantic. Trans Am Fish Soc 142:95–104. https://doi.org/10.1080/00028487.2012.720629
Lin CH, Lin JS, Chen KS, Chen MH, Chen CY, Chang CW (2020) Feeding habits of bigeye tuna (Thunnus obesus) in the western Indian Ocean reveal a size-related shift in its fine-scale piscivorous diet. Front Mar Sci 7:582571. https://doi.org/10.3389/fmars.2020.582571
Loor-Andrade P, Pincay-Espinoza J, Rosas-Luis R (2017) Diet of the blue shark Prionace glauca in the Ecuadorian Pacific Ocean during the years 2013 to 2015. J Appl Ichthyol 33:558–562. https://doi.org/10.1111/jai.13329
Lopez S, Meléndez R, Barría P (2010) Preliminary diet analysis of the blue shark Prionace glauca in the eastern South Pacific. Rev Biol Mar Oceanogr 45:745–749
Lorance P, Trenkel VM (2006) Variability in natural behaviour, and observed reactions to an ROV, by mid-slope fish species. J Exp Mar Biol Ecol 332:106–119. https://doi.org/10.1016/j.jembe.2005.11.007
Lowry M, Williams D, Metti Y (2007) Lunar landings—Relationship between lunar phase and catch rates for an Australian gamefish-tournament fishery. Fish Res 88:15–23. https://doi.org/10.1016/j.fishres.2007.07.011
Lubitz N, Bradley M, Sheaves M, Hammerschlag N, Daly R, Barnett A (2022) The role of context in elucidating drivers of animal movement. Ecol Evol 12:e9128. https://doi.org/10.1002/ece3.9128
Maiorano P, Sabatella RF, Marzocchi BM (2019) Annuario sullo stato delle risorse e sulle strutture produttive dei mari italiani
Midway SR, Wagner T, Burgess GH (2019) Trends in global shark attacks. PLoS ONE 14:e0211049. https://doi.org/10.1371/journal.pone.0211049
Musick JA, Burgess G, Cailliet G, Camhi M, Fordham S (2000) Management of sharks and their relatives (Elasmobranchii). Fisheries 25:9–13. https://doi.org/10.1577/1548-8446(2000)025%3c0009:MOSATR%3e2.0.CO;2
Musyl MK, Brill R, Curran DS, Fragoso NM, McNaughton L, Nielsen A, Kikkawa BS, Moyes CD (2011) Post-release survival, vertical and horizontal movements, and thermal habitats of five species of pelagic sharks in the central Pacific Ocean. Fish Bull 109:341–368
Nakamura I, Sato K (2014) Ontogenetic shift in foraging habit of ocean sunfish Mola mola from dietary and behavioral studies. Mar Biol 161:1263–1273. https://doi.org/10.1007/s00227-014-2416-8
Nakano H, Stevens, JD (2008) The biology and ecology of the blue shark, Prionace Glauca. In: Pitcher TJ, Camhi MD, Pikitch EK, Babcock EA (eds) Sharks of the Open Ocean. https://doi.org/10.1002/9781444302516.ch12
Niedermüller S, Ainsworth G, de Juan S, Garcia R, Ospina-Alvarez A, Pita P, Villasante S (2021) The shark and ray meat network a deep dive into a global affair. WWF MMI, Rome
Orbesen ES, Snodgrass D, Shideler GS, Brown CA, Walter JF (2017) Diurnal patterns in Gulf of Mexico epipelagic predator interactions with pelagic longline gear: implications for target species catch rates and bycatch mitigation. Bull Mar Sci 93:573–589. https://doi.org/10.5343/bms.2016.1008
Otway N (2020) Ultrasound-guided sampling of the lateral abdominal vein in the grey nurse shark (Carcharias taurus, Rafinesque 1810). Vet Med Sci 6:579–586. https://doi.org/10.1002/vms3.272
Poisson F, Gaertner JC, Taquet M, Durbec JP, Bigelow K (2010) Effects of lunar cycle and fishing operations on longline-caught pelagic fish: fishing performance, capture time, and survival of fish. Fish Bull 108:268–281
Poisson F, Vernet AL, Séret B, Dagorn L (2012). Good practices to reduce the mortality of sharks and rays caught incidentally by the tropical tuna purse seiners. EU FP7 project, 210496. Montpellier, 15–18 October 2012
Poisson F, Demarcq H, Coudray S, Bohn J, Camiñas JA, Groul J-M, March D (2024) Movement pathways and habitat use of blue sharks (Prionace glauca) in the Western Mediterranean Sea: distribution in relation to environmental factors, reproductive biology, and conservation issues. Fish Res 270:106900. https://doi.org/10.1016/j.fishres.2023.106900
Prihartato PK, Irigoien X, Genton MG, Kaartvedt S (2016) Global effects of moon phase on nocturnal acoustic scattering layers. Mar Ecol Prog Ser 544:65–75. https://doi.org/10.3354/meps11612
Queiroz N, Humphries NE, Noble LR, Santos AM, Sims DW (2010) Short-term movements and diving behaviour of satellite-tracked blue sharks Prionace glauca in the northeastern Atlantic Ocean. Mar Ecol Prog Ser 406:265–279. https://doi.org/10.3354/meps08500
Queiroz N, Humphries NE, Noble LR, Santos AM, Sims DW (2012) Spatial dynamics and expanded vertical niche of blue sharks in oceanographic fronts reveal habitat targets for conservation. PLoS ONE 7:1–12. https://doi.org/10.1371/journal.pone.0032374
Queiroz N, Humphries NE, Mucientes G, Hammerschlag N, Lima FP, Scales KL, Miller PI, Sousa LL, Seabra R, Sims DW (2016) Ocean-wide tracking of pelagic sharks reveals extent of overlap with longline fishing hotspots. Proc Natl Acad Sci 113:1582–1587. https://doi.org/10.1073/pnas.1510090113
Queiroz N, Vila-Pouca C, Couto A, Southall EJ, Mucientes G, Humphries NE, Sims DW (2017) Convergent foraging tactics of marine predators with different feeding strategies across heterogeneous ocean environments. Front Mar Sci 4:239. https://doi.org/10.3389/fmars.2017.00239
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ratsimandresy A, Hamoutene D, Lang C, MacSween C, Marshall K, Kenny S, Kealey B, Kealey J (2017) Arctic charr (Salvelinus alpinus) distribution in seawater cages in relation to environmental conditions. Can Technol Rep Fish Aquat Sci Fs97–6/3203E-PDF
Rigby CL, Barreto R, Carlson J, Fernando D, Fordham S, Francis MP, Herman K, Jabado RW, Liu KM, Marshall A, Pacoureau N, Romanov E, Sherley RB, Winker H (2019) Prionace glauca. The IUCN Red List of Threatened Species 2019: e.T39381A2915850. https://doi.org/10.2305/IUCN.UK.2019-3.RLTS.T39381A2915850.en. Accessed 20 July 2022
Rochman F, Arnenda GL, Wujdi A, Kurniawan R (2021) Vertical distribution of blue Shark (Prionace glauca) in the Indian Ocean. E3S Web Conf 322:01009. https://doi.org/10.1051/e3sconf/202132201009
Rodríguez-Cabello C, González-Pola C, Sánchez F (2016) Migration and diving behavior of Centrophorus squamosus in the NE Atlantic. Combining electronic tagging and Argo hydrography to infer deep ocean trajectories. Deep Sea Res Part I Oceanogr Res 115:48–62. https://doi.org/10.1016/j.dsr.2016.05.009
Rondon-Medicci M, Cardoso LG, Mourato B, Dalla Rosa L (2023) Blue shark (Prionace glauca) occurrence and relative abundance in the western South Atlantic Ocean influenced by spatiotemporal variability, environmental variables, and oceanographic processes. Mar Environ Res 183:105842. https://doi.org/10.1016/j.marenvres.2022.105842
Ryan JP, Green JR, Espinoza E, Hearn AR (2017) Association of whale sharks (Rhincodon typus) with thermo-biological frontal systems of the eastern tropical Pacific. PLoS ONE 12:e0182599. https://doi.org/10.1371/journal.pone.0182599
Rykaczewski RR, Checkley DM Jr (2008) Influence of ocean winds on the pelagic ecosystem in upwelling regions. PNAS 105:1965–1970. https://doi.org/10.1073/pnas.0711777105
Schaefer KM, Fuller DW, Block BA (2009) Vertical movements and habitat utilization of skipjack (Katsuwonus pelamis), yellowfin (Thunnus albacares), and bigeye (Thunnus obesus) tunas in the equatorial eastern Pacific Ocean, ascertained through archival tag data. In: Nielsen JL, Arrizabalaga H, Fragoso N, Hobday A, Lutcavage M, Sibert J (eds) Tagging and tracking of marine animals with electronic devices. Reviews: methods and technologies in fish biology and fisheries, vol 9. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-9640-2_8
Schlaff AM, Heupel MR, Simpfendorfer CA (2014) Influence of environmental factors on shark and ray movement, behaviour and habitat use: a review. Rev Fish Biol Fish 24:1089–1103. https://doi.org/10.1007/s11160-014-9364-8
Sciarrotta TC, Nelson DR (1977) Diel behavior of the blue shark, Prionace glauca, near Santa Catalina Island, California. Fish Bull 75(3):519–528
Sepúlveda M, Pérez-Álvarez MJ, Santos-Carvallo M, Pavez G, Olavarría C, Moraga R, Zerbini AN (2018) From whaling to whale watching: identifying fin whale critical foraging habitats off the Chilean coast. Aquat Conserv Mar Freshw 28:821–829. https://doi.org/10.1002/aqc.2899
Shepard EL, Ahmed MZ, Southall EJ, Witt MJ, Metcalfe JD, Sims DW (2006) Diel and tidal rhythms in diving behaviour of pelagic sharks identified by signal processing of archival tagging data. Mar Ecol Prog Ser 328:205–213. https://doi.org/10.3354/meps328205
Sims DW (2003) Tractable models for testing theories about natural strategies: foraging behaviour and habitat selection of free-ranging sharks. J Fish Biol 63:53–73. https://doi.org/10.1111/j.1095-8649.2003.00207.x
Sims DW, Wearmouth VJ, Southall EJ, Hill JM, Moore P, Rawlinson K, Hutchinson N, Budd GC, Righton D, Metcalfe JD, Nash JP, Morritt D (2006) Hunt warm, rest cool: bioenergetic strategy underlying diel vertical migration of a benthic shark. J Anim Ecol 75:176–190. https://doi.org/10.1111/j.1365-2656.2005.01033.x
Sims D, Fowler SL, Ferretti F, Stevens J (2016) Prionace glauca. The IUCN Red List of Threatened Species 2016: e.T39381A16553182. Accessed 20 July 2022
STECF (2019) The 2019 annual economic report on the EU Fishing Fleet (STECF 19-06). In: Carvalho N, Keatinge M, Guillen Garcia J (eds) EUR 28359 EN, Publications Office of the European Union, Luxembourg, ISBN 978-92-76-09517-0. https://doi.org/10.2760/911768, JRC117567
Stevens JD, Bradford RW, West GJ (2010) Satellite tagging of blue sharks (Prionace glauca) and other pelagic sharks off eastern Australia: depth behaviour, temperature experience and movements. Mar Biol 157:575–591. https://doi.org/10.1007/s00227-009-1343-6
Strasburg DW (1958) Distribution, abundance, and habits of pelagic sharks in the Central Pacific Ocean. Fish Bull 58:335–361
Tolotti MT, Filmalter JD, Bach P, Travassos P, Seret B, Dagorn L (2015) Banning is not enough: the complexities of oceanic shark management by tuna regional fisheries management organizations. Glob Ecol Conserv 4:1–7. https://doi.org/10.1016/j.gecco.2015.05.003
Trenkel VM, Lorance P, Mahévas S (2004) Do visual transects provide true population density estimates for deepwater fish? ICES. J Mar Sci 61:1050–1056. https://doi.org/10.1016/j.icesjms.2004.06.002
Ubeda AJ, Simpfendorfer CA, Heupel MR (2009) Movements of bonnetheads, Sphyrna tiburo, as a response to salinity change in a Florida estuary. Environ Biol Fish 84:293–303. https://doi.org/10.1007/s10641-008-9436-5
Uiblein F, Lorance P, Latrouite D (2002) Variation in locomotion behaviour in northern cutthroat eel (Synaphobranchus kaupi) on the Bay of Biscay continental slope. Deep Sea Res Part I Oceanogr Res Pap 49:1689–1703. https://doi.org/10.1016/S0967-0637(02)00065-1
Vedor M, Mucientes G, Hernández-Chan S, Rosa R, Humphries N, Sims DW, Queiroz N (2021) Oceanic diel vertical movement patterns of blue sharks vary with water temperature and productivity to change vulnerability to fishing. Front Mar Sci 8:1–16. https://doi.org/10.3389/fmars.2021.688076
Vilibić I, Orlić M (2002) Adriatic water masses, their rates of formation and transport through the Otranto Strait. Deep Sea Res Part I Oceanogr Res Pap 49:1321–1340. https://doi.org/10.1016/S0967-0637(02)00028-6
Ward P, Hindmarsh S (2007) An overview of historical changes in the fishing gear and practices of pelagic longliners, with particular reference to Japan’s Pacific fleet. Rev Fish Biol Fish 17:501–516. https://doi.org/10.1007/s11160-007-9051-0
Watanabe YY, Sato K, Watanuki Y, Takahashi A, Mitani Y, Amano M, Aoki K, Narazaki T, Iwata T, Minamikawa S, Miyazaki N (2011) Scaling of swim speed in breath-hold divers. J Anim Ecol 80:57–68. https://doi.org/10.1111/j.1365-2656.2010.01760.x
Watanabe YY, Payne NL, Semmens JM, Fox A, Huveneers C (2019) Swimming strategies and energetics of endothermic white sharks during foraging. J Exp Biol 222:jeb185603. https://doi.org/10.1242/jeb.185603
Watanabe YY, Nakamura I, Chiang WC (2021) Behavioural thermoregulation linked to foraging in blue sharks. Mar Biol 168:161. https://doi.org/10.1007/s00227-021-03971-3
Wildlife Computer (2007) Location processing (GPE3 & Fastloc GPS®) in the Wildlife Computers Data Portal User Guide. https://static.wildlifecomputers.com/Location-Processing-UserGuide.pdf
Williamson MJ, Tebbs EJ, Dawson TP, Jacoby DMP (2019) Satellite remote sensing in shark and ray ecology, conservation and management. Front Mar Sci 6:1–23. https://doi.org/10.3389/fmars.2019.00135
Wood SN (2006) Generalized additive models: an introduction with R. Chapman and Hall, CRC, London
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Ser B Stat Methodol 73:3–36. https://doi.org/10.1111/j.1467-9868.2010.00749.x
Wood SN (2017) Generalized additive models: an introduction with R (2nd edition). Chapman and Hall/CRC
WWF (2022) IL PROGETTO SafeSharks. https://www.wwf.it/cosa-facciamo/progetti/large-pelagics/
Acknowledgements
The authors warmly acknowledge the Captain Onofrio Comes and the crew of the Galeone vessel.
Funding
The study was funded by: “Med By-Catch Project” (Understanding Mediterranean Multi-Taxa By-Catch of Vulnerable Species and Testing Mitigation: A Collaborative Approach) and “SafeShark project” supported by MAVA Fondation Pour la Nature, and “Shaping ecosystem based fisheries management (SEAwise) project” (H2020 BG-10-2020: Fisheries in the full ecosystem context; Grant Agreement Number 101000318) supported by EU Commission.
Author information
Authors and Affiliations
Contributions
Conceptualization: PC, GP, SA, UK, SN, WZ; Methodology: PC, GP, SA, MB, UK, CN, SN, WZ; Formal analysis and investigation: PC, TH, CN, LT, WZ; Writing—original draft preparation: PC, TH; Writing—review and editing: PC, GP, SA, MB, TH, UK, CN, SN, LT, WZ; Funding acquisition: GP, SN.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical standards
Animals used in this study were caught as unwanted bycatch from legal commercial fishing operations. The sampling design and handling methods were reviewed and approved by the Committee on the Ethics of Animal Experiments of COISPA (Italian Ministry of Health 17/2022-UT).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Carbonara, P., Prato, G., Alfonso, S. et al. Blue shark vertical movement patterns in the Central Mediterranean: bycatch mitigation windows revealed from pop-up satellite archival tag data. Rev Fish Biol Fisheries (2024). https://doi.org/10.1007/s11160-024-09879-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11160-024-09879-7