Abstract
Worldwide, there is a dramatic decline in freshwater eel populations (Anguilla spp.), resulting in an urgent need to improve eel management and artificial reproduction protocols. Unfortunately, eels in captivity do not reproduce spontaneously as they remain in a (pre)-pubertal state resulting from a strong neural blockage. Eel propagation is possible to some extent by applying extensive, expensive, unnatural hormonal treatments. However, the success rates are still far too low to support a sustainable farming industry, due to low gamete quality and low survival rates of larvae. Artificial reproduction of eels has been pursued for almost 80 years, and maturation protocols have changed little. In order to improve current protocols it is clear that a different approach towards stimulating sexual maturation is required. In many fish species, changes in external environmental cues, such as photoperiod and temperature, are crucial to induce gonadal recrudescence and development. Still, the natural triggers involved in the gametogenesis of eels are poorly understood. The time-keeping hormone melatonin is a well-known transmitter of external cues, and influences various physiological processes, including reproduction. In eels, we hypothesize that melatonin is an important key player in the regulation of sexual maturation. Thus far, its mode of action is still an area which needs to be explored. In this review, we provide an overview of the current knowledge of studied natural cues possibly affecting reproductive function and the plausible role of melatonin in the regulation of puberty in eels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The enigmatic freshwater eels (Anguilla spp.) are distributed over a large part of the world. After spending most of their life in continental waters, they migrate for hundreds to thousands of kilometers to their spawning area in oceanic waters (Fig. 1, Schmidt 1923; Tesch 2003; Tsukamoto et al. 2003, 2011; van Ginneken and Maes 2005; Aoyama 2009; Tsukamoto 2009). Worldwide, populations of the ecologically and economically valuable anguillids have shown a dramatic decline since the 1980s (Richkus and Whalen 2000; Dekker 2003; Dekker et al. 2003; Stone 2003; Hanel et al. 2014). Several Anguilla species were added to the IUCN Red list as endangered or critically endangered (Jacoby and Gollock 2014; Jacoby et al. 2015), and all species are now considered for inclusion on that list (Dekker and Casselman 2014). Therefore, to restore wild populations and reduce pressure from fisheries, there is an urgent need to improve the management of natural eel stocks (c.f., ICES 2012) and to close their life cycle in captivity. However, eels in captivity do not mature spontaneously and when prevented from their reproductive migration, remain in a (pre)-pubertal state (Dufour et al. 2003; Rousseau et al. 2009).
The life cycle of the European eel. After hatching, presumably in the Sargasso Sea, cylindrical larvae develop into leaf-shaped leptocephalus larvae, which after drifting on the Gulf Stream for approximately 1 year metamorphose into glass eels close to the European coast. The glass eels may stay at the coast or migrate upriver, where they stay as juveniles (elvers and yellow eels) for many years (depending on the region: males 4–6 years, females 8–12 years). Finally, they develop into migrating silver eels; the cause and timing of silvering is not well understood. They mature during or after migration to the spawning grounds. Reproduced and with permission from Henkel et al. (2012a)
Eels can be propagated artificially by applying extensive hormonal treatments, but the success rates remain far too low for uptake of this technology to create a sustainable eel aquaculture industry (see below). In order to improve the currently applied protocols for artificial maturation of eels with respect to gamete quality and welfare, but also with respect to expenses, the hormonal treatments need to be replaced by manipulation of external cues, which are generally used to affect reproduction of many other fish species. This can be achieved by further extending our knowledge and understanding of the reproductive physiology of eels. In this review, we present a holistic overview of the current knowledge about the principal environmental cues that affect the reproductive system of freshwater eels and discuss the possible role of one of the key regulators of onset of puberty, the so-called ‘time-keeping hormone’ melatonin.
Artificial control of reproduction
In captivity, gonadal development in eels does not spontaneously progress beyond the peri-pubertal stage due to dopaminergic inhibition and a deficient secretion of key hormones of the reproductive axis, i.e. gonadotropin-releasing hormone (Gnrh), follicle-stimulating hormone (Fsh) and luteinizing hormone (Lh) (Fig. 2; Dufour et al. 1988, 1993; Vidal et al. 2004; Weltzien et al. 2006, 2009; Rousseau et al. 2013). To some extent, full sexual maturity can be induced artificially by circumventing this strong neural blockage using expensive hormonal treatments, i.e. administering salmon or carp pituitary extracts (SPE or CPE) to females and human chorionic gonadotropin (hCG) to males (e.g. Fontaine 1936; Fontaine et al. 1964; Yamamoto and Yamauchi 1974; Boëtius and Boëtius 1980; Ohta et al. 1996, 1997; Lokman and Young 2000; Tanaka et al. 2001, 2003; Pedersen 2003, 2004; Palstra et al. 2005; Oliveira and Hable 2010; Burgerhout et al. 2011a; Butts et al. 2014, 2016; Okamura et al. 2014; Sørensen et al. 2016). In general, achieving full sexual maturity appears to be easier in male eels than in female eels. A single hCG injection can result in acquisition of sperm (e.g. Miura et al. 1991; Peñaranda et al. 2010), whereas up to 29 weekly injections with pituitary extracts are required to obtain fully mature female eels (e.g. Lokman and Young 2000; Pedersen 2004). Over the last decades, steady progress has been achieved with regard to the reproduction of eels (reviewed by Ijiri et al. 2011; Okamura et al. 2014), but the method of maturing eels using hormonal treatment has changed little, except perhaps with regard to the use of slow-release osmotic pumps to provide constant plasma hormone levels instead of weekly injections in some studies (Kagawa et al. 2009, 2013). The most notable advance during the last decade, however, has arguably been the optimization of spontaneous spawning by artificially matured brood stock—as opposed to strip-spawning approaches—highlighting that mimicking of “natural” cues may well prove effective to artificially propagate the eel in the future.
Schematic representation of the proposed neurohormonal control of puberty in eels. External cues, such as photoperiod, temperature and salinity, regulate the daily synthesis and release of melatonin (MEL), which transmits the external cues on all levels of the brain–pituitary–gonad (BPG) axis. Subsequently, MEL stimulates dopamine (DA) synthesis (Sébert et al. 2008). DA inhibits gonadotropin releasing hormone (GnRH) and synthesis and release of gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which regulate gonadal activity (steroidogenesis and gametogenesis) (Dufour et al. 1988, 1993; Vidal et al. 2004; Weltzien et al. 2006, 2009; Rousseau et al. 2013). Sex steroids (e.g. 17β-estradiol, 11-ketotestosterone, testosterone) exert positive and negative feedbacks on different levels in the BPG axis and liver. It is expected that MEL affects the KiSS/GPR54 system (KiSS) and/or GnRH, directly or indirectly via gonadotropin inhibiting hormone (GnIH), and thereby inducing puberty (Dufour et al. 2010; Migaud et al. 2010; Falcón and Zohar 2018). Decreasing melatonin is expected to result in an inhibition of DA and thereby stimulate sexual maturation
Although the life cycle of the Japanese eel (A. japonica) was recently closed (Masuda et al. 2012), numbers of offspring that reach the glass eel stage have remained extremely low, mainly due to low egg quality and fertilization rates, and high mortality of the larvae (Adachi et al. 2003; Ijiri et al. 2011; Okamura et al. 2014; Izumi et al. 2016). These major problems are most likely caused by the unnatural stimulation of gametogenesis, resulting in, amongst others, handling stress, physiological stress associated with greatly fluctuating hormone levels (Sato et al. 2000, 2003) and possibly, asynchronous oocyte development (Palstra et al. 2005). Additionally, the gonadotropins Fsh and Lh differentially regulate full sexual maturity in eels and are under positive and negative feedback control by sex steroids (Quérat et al. 1991; Suetake et al. 2002, 2003; Schmitz et al. 2005; Jeng et al. 2007; Kazeto et al. 2008). The Lh receptor can only be activated by Lh, while the Fsh receptor was found to be activated by both Fsh and Lh (Kazeto et al. 2008; Minegishi et al. 2012). Besides, Fsh is likely to be the principal gonadotropin during early stages of vitellogenesis in eels, at least in wild-caught midvitellogenic A. dieffenbachii (Saito et al. 2003). As pituitary extracts often contain both Fsh and Lh (e.g. Suetake et al. 2002; Aroua et al. 2005; Schmitz et al. 2005), they may therefore compromise gametogenesis and potentially contribute to the observed low gamete quality. Aside from these concerns about gonadotropin signaling, pituitary extracts also contain many other bioactive compounds (e.g., prolactin, growth hormone, adrenocorticotropin) that conceivably exert their effects during gametogenesis. Moreover, it has already been shown earlier that exposure of teleost fish to stressors negatively affects the rates of reproductive success (e.g. Barton and Iwama 1991; Schreck 2010).
Pre-treatment of oogenesis
There is a wide range in responsiveness to hormonal treatments (Lokman and Young 2000; Pedersen 2003, 2004; Palstra et al. 2005) and often > 50% of female broodstock do not reach full maturity (Palstra and van den Thillart 2009; Burgerhout et al. unpublished data). The differential response to hormonal treatments is at least in part due to differences in the initial stage of oogenesis (Ijiri et al. 1995, 1998; Durif et al. 2006; Okamura et al. 2008; Dirks et al. 2014; Burgerhout et al. 2016). For example, it was shown that wild Japanese eels in the yellow stage do not respond to hormonal treatments, while silver stage 1 and stage 2 eels respond with approximately 80% and 100% efficacy, respectively (Okamura et al. 2008). The response to hormone treatment in farmed eels is, in general, slower and egg quality is lower compared to wild female eels (Ijiri et al. 1995, 1998), also indicating a difference in initial body and/or ovarian composition (e.g. Adachi et al. 2003). Recently, it was found that the response to the hormonal treatment in wild female European eels may be dependent on the transcriptional dynamics of ribosomal genes, with the 5S/18S ratio of non-responding fish corresponding to that of previtellogenic stage fish (Rojo-Bartelomé et al. 2017).
Over the last decade, studies on the effects of broodstock diet on egg and larval quality received increased attention (Furuita et al. 2007, 2009; Heinsbroek et al. 2013; da Silva et al. 2016; Støttrup et al. 2016). Moreover, feminization of eels by administering 17β-estradiol (E2) with the diet during the early growth phase resulted in a better response to the hormone treatments, albeit still below the response shown by wild female anguillids, as shown for Japanese eels (Ijiri et al. 1998; Chai et al. 2010). In addition, pre-treatment with androgens (17-methyltestosterone and 11-ketotestosterone (11-KT)) also has been shown to affect the response to hormonal treatment in wild New Zealand short-finned eels (A. australis), as it resulted in a reduced number of CPE injections needed to reach the pre-ovulatory stage (Lokman et al. 2015). Similarly, androgen co-treatment in European eel females also resulted in a reduction of time to spawning, and further, in increased fertilization, hatching and survival rates (di Biase et al. 2017; Mordenti et al. 2018). It is, therefore, of great importance to elucidate the hormonal mechanisms and the cues initiating silvering in farmed eels.
Natural conditions
Although the artificial control of reproduction of freshwater eels has been pursued for approximately 80 years, it is surprising that the natural cues that may induce vitellogenesis, final oocyte maturation and ovulation in eels are still insufficiently studied. This may be due to the fact that their oceanic life phase is still largely unknown, and information about the environmental conditions encountered during the reproductive migration is scarce.
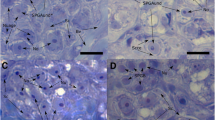
Full sexual maturity needs to occur during or after the spawning migration (Dufour et al. 2003). Recently, it has been proposed that vitellogenesis and final maturation may take place at or near the spawning grounds as swimming exercise suppressed hepatic vitellogenesis in European eels (A. anguilla) (Palstra et al. 2010). It was suggested that this suppression may be a strategy to avoid drag associated with increased abdominal girth and precocious muscle atrophy during long-distance swimming (Palstra et al. 2010). However, several other Anguilla spp., such as the New Zealand long-finned eel A. dieffenbachii and A. celebesensis, show developmental stages up to mid-vitellogenesis (gonadosomatic index (GSI) up to around 10%) prior to the onset of their migration (e.g. Lokman et al. 1998; Hagihara et al. 2012). An advanced stage of gonadal development at the onset of the spawning migration appears to be negatively correlated with the migration distance (Todd 1981a; Dufour et al. 2003). Indeed, vitellogenesis in temperate fish species is a slow process that can comprise several months (Patiño et al. 2001; Wang et al. 2010), which appears to correlate with the average period necessary to artificially mature females by hormone treatments (ca. 2–6 months, e.g. Ohta et al. 1996; Lokman and Young 2000; Tanaka et al. 2001; Palstra et al. 2005; Oliveira and Hable 2010). Additionally, by applying hormonal treatments, mid-vitellogenic stages (even GSI > 10%) can be obtained without an increase in maximum body girth in European eel (Burgerhout, Brittijn, Dirks, van den Thillart, unpublished data), suggesting that up to this stage an increase in drag is most likely not an issue. On the other hand, some silver A. dieffenbachii (GSI unknown, but likely to be in the 5–10% range) clearly show an increased body girth (Fig. 3), which may suggest the difference between the natural situation and the laboratory or may indicate species-specific differences.
Currently, reproduction of many fish species can be controlled by manipulation of environmental conditions, especially photoperiod and temperature (Taranger et al. 2010; Wang et al. 2010). Major barriers at several phases of the reproductive cycle, such as the induction of puberty (e.g. gonadal growth, vitellogenesis), can be expected to be overcome using natural cues.
The melatonin system and reproduction
Melatonin, the time-keeping hormone, is produced by the pineal gland and the retina (Ekström and Meissl 1997). Pineal melatonin shows an increased synthesis and release during the night and corresponding decreases during the day, as well as season-dependent fluctuations, thus providing information about time of day and year (Zachmann et al. 1992a; Falcón et al. 1992, 2007, 2010a, b; Gern et al. 1991; Reiter 1993; Ekström and Meissl 1997; Migaud et al. 2010; Falcón and Zohar 2018; Saha et al. 2018). Melatonin is produced from serotonin via tryptophan and regulated by the rate-limiting enzyme aralkylamine N-acetyltransferase (AANAT, Fig. 4) (Falcón et al. 2010a,b; Falcón and Zohar 2018). In teleost fish there are two AANATs present; the retinal AANAT1 and pineal AANAT2 (Falcón et al. 1996, 2003). Pineal AANAT2 responds to temperature, and therefore is the amplitude of the pineal melatonin production temperature-dependent (Zachmann et al. 1992b; Falcón et al. 2010a,b; Falcón and Zohar 2018; Saha et al. 2018). Melatonin produced by the retina, on the other hand, is not per se dependent on the night; it is catabolized in situ and used for autocrine/paracrine purposes (Falcón and Zohar 2018).
Melatonin has a key role in transmitting external cues (Fig. 2), thereby synchronizing behavioural and physiological processes, such as gonadal development, in all classes of vertebrates (Underwood, 1989; Falcón et al. 1992, 2010a, b; Zachmann et al. 1992a; Mayer et al. 1997; Pandi-Perumal et al. 2006; Li and Zhou 2015; Falcón and Zohar 2018; Saha et al. 2018). Recently, Falcón and Zohar (2018) reviewed in great detail the complex actions of melatonin on all levels within the reproductive system in fish (see particularly Fig. 2 of that review).
Substantial evidence from numerous studies on vertebrates shows that the daily changes in melatonin levels are crucial for the onset of puberty and final oocyte maturation (Malpaux et al. 2001). Melatonin can further affect the secretion of Gnrh and Lh, as well as the synthesis of testosterone (Fig. 2, Falcón et al. 2007, 2010a, b; Migaud et al. 2010; Li and Zhou 2015; Falcón and Zohar 2018). Depending on the timing of the onset of gonadal recruitment (autumn–winter or spring–summer) either an increase or a decrease in melatonin is necessary to activate the reproductive axis (Malpaux et al. 2002; Anand et al. 2007). Moreover, melatonin is hypothesized to stimulate the KiSS/GPR54 system (Migaud et al. 2010), which was shown to be involved in the reproductive cycle acting upstream of GnRH and subsequently on gonadotropins. Kisspeptin is considered an important gatekeeper for puberty in mammals, as well as in fish (Elizur 2009; Zohar et al. 2010; Tena-Sempere et al. 2012), although based on gene knockout studies it was shown that in zebrafish this system is not essential for the development of the reproductive system (Tang et al. 2014). Intriguingly, studies in medaka (Oryzias latipes) showed an important role for kisspeptin during embryonic development, as severe effects on morphology and survival resulted from gene knockdown (Hodne et al. 2013).
Recently, it was shown in zebrafish (Danio rerio) and male European sea bass (Dicentrarchus labrax) that melatonin affects the kiss expression levels (Carnevali et al. 2011; Alvarado et al. 2015). Kisspeptin selectively inhibited lhb expression in primary cultures of eel pituitary cells, while no effect was found regarding fshb and tshb expression. It was suggested that the inhibitory effect of kisspeptin on lhb expression may be part of the species specific pre-pubertal blockage found in eels (Pasquier et al. 2011). However, kisspeptin may have a different effect in vivo due to possible feedback mechanisms, which to date still needs to be elucidated. Gonadotropin inhibiting hormone (Gnih), a neuropeptide that affects the expression of Gnrh, appears also regulated by melatonin (Falcón and Zohar 2018). Currently, there is no data available regarding the function of Gnih and its interaction with melatonin in the reproduction of freshwater eels. Understanding its role within the reproduction of eels will be of great interest for future research.
The melatonin mechanism of action in eels is thus far an unexplored field with only one publication in the context of reproduction by Sébert et al. (2008). In that study, the effect of melatonin on puberty in female European eels was examined. Administration of melatonin resulted in a stimulation of the dopaminergic system, thereby inhibiting the reproductive function as shown by a significant decrease in expression of fshb and lhb in the pituitary and a reduction in levels of sex steroids in plasma. However, administration of melatonin to zebrafish resulted in a positive effect on the reproductive axis, as shown by an increase in GSI, and increases in mRNA levels of, for example, kiss1, kiss2, gnrh3 and lhb (Carnevali et al. 2011). Positive effects of melatonin administration on the reproductive axis were also found in other fish, including masu salmon (Oncorhynchus masou) (Amano et al. 2000), Indian major carp (Labeo rohita/Catla catla) (Chattoraj et al. 2005; Maitra et al. 2005), and mummichog (Fundulus heteroclitus) (Lombardo et al. 2012, 2014). In male European sea bass, on the other hand, a catadromous species like the eel, administration of melatonin resulted in a decrease of expression levels of gnrh1, gnrh3 and gnrh receptors (Servili et al. 2013). Moreover, melatonin administration resulted in suppression of gonadogenesis, and a decrease in serum levels of steroids and gonadotropin (Alvarado et al. 2015). Also, in whole brain of the sapphire devil (Chrysiptera cyanea) the dopaminergic activity was increased after treatment with melatonin (Badruzzaman et al. 2013). The same study showed a decrease in GSI and in the number of vitellogenic oocytes after dietary melatonin administration.
Clearly, the melatonin mechanism with respect to reproduction appears to be species-specific, which may reflect the differences in life cycles and timing of spawning of the species. We therefore hypothesized that in freshwater eels a decrease of melatonin levels may result in the release of the dopaminergic inhibition and thereby stimulate the reproductive axis. Preliminary research based on the recently published draft genome sequences of the European and Japanese eels (Henkel et al. 2012a, b; Jansen et al. 2017) revealed the nucleotide sequences of seven melatonin receptors (Table 1, unpublished data). In addition, sequences of the putative genes within the biosynthetic pathway of melatonin, which is synthesized from tryptophan via four enzymatic steps (Fig. 4), are now available (Table 2, unpublished data).
Environmental factors affecting the melatonin system
Annual cycles of reproduction are often linked to changes in photoperiod and temperature regimes. For many fish species, such as salmonids, percids and cyprinids, the effects of photothermal exposure on the induction of vitellogenesis or final maturation have been extensively studied and used to manipulate the reproductive cycle (Taranger et al. 2010; Wang et al. 2010). In general, photoperiod is assumed to be the principal and temperature the secondary determinant to entrain and synchronize reproductive development in temperate fish (Bromage et al. 2001; Pankhurst and Porter 2003; Migaud et al. 2010).
The onset of silvering and the eel’s reproductive migration in autumn appear to coincide with a decrease in photoperiod and in water temperature, and with an increase of the river’s water discharge, and is found dependent on wind speed and lunar phase (for examples, see Deelder 1954; Todd 1981b; Vøllestad et al. 1994; Haro 2003; Durif et al. 2005; van Ginneken et al. 2007a; Bruijs and Durif 2009; Sandlund et al. 2017; Sudo et al. 2017). European eels mostly migrate between a temperature range of 6 °C to 15 °C, and migration proceeds more quickly when daylight decreases. Japanese eels migrate between temperatures of 10 °C and 20 °C (Sudo et al. 2017). It has further been proposed that decreasing photoperiod and/or a decrease in temperature accelerates the last stages of the silvering process (Vøllestad et al. 1994; Durif et al. 2005; Bruijs and Durif 2009).
During their oceanic migration, eels show notable diel vertical migrations (DVMs) (Jellyman and Tsukamoto 2002, 2005, 2010; Aarestrup et al. 2009; Manabe et al. 2011; Schabetsberger et al. 2013, 2015; Béguer-Pon et al. 2015; Chow et al. 2015; Wysujack et al. 2015; Amilhat et al. 2016), a behaviour also found in other fish species (see e.g. Beamish 1966; Neilson and Perry 1990; Watanabe et al. 1999). Based on the information derived from these field studies it can be deduced that eels clearly exhibit a daily rhythm, and thereby encounter daily fluctuations in temperature, as well as hydrostatic pressure and presumably, photoperiod, oxygen content and salinity.
Photoperiod
During the transition from yellow to silver eel, the eye diameter increases and by switching from freshwater to seawater, opsin the retinal pigment becomes more sensitive to blue wavelengths (Beatty 1975; Pankhurst 1982; Pankhurst and Lythgoe 1983; Archer et al. 1995; Zhang et al. 2000; Durif et al. 2005; Thomson-Laing et al. 2018). Anadromous salmonids also show plasticity of the photoreceptors (Cheng and Flamarique 2004), reflected in a switch in the composition of visual pigments towards red during their reproductive migration from seawater to freshwater (e.g. Beatty 1966; Flamarique 2005). In eels, the switch indicates that they pre-adapt to the photic environments of mesopelagic oceanic waters, which is also reflected in the clear DVM behaviour associated with sunrise and sunset (Jellyman and Tsukamoto 2002, 2005, 2010; Aarestrup et al. 2009; Manabe et al. 2011; Schabetsberger et al. 2013, 2015; Béguer-Pon et al. 2015; Chow et al. 2015; Wysujack et al. 2015; Amilhat et al. 2016). Interestingly, a recent telemetry study showed that there was also a direct impact of the lunar cycle on swimming depths of A. marmorata (Fig. 5); during full moon eels were found at greater depths (ca. 230 m) than during new moon (ca. 170 m) (Schabetsberger et al. 2013). It was shown for other fish species that moonlight can also alter melatonin production and expression of melatonin receptors (e.g. Takemura et al. 2010a; Park et al. 2014). In addition, it was reported that blue wavelengths decrease plasma melatonin levels in European sea bass (Bayarri et al. 2002) and zebrafish (Ziv et al. 2007). Although predator avoidance is supposed to be the primary driver for the DVM (reviewed by Righton et al. 2012), this behaviour may well also be involved in enhancing gonadal development instead of delaying it as suggested by Boëtius and Boëtius (1967).
Diel vertical migration (a) and night-time upper migration depths (b) (daily median: thin blue; spline curve: bold blue) of Anguilla marmorata followed over 3 months using pop-up satellite tags. The daily and monthly/seasonal rhythms (new and full moon) are clearly visible, indicating an effect of photoperiod and or light intensity on the migratory behaviour of eels. In a, solid vertical lines correspond to sunset and sunrise; the dotted line indicates midnight. In b, moon phase (dotted black line), and spectral irradiance at 490 nm (daily Kd estimates: red dots; spline curve: bold red) along a straight line between release and pop-up location (Schabetsberger et al. 2013. With permission)
Through these DVMs, the photoperiod and light intensity eels encounter may change, coinciding with a possible reduction in daily melatonin production, both with respect to peak levels and duration. In Atlantic salmon, for example, additional illumination during night time resulted in a decrease of melatonin levels during the dark phase, resulting in a significantly lower number of fish that matured (Porter et al. 1998). Also, constant illumination or relatively long photoperiods can delay sexual development and ovulation in the likes of Atlantic salmon (Taranger et al. 1998), Atlantic cod (Hansen et al. 2001; Davie et al. 2003, 2007) and European sea bass (Bayarri et al. 2004). It is hypothesized that the inhibition of maturation in Atlantic salmon was mediated through a reduction in the plasma melatonin amplitude below a certain threshold level during the dark phase (Porter et al. 1998). In eels, the opposite to that seen in salmonids may occur, i.e. the onset of vitellogenesis is induced when the amplitude of melatonin plasma levels gets below a certain threshold. An argument for this hypothesis is that salmonids are typically autumn and winter spawners (Pankhurst and King 2010), while eels are considered spring and summer spawners (Schmidt 1923; McCleave 2003; Tsukamoto et al. 2003; Tsukamoto 2009).
The effect of photoperiod on gonadal recruitment in wild female European eels was investigated in combination with hormonal stimuli (Mordenti et al. 2012; Parmeggiani et al. 2015). Briefly, eels were subjected either to ‘light’ 14:10 L:D (400 lx at the bottom of the tank without water) or to continuous ‘dark’ (40 lx at the bottom of the tank without water). For comparison, the light intensity of a full moon on a clear night is < 0.20 lx (e.g. Fraser and Metcalfe 1997), which eels can sense at over 200 m depth (Schabetsberger et al. 2013). It was found that under ‘dark’ conditions, females showed a significantly higher GSI after 16 hormone injections and an increase in the number of eggs released (Mordenti et al. 2012). Parmeggiani and co-workers (2015) showed that eels from the ‘dark’ group displayed higher blood plasma E2 and testosterone levels, and a higher GSI compared to the ‘light’ group. However, based on histology, no differences were found on the stage of gonadal development between the two groups. It is clear that the effects of photoperiod or light intensity, among the best-known cues regulating the reproductive axis in fish, should be investigated in more depth.
Temperature
To entrain and synchronize full sexual maturity, temperature is considered the secondary determinant (Bromage et al. 2001; Pankhurst and Porter 2003; Migaud et al. 2010), and it has been shown that temperature affects melatonin profiles in fish (e.g. Zachmann et al. 1992b; Iigo and Aida 1995; Porter et al. 2001; Vera et al. 2007). The amplitude of the nocturnal surge of melatonin is controlled by temperature through the rate-limiting enzyme in the biosynthetic pathway, aralkylamine N-acetyltransferase (AANAT, Fig. 4) (Falcón et al. 2010a, b; Falcón and Zohar 2018; Saha et al. 2018).
During their oceanic migration, eels encounter daily fluctuations in water temperatures (Aarestrup et al. 2009; Jellyman and Tsukamoto 2002, 2005, 2010; Manabe et al. 2011; Schabetsberger et al. 2013, 2015; Tesch 2003; Chow et al. 2015; Wysujack et al. 2015; Amilhat et al. 2016). For example, during the first ~ 1300 km of their migration, European eels showed a temperature range between 8 and 13 °C, with a daily average of 10.1 °C (Aarestrup et al. 2009). Interestingly, during the migration in the Mediterranean Sea, the temperature did not change despite the DVMs (13.2–13.4 °C; Amilhat et al. 2016). A much wider temperature range was found for the Japanese eel (A. japonica), namely between 4 and 10 °C during the day and 15–24 °C during the night (Manabe et al. 2011). Also, A. marmorata shows a range similar to that of the Japanese eel (Schabetsberger et al. 2013). These species-specific differences should probably be taken into account with respect to the artificial control of reproduction. It was hypothesized that the relatively low temperatures encountered during parts of the oceanic journey may delay gonadal development (Boëtius and Boëtius 1967). However, a so-called cold or chilling period—a period maintaining a temperature below a certain threshold, above which gametogenesis would be impaired—is necessary or suggested to advance vitellogenesis in several fish species, such as striped bass, European sea bass and Atlantic cod (Wang et al. 2010), and was recently also proposed for pike perch (Hermelink et al. 2011, 2013). In eels a similar mechanism may be involved, and as in other fish species, it appears that temperature affects melatonin levels. Sébert et al. (2008) reported that low temperature down-regulates the secretion of melatonin in European eels.
Several studies have addressed the effects of temperature on artificial induction of full sexual maturity in male (Gallego et al. 2012; Baeza et al. 2014) and female eels (Sato et al. 2006; Dou et al. 2008; Pérez et al. 2011; Mazzeo et al. 2014) or they evaluated whether temperature advanced early oogenesis (Zadmajid et al. 2015). It needs to be noted that temperature manipulations were performed in combination with hormonal treatments, which stimulate gonadogenesis unnaturally by circumventing the endogenous regulatory processes. Still, Sato et al. (2006) concluded that in Japanese eels: “… water temperature is an important factor for the artificial induction of ovarian maturation, and an effective temperature for the induction of ovarian development is 20 °C”. As compared to 20 °C, ovarian development was slow at 10 °C, and final maturation and ovulation could not be induced within 13 weeks. However, in the study of Pérez et al. (2011), using female European eels, a temperature regime from 10 to 20 °C accelerated oocyte development until week 8 (Fig. 6). In addition, higher levels of fshb, lhb and estrogen receptor 1 (esr1) mRNA, and of plasma E2 were observed. In male European eels, the best results concerning various sperm parameters, such as volume, density and motility, were obtained at 20 °C, compared to 10 °C or 15 °C. Hence, it was suggested that the optimum temperature to induce maturation in male eels via weekly injections with hCG is around 20 °C (Gallego et al. 2012).
Effect of temperature on vitellogenesis during hormonal treatment in the European eel. A. Water temperature treatments during treatment with carp pituitary extract (CPE) in female silver eels. Arrows indicate the sampling times at weeks 0th, 4th, 8th and 12th. Line indicates the period when ovulations were obtained in T10 group. B. Percentage of the different stages of ovarian development at 0th, 4th, 8th and 12th weeks of CPE treatment (n = 10/week) in each temperature treatment. PV, previtellogenic stage; EV, early vitellogenic stage; MV, mid-vitellogenic stage; LV, late vitellogenic stage (Pérez et al. 2011. With permission)
Simulating change of water temperatures encountered during migration may be of notable importance to stimulate the reproductive axis. By changing temperatures between 5 and 15 °C, one female Japanese eel showed an increase in gonad development (GSI = 8.5%) without hormonal treatment (Mikawa et al. 2008). After repeating this experiment in combination with swimming exercise, no apparent change was found (K. Tsukamoto, pers. comm.). The effects of temperature decrease on the onset of vitellogenesis of farmed female Japanese eels were studied in order to simulate the downstream migration (Sudo et al. 2011a). It was shown that oocyte diameter increased and that they had accumulated oil droplets when the temperature was decreased over a 50-day period from 25 to 15 °C. Although fshb and lhb expression in the pituitary were reduced, blood levels of 11-KT increased. 11-KT can induce previtellogenic oocyte growth and is considered an important factor to stimulate early gonadal development (Lokman et al. 1998, 2007; Rohr et al. 2001; Matsubara et al. 2003; Sudo et al. 2011b). Further, advancement of oogenesis (i.e. vitellogenesis) was not observed. Therefore, it was suggested that other environmental cues participating in the process, such as photoperiod or salinity, are required (Sudo et al. 2011a). However, in temperate fish species, vitellogenesis takes up to several months (Patiño et al. 2001; Wang et al. 2010), suggesting that during a prolonged chilling period the growth of oocytes may have occurred. In addition, there may be a temperature threshold above which gametogenesis is impaired and a threshold below which it is stimulated (Wang et al. 2010).
Environmental factors potentially affecting the melatonin system
In teleost fish, photoperiod and temperature are proposed as the major environmental cues affecting the melatonin system (Zachmann et al. 1992a; Falcón et al. 1992, 2007, 2010a, b; Gern et al. 1991; Reiter 1993; Migaud et al. 2010; Falcón and Zohar 2018). Eels pre-adapt to mesopelagic oceanic life during silvering (van Ginneken and Maes 2005; Tsukamoto 2009). Changes in salinity and hydrostatic pressure could, therefore, also have an effect on the melatonin system and may contribute to stimulating sexual maturation in eels.
Salinity
The effect of changing salinity on gonadogenesis has hardly been studied, most studies instead having been conducted on eels in seawater. One study assessed the effect of salinity on induced maturation in female eels (Kagawa et al. 1998; Kagawa 2003). Briefly, farmed female Japanese eels were subjected to seawater for 1 week or for 1 or 3 months. After 3 months GSI, oocyte diameter and oocyte stage (early vitellogenesis) had increased compared to groups exposed to seawater for 1 week or 1 month. When subjected to hormonal treatment, females held in seawater for 3 months needed a significantly smaller number of weekly injections to complete vitellogenesis than those held in seawater for only 1 week or 1 month. These results indicated that rearing in seawater advanced gonadal development and increased responsiveness to treatment with pituitary extracts in farmed female Japanese eels. In female European eels, plasma E2 concentrations had significantly increased 2 months after seawater transfer (Quérat et al. 1987) and seawater acclimation resulted in an increase in fshb mRNA levels in the pituitary (albeit not significantly) in female Japanese eels (Suetake et al. 2002, 2003).
Interestingly, salinity was seen to affect the production of melatonin and its receptors in European sea bass. Increasing salinity resulted in a significant reduction in melatonin plasma levels during the night. In addition, melatonin receptor densities significantly decreased in the optic tectum and retina (López-Olmeda et al. 2009). In eels, acclimation to seawater triggered early vitellogenesis (Kagawa et al. 1998; Kagawa 2003), which may have been mediated by a reduction in plasma melatonin levels. Clearly, additional studies are needed to clarify the effects of salinity on gonadal development in eels.
Hydrostatic pressure
Eels show DVMs over several hundreds of meters, resulting in strong daily fluctuations in hydrostatic pressures (HP) (Aarestrup et al. 2009; Jellyman and Tsukamoto 2002, 2005, 2010; Manabe et al. 2011; Schabetsberger et al. 2013, 2015; Tesch 2003; Chow et al. 2015; Wysujack et al. 2015). Within approximately an hour, eels are able to descent or ascent between 200 and 500 m (Chow et al. 2015). Similar DVM behaviour within the same depth range has also been observed in, for example, bigeye tunas (Thunnus obesus) (Dagorn et al. 2000). It has been shown that eels are able to withstand HP up to 100 ATA, which is equivalent to the pressure at 1000 m depth (Sébert et al. 1987, 1990; Simon et al. 1992). The effect of HP on vitellogenesis in eels, has to date only been explored in two studies (Fontaine et al. 1985; Sébert et al. 2007). In the first study, caged female European eels were sunk to a depth of 450 m in the Mediterranean Sea for 3 months, which resulted in increased GSI and pituitary LH content (Fontaine et al. 1985). In the second study, females and males were subjected to 101 ATA for respectively 3 and 7 weeks. Females of the HP group showed significantly greater oocyte diameters and higher E2, 11-KT and vitellogenin levels in plasma as compared to the control group. In addition, the lhb/fshb ratio in females of the HP group was significantly higher; fshb expression was lower than in the control group, but not significantly so. Males subjected to HP showed a higher plasma 11-KT level, accompanied by higher pituitary lhb and lower fshb expression; however, these changes were not statistically significant. Based on these results, it was concluded that HP plays a positive role in the onset of gonadogenesis of eels, but other factors are needed for completing sexual maturation (Sébert et al. 2007). Interestingly, in the three-spot wrasse (Halichoeres trimaculatus), it was found that dopaminergic activity was decreased when fish were kept at a depth of 3 m compared to 0 m. Although this wrasse is a tropical species that spawns daily, it shows that HP due to daily tidal changes affects the reproductive axis (Takemura et al. 2010a, b, 2012). Also, in flounder (Platichthys flesus) subjected to daily fluctuating HP, a decrease in dopamine levels was found (Damasceno-Oliveira et al. 2007). Moreover, it was reported that daily fluctuating HP in female flounder resulted in an increase in pituitary FSH protein, correlated with vitellogenic oocyte development (Damasceno-Oliveira et al. 2014). However, a decrease in levels of metabolites of the maturation-inducing hormone 17α,20β-dihydroxy-4-pregnen-3-one (DHP) was also observed. Therefore, it was suggested that HP stimulates ovarian development, but may delay final oocyte maturation (Damasceno-Oliveira et al. 2012). Strong rhythmic, daily changes in HP also occur during the migration of eels, and evaluation of the daily effects of HP on the melatonin system and the reproductive axis would be of great interest.
Combination of environmental factors
Puberty of eels is most likely induced and facilitated by a combination of the previously discussed environmental factors. Thus far, the effects of manipulation of multiple environmental factors on gonadogenesis in eels has received little attention (e.g. Boëtius and Boëtius 1967; Nilsson et al. 1981). Recently, attempts were made to simulate the freshwater downstream migration over 2 weeks and the oceanic migration over 9 weeks (total distance swam of 3792 km) in order to study the combined effects of photothermal regime, salinity and swimming exercise on farmed male and female European eels (Mes et al. 2016). No major changes occurred during this period with regard to gonadogenesis. While photothermal regime and salinity are expected to be involved in inducing gonadogenesis, the effect of swimming exercise remains unclear. When subjected to swimming exercise, a suppression of vitellogenesis was found in wild female European eels (Palstra et al. 2008a, 2010) and no changes in reproduction-related end points, such as GSI and plasma levels of testosterone and Lh, were found in farmed male European eels (Burgerhout et al. 2013a). Only a relatively small study (n = 6 per group) yielded increased GSI and lhb mRNA levels in wild male silver European eels subjected to swimming exercise for 3 months at relatively low speed of ca. 0.12 m s−1 compared to resting eels (Palstra et al. 2008a). The difference in response found between the study of Palstra et al. (2008a) and Burgerhout et al. (2013a) is possibly due to differences in the initial maturity status of the eels at the start of the trials, as reflected in a GSI ≤ 0.3 in the former (Palstra et al. unpublished data) and a GSI ≤ 0.09 in the latter study (Burgerhout et al. 2013a). This once again reinforces the hypothesis that the initial maturation status appears to be of great importance (Durif et al. 2006; Okamura et al. 2008; Dirks et al. 2014; Burgerhout et al. 2016; Rojo-Bartolomé et al. 2017).
In addition, it is expected that migrating eels are actively swimming during their migration and experiments involving swimming exercise are commonly performed against a current. However, the downstream migration of silver eels is found to increase with increased river discharge, indicating the use of water currents (Deelder 1954; Durif et al. 2003; Tesch 2003; see also Bruijs and Durif 2009). Moreover, swimming speeds found for eels equipped with pop-up satellite tags during their oceanic migration (A. anguilla: up to 0.29 m s−1 (Aarestrup et al. 2009), A. dieffenbachii: up to 0.36 m s−1 (Jellyman and Tsukamoto 2002); A. japonica, up to 0.17 m s−1 (Manabe et al. 2011) were lower than the optimal swimming speeds (i.e., the speed with the minimal cost of transport) found under laboratory conditions (A. anguilla: up to 0.68 m s−1 (Palstra et al. 2008b; Burgerhout et al. 2011b, 2013b; Tudorache et al. 2014, 2015); A. australis: up to 0.51 m s−1 (Tudorache et al. 2015)). The differences in swimming speeds observed between eels in the field and in the laboratory could very well be due to the increased drag resulting from the tag (Burgerhout et al. 2011b; Methling et al. 2011; Tudorache et al. 2014). However, it seems equally plausible that eels use oceanic currents during their journey, as suggested by Fricke and Kaese (1995). Although it has been shown that eels are highly efficient swimmers (van Ginneken and van den Thillart 2000; van Ginneken et al. 2005, 2007b; Palstra et al. 2008b; Burgerhout et al. 2013a, b), any reduction in energy use during migration is likely to benefit reproductive success at the spawning grounds.
Summary and future recommendations
In order to improve current protocols of artificial control of propagation of freshwater eels, we suggest that the hormonal treatments need to be replaced by use of simulated environmental conditions. However, the environmental cues inducing full sexual maturity have still been insufficiently studied, which probably reflects the limited knowledge of the natural conditions encountered during the oceanic spawning migration. Moreover, the majority of studies involving the examination of environmental factors are, unfortunately, combined with the hormonal treatments (Pérez et al. 2011; Gallego et al. 2012; Baeza et al. 2014; Mordenti et al. 2012; Parmeggiani et al. 2015).
The major crux for inducing or advancing gonadogenesis in eels is overcoming the inhibition by dopamine, which is rather extreme as compared to other fish species (Vidal et al. 2004; Dufour et al. 2005, 2010). Knowledge of the (neuro)-endocrine pathways that control spontaneous gonadal development is of crucial importance to understand how dopaminergic inhibition may be exerted, and consequently, to override this inhibition. Information on entry into vitellogenesis may be obtained by studying closely related eel species that are far more advanced at the onset of their oceanic migration, such as New Zealand longfinned eels, A. dieffenbachii (Lokman et al. 1998) or the Indonesian A. celebesensis (Hagihara et al. 2012).
Some insights into the dopaminergic inhibition of the eel BPG axis are provided by administration of melatonin to female eels, which resulted in stimulation of the inhibitory tone (Sébert et al. 2008). It is therefore suggested that a decrease of melatonin levels, below a certain threshold, may result in the release of the dopaminergic inhibition and thereby stimulate maturation. Artificially decreasing melatonin levels using an antagonist would be an experiment we would like to recommend to conduct. Further, melatonin levels can most likely be decreased by changes in environmental factors, such as salinity (López-Olmeda et al. 2009), temperature (Zachmann et al. 1992a, b; Porter et al. 2001; Sébert et al. 2008), photoperiod (Taranger et al. 1998; Porter et al. 1998; Hansen et al. 2001; Bayarri et al. 2004), blue light (Bayarri et al. 2002; Ziv et al. 2007) or a combination of multiple parameters. Melatonin is known to be mainly regulated by photoperiod and temperature, and therefore it is highly recommended for future studies to investigate the effects of those two environmental factors on the induction of full sexual maturity of freshwater eels.
Thus far, melatonin and its interaction with the reproductive axis in eels is still a little-explored field. Understanding the effects of melatonin and the daily rhythms of the involved natural factors on puberty will have great potential for creating important new insights into the eel reproduction problem and may prove to be essential for eel aquaculture.
References
Aarestrup K, Økland F, Hansen MM, Righton D, Gargan P, Castonguay M et al (2009) Oceanic spawning migration of the European eel (Anguilla anguilla). Science 325:1660
Adachi S, Ijiri S, Kazeto Y, Yamauchi K (2003) Oogenesis in the Japanese eel, Anguilla japonica. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer, Tokyo, pp 301–317
Alvarado MV, Carrillio M, Felip A (2015) Melatonin-induced changes in kiss/gnrh gene expression patterns in the brain of male sea bass during spermatogenesis. Comp Biochem Physiol A 185:69–79
Amano M, Iigo M, Ikuta K, Kitamura S, Yamada H, Yamamori K (2000) Roles of melatonin in gonadal maturation of underyearling precocious male masu salmon. Gen Comp Endocrinol 120:190–197
Amilhat E, Aarestrup K, Faliex E, Simon G, Westerberg H, Righton D (2016) First evidence of European eels exiting the Mediterranean Sea during their spawning migration. Sci Rep 6:21817
Anand S, Losee-Olson S, Turek FW, Horton T (2007) Differential regulation of luteinizing hormone and follicle-stimulating hormone in male Siberian hamsters by exposure to females and photoperiod. Endocrinology 143:2178–2188
Aoyama J (2009) Life history and evolution of migration in catadromous eels (genus Anguilla). Aqua-BioSci Monogr 2:1–42
Archer S, Hope T, Partridge JC (1995) The molecular basis for the green-blue sensitivity shift in rod visual pigments of the European eel. Proc R Soc Lond B 262:289–295
Aroua S, Schmitz M, Baloche S, Vidal B, Rousseau K, Dufour S (2005) Endocrine evidence that silvering, a secondary metamorphosis in the eel, is a pubertal rather than a metamorphic event. Neurendocrinology 82:221–232
Badruzzaman M, Bapary MAJ, Takemura A (2013) Possible roles of photoperiod and melatonin in reproductive activity via changes in dopaminergic activity in the brain of a tropical damselfish, Chrysiptera cyanea. Gen Comp Endocrinol 194:240–247
Baeza R, Mazzeo I, Vílchez MC, Gallego V, Peñaranda DS, Pérez L et al (2014) Effect of thermal regime on fatty acid dynamics in male European eels (Anguilla anguilla) during hormonally-induced spermatogenesis. Aquaculture 430:86–97
Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis 1:3–26
Bayarri MJ, Madrid JA, Sanchez-Vazquez FJ (2002) Influence of light intensity, spectrum and orientation on sea bass plasma and ocular melatonin. J Pineal Res 32:34–40
Bayarri MJ, Rodríguez L, Zanuy S, Madrid JA, Sanchez-Vazquez FJ, Kagawa H et al (2004) Effect of photoperiod manipulation on the daily rhythms of melatonin and reproductive hormones in caged European sea bass (Dicentrarchus labrax). Gen Comp Endocrinol 136:72–81
Beamish FWH (1966) Vertical migration by demersal fish in the Northwest Atlantic. J Fish Board Can 23:109–139
Beatty DD (1966) A study of the succession of visual pigments in pacific salmon (Oncorhynchus). Can J Zool 44:429–455
Beatty DD (1975) Visual pigments of the American eel Anguilla rostrate. Vis Res 15:771–776
Béguer-Pon M, Castonguay M, Shan S, Benchetrit J, Dodson JJ (2015) Direct observations of American eels migrating across the continental shelf to the Sargasso Sea. Nat Commun 6
Boëtius I, Boëtius J (1967) Studies in the European eel, Anguilla anguilla (L.). Experimental induction of the male sexual cycle, its relation to temperature and other factors. Meddelser fra Danmarks Fiskeri- og Havunderogelser 4:339–405
Boëtius I, Boëtius J (1980) Experimental maturation of female silver eels, Anguilla anguilla. Estimates of fecundity and energy reserves for migration and spawning. Dana 1:1–28
Bromage N, Porter M, Randall C (2001) The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture 197:63–98
Bruijs MCM, Durif CMF (2009) Silver eel migration and behaviour. In: van den Thillart G, Dufour S, Rankin JC (eds) Spawning migration of the European eel, reproduction index, a useful tool for conservation management. Springer, Dordrecht, pp 65–95
Burgerhout E, Brittijn SA, Kurwie T, Decker P, Dirks RP, Palstra AP et al (2011a) First artificial hybrid of the eel species Anguilla australis and Anguilla anguilla. BMC Dev Biol 11:16
Burgerhout E, Manabe R, Brittijn SA, Aoyama J, Tsukamoto K, van den Thillart GEEJM (2011b) Dramatic effect of pop-up satellite tags on eel swimming. Naturwissenschaften 98:631–634
Burgerhout E, Brittijn SA, Tudorache C, de Wijze DL, Dirks RP, van den Thillart GEEJM (2013a) Male European eels are highly efficient long distance swimmers: effects of endurance swimming on maturation. Comp Biochem Physiol A 166:522–527
Burgerhout E, Tudorache C, Brittijn SA, Palstra AP, Dirks RP, van den Thillart GEEJM (2013b) Schooling reduces energy consumption in swimming male European eels, Anguilla anguilla L. J Exp Mar Biol Ecol 448:66–71
Burgerhout E, Minegishi Y, Brittijn SA, de Wijze DL, Henkel CV, Jansen HJ et al (2016) Changes in ovarian gene expression profiles and plasma hormone levels in maturing European eel (Anguilla anguilla); biomarkers for broodstock selection. Gen Comp Endocrinol 225:185–196
Butts IA, Sørensen SR, Politis SN, Pitcher TE, Tomkiewicz J (2014) Standardization of fertilization protocols for the European eel, Anguilla anguilla. Aquaculture 426:9–13
Butts IA, Sørensen SR, Politis SN, Tomkiewicz J (2016) First-feeding by European eel larvae: a step towards closing the life cycle in captivity. Aquaculture 464:451–458
Carnevali O, Gioacchini G, Maradonna F, Olivotto I, Migliarini B (2011) Melatonin induces follicle maturation in Danio rerio. PLoS ONE 6:e19978
Chai Y, Tosaka R, Abe T, Sago K, Sago Y, Hatanaka et al (2010) The relationship between the developmental stage of oocytes in various seasons and the quality of egg obtained by artificial maturation in the feminized Japanese eel Anguilla japonica. Aquac Sci 58:269–278
Chattoraj A, Bhattacharyya S, Basu D, Bhattacharya S, Bhattacharya S, Maitra SK (2005) Melatonin accelerates maturation inducing hormone (MIH): induced oocyte maturation in carps. Gen Comp Endocrinol 140:145–155
Cheng CL, Flamarique IN (2004) Opsin expression: new mechanism for modulating colour vision. Nature 428:279
Chow S, Okazaki M, Watanabe T, Segawa K, Yamamoto T, Kurogi H et al (2015) Light-sensitive vertical migration of the Japanese eel Anguilla japonica revealed by real-time tracking and its utilization for geolocation. PLoS ONE 10:e0121801
da Silva FF, Støttrup JG, Kjørsvik E, Tveiten H, Tomkiewicz J (2016) Interactive effects of dietary composition and hormonal treatment on reproductive development of cultured female European eel, Anguilla anguilla. Animal Reproduction Science 171:17–26
Dagorn L, Bach P, Joss E (2000) Movement patterns of large bigeye tuna (Thunnus obesus) in the open ocean, determined using ultrasonic telemetry. Mar Biol 136:361–371
Damasceno-Oliveira A, Fernández-Durán B, Gonçalves J, Serrão P, Soares-da-Silva P, Reis-Henriques MA et al (2007) Effects of cyclic hydrostatic pressure on the brain biogenic amines concentrations in the flounder, Platichthys flesus. Gen Comp Endocrinol 153:385–389
Damasceno-Oliveira A, Fernández-Durán B, Gonçalves J, Couto E, Canario AV, Coimbra J (2012) Plasma steroid hormone levels in female flounder Platichthys flesus and the influence of fluctuating hydrostatic pressure. Comp Biochem Physiol A 163:272–277
Damasceno-Oliveira A, Levavi-Sivan B, Aizen J, Gonçalves J, Fernández-Durán B, Coimbra J (2014) Pituitary follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels in maturing female flounder Platichthys flesus under hydrostatic pressure simulating vertical migrations. Mar Biol Res 10:85–92
Davie A, Porter MJR, Bromage NR (2003) Photoperiod manipulation of maturation and growth of Atlantic cod (Gadus morhua). Fish Physiol Biochem 28:399–401
Davie A, Porter MJ, Bromage NR, Migaud H (2007) The role of seasonally altering photoperiod in regulating physiology in Atlantic cod (Gadus morhua). Part I. Sexual maturation. Can J Fish Aquat Sci 64:84–97
Deelder CL (1954) Factors affecting the migration of the silver eel in Dutch inland waters. J du Conceil Permanent International Pour l’Exploration de la Mer 20:177–185
Dekker W (2003) Did lack of spawners cause the collapse of the European eel, Anguilla anguilla? Fish Manag Ecol 10:365–376
Dekker W, Casselman JM (2014) The 2003 Québec declaration of concern about eel declines—11 years later: are eels climbing back up the slippery slope? Fisheries 39:613–614
Dekker W, Casselman JM, Cairns DK, Tsukamoto K, Jellyman D, Lickers H (2003) Worldwide decline of eel resources necessitates immediate action. Fisheries 28:28–30
Di Biase A, Lokman PM, Govoni N, Casalini A, Emmanuele P, Parmeggiani A, Mordenti O (2017) Co-treatment with androgens during artificial induction of maturation in female eel, Anguilla anguilla: effects on egg production and early development. Aquaculture 479:508–515
Dirks RP, Burgerhout E, Brittijn SA, de Wijze DL, Ozupek H, Tuinhof-Koelma N et al (2014) Identification of molecular markers in pectoral fin to predict artificial maturation of female European eels (Anguilla anguilla). Gen Comp Endocrinol 204:267–276
Dou S-Z, Yamada Y, Okamura A, Shinoda A, Tanaka S, Tsukamoto K (2008) Temperature influence on the spawning performance of artificially-matured Japanese eel, Anguilla japonica, in captivity. Environ Biol Fishes 82:151–164
Dufour S, Lopez E, Le Menn F, Le Belle N, Baloche S, Fontaine YA (1988) Stimulation of gonadotropin release and of ovarian development, by the administration of a gonadoliberin agonist and of dopamine antagonists, in female silver eel pretreated with estradiol. Gen Comp Endocrinol 70:20–30
Dufour S, Montero M, Le Belle N, Bassompiere M, King JA, Millar RP (1993) Differential distribution and response to experimental maturation of two forms of brain gonadotropin-releasing hormone (GnRH) in the European eel, Anguilla anguilla. Fish Physiol Biochem 11:99–106
Dufour S, Burzawa-Gerard E, Le Belle N, Shaihi M, Vidal B (2003) Reproductive endocrinology of the European eel, Anguilla anguilla. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer, Tokyo, pp 373–383
Dufour S, Weltzien F-A, Sébert M-E, Le Belle N, Vidal B, Vernier P et al (2005) Dopaminergic inhibition of reproduction in teleost fishes—ecophysiological and evolutionary implications. Ann N Y Acad Sci 1040:9–21
Dufour S, Sebert M-E, Weltzien F-A, Rousseau K, Pasqualini C (2010) Neuroendocrine control by dopamine of teleost reproduction. J Fish Biol 76:129–160
Durif C, Elie P, Gosset C, Rives J, Travade F (2003) Behavioural study of downstream migrating eels by radiotelemetry at a small hydroelectric power plant. In: Dixon DA (ed) Biology, management, and protection of catadromous eels. American Fisheries Society, Bethesda, pp 343–356
Durif C, Dufour S, Elie P (2005) The silvering process of Anguilla anguilla: a new classification from the yellow resident to the silver migrating stage. J Fish Biol 66:1025–1043
Durif C, Dufour S, Elie P (2006) Impact of silvering stage, age, body size and condition on reproductive potential of the European eel. Mar Ecol Prog Ser 327:171–181
Ekström P, Meissl H (1997) The pineal organ of teleost fish. Rev Fish Biol Fisheries 7:199–284
Elizur A (2009) The KiSS1/GPR54 system in fish. Peptides 30:164–170
Falcón J, Zohar Y (2018) Photoperiodism in fish. In: Skinner M (ed) Encyclopedia of reproduction, 2nd edn. Academic Press, Oxford, pp 400–408
Falcón J, Thibault C, Begay V, Zachmann A, Collin JP (1992) Regulation of the rhythmic melatonin secretion by fish pineal photoreceptor cells. In: Ali MA (ed) Rhythms in fishes. Plenum Press, New York, pp 167–198
Falcón J, Bolliet V, Collin JP (1996) Partial characterization of serotonin N-acetyltransferases from northern pike (Esox lucius, L.) pineal organ and retina: effects of temperature. Pflügers Archiv 432:386–393
Falcón J, Gothilf Y, Coon SL, Boeuf G, Klein DC (2003) Genetic, temporal and developmental differences between melatonin rhythm generating systems in teleost fish pineal organ and retina. J Neuroendocrinol 15:378–382
Falcón J, Besseau L, Sauzet S, Boeuf G (2007) Melatonin effects on the hypothalamo–pituitary axis in fish. Trends Endocrinol Metab 18:81–88
Falcón J, Migaud H, Muñoz-Cueto JA, Carrillo M (2010a) Current knowledge on the melatonin system in teleost fish. Gen Comp Endocrinol 165:469–482
Falcón J, Besseau L, Manganou E, Sauzet S, Fuentès M, Boeuf G (2010b) The pineal organ of fish. In: Kulczykowska E, Popek W, Kapoor BG (eds) Biological clock in fish. CRC Press, Boca Raton
Flamarique IN (2005) Temporal shifts in visual pigment absorbance in the retina of Pacific salmon. J Comp Physiol A 191:37–49
Fontaine M (1936) Sur la maturation complete des organs génitaux de l’anguille male et l’émission spontanée. Comptes Rendus Hebdomadaires des Séances de l’Academie des Sciences Paris 202:132–134
Fontaine M, Betrand E, Lopez E, Callamand O (1964) Sur la maturation des organs génitaux de l’anguille femelle (Anguilla anguilla L.) et l’émission spontanée des oeufs en aquarium. Comptes Rendus de l’Academie des Sciences 259:2907–2910
Fontaine YA, Dufour S, Alinat J, Fontaine M (1985) L’immersion prolongée en profondeur stimule la fonction hypophysaire de l’Anguille européenne (Anguilla anguilla L.) femelle. Comptes Rendus de l’Academie des Sciences Paris série III 2:83–87
Fraser NHC, Metcalfe NB (1997) The costs of becoming nocturnal: feeding efficiency in relation to light intensity in juvenile Atlantic salmon. Funct Ecol 11:385–391
Fricke H, Kaese R (1995) Tracking of artificially matured eels (Anguilla anguilla) in the Sargasso Sea and the problem of the eel’s spawning site. Naturwissenschaften 82:32–36
Furuita H, Hori K, Sugita T, Yamamoto T (2007) Effect of n-3 and n-6 fatty acids in broodstock diet on reproduction and fatty acid composition of broodstock and eggs in the Japanese eel Anguilla japonica. Aquaculture 267:55–61
Furuita H, Unuma T, Nomura K, Tanaka H, Sugita T, Yamamoto T (2009) Vitamin contents of eggs that produce larvae showing a high survival rate in the Japanese eel Anguilla japonica. Aquac Res 40:1270–1278
Gallego V, Mazzeo I, Vílchez M, Peñaranda DS, Carneiro PCF, Pérez L et al (2012) Study of the effects of thermal regime and alternative hormonal treatments on the reproductive performance of European eel males (Anguilla anguilla) during induced sexual maturation. Aquaculture 354–355:7–16
Gern W, Falcón J, Meissl H, Ali MA (1991) Melatonin synthesis by the teleost pineal: an overview. In: Ali MA (ed) Rhythms in fishes. Plenum Press, New York, pp 219–222
Hagihara S, Aoyama J, Limbong D, Tsukamoto K (2012) Morphological and physiological changes of female tropical eels, Anguilla celebesensis and Anguilla marmorata, in relation to downstream migration. J Fish Biol 81:408–426
Hanel R, Stepputtis D, Bonhommeau S, Castonguay M, Schaber M, Wysujack K et al (2014) Low larval abundance in the Sargasso Sea: new evidence about reduced recruitment of the Atlantic eels. Naturwissenschaften 101:1041–1054
Hansen T, Karlsen Ø, Taranger GL, Hemre G-I, Holm JS, Kjesbu OS (2001) Growth, gonadal development and spawning time of Atlantic cod (Gadus morhua) reared under different photoperiods. Aquaculture 203:51–67
Haro A (2003) Downstream migration of silver-phase anguillid eels. In: Biology Eel (ed) Aida K, Tsukamoto K, Yamauchi K. Springer-Verlag, Tokyo, pp 215–222
Heinsbroek LTN, Støttrup JG, Jacobsen C, Corraze G, Kraiem MM, Holst LK et al (2013) A review on broodstock nutrition of marine pelagic spawners: the curious case of the freshwater eels (Anguilla spp.). Aquac Nutr 19:1–24
Henkel CV, Burgerhout E, de Wijze DL, Dirks RP, Minegishi Y, Jansen HJ et al (2012a) Primitive duplicate Hox clusters in the European eel’s genome. PLoS ONE 7:e32231
Henkel CV, Dirks RP, de Wijze DL, Minegishi Y, Aoyama J, Jansen HJ et al (2012b) First draft genome sequence of the Japanese eel, Anguilla japonica. Gene 511:195–201
Hermelink B, Wuertz S, Trubiroha A, Rennert B, Kloas W, Schulz C (2011) Influence of temperature on puberty and maturation of pikeperch, Sander lucioperca. Gen Comp Endocrinol 172:282–292
Hermelink B, Wuertz S, Rennert B, Kloas W, Schultz C (2013) Temperature control of pikeperch (Sander lucioperca) maturation in recirculating aquaculture systems—induction of puberty and course of gametogenesis. Aquaculture 400–401:36–45
Hodne K, Weltzien FA, Oka Y, Okubo K (2013) Expression and putative function of kisspeptins and their receptors during early development in medaka. Endocrinology 154:3437–3446
ICES (2012) Report of the Joint EIFAAC/ICES Working Group on Eels (WGEEL), 3–9 September 2012, Copenhagen, Denmark. ICES CM 2012/ACOM 18:824
Iigo M, Aida K (1995) Effects of season, temperature, and photoperiod on plasma melatonin rhythms in the goldfish, Carassius auratus. J Pineal Res 18:62–68
Ijiri S, Kazeto Y, Takeda N, Chiba H, Adachi S, Yamauchi K (1995) Changes in serum steroid hormones and steroidogenic ability of ovarian follicles during artificial maturation of cultivated Japanese eel, Anguilla japonica. Aquaculture 135:3–16
Ijiri S, Kayaba T, Takeda N, Tachiki H, Adachi S, Yamauchi K (1998) Pretreatment reproductive stage and oocyte development induced by salmon pituitary homogenate in the Japanese eel Anguilla japonica. Fish Sci 64:531–537
Ijiri S, Tsukamoto K, Chow S, Kurogi H, Adachi S, Tanaka H (2011) Controlled reproduction in the Japanese eel (Anguilla japonica), past and present. Aquaculture Europe 32:13–17
Izumi H, Gen K, Horiuchi M, Matsuya N, Ijiri S, Adachi S (2016) Quantitative comparisons of maternal transcripts related to cell division between good and poor quality eggs from artificially matured Japanese eel Anguilla japonica. Aquaculture Science 64:29–42
Jacoby D, Gollock M (2014) Anguilla anguilla. The IUCN Red List of Threatened Species. Version 2014.3. http://www.iucnredlist.org
Jacoby DMP, Casselman JM, Crook V, DeLucia M-B, Ahn H, Kaifu K et al (2015) Synergistic patterns of threat and the challenges facing global anguillid eel conservation. Glob Ecol Conserv 4:321–333
Jansen HJ, Liem M, Jong-Raadsen SA, Dufour S, Weltzien F-A, Swinkels W et al (2017) Rapid de novo assembly of the European eel genome from nanopore sequencing. Sci Rep 7:7213
Jellyman D, Tsukamoto K (2002) First use of archival transmitters to track migrating freshwater eels Anguilla dieffenbachii at sea. Mar Ecol Prog Ser 233:207–215
Jellyman D, Tsukamoto K (2005) Swimming depths of offshore migrating longfin eels Anguilla dieffenbachii. Mar Ecol Prog Ser 286:261–267
Jellyman D, Tsukamoto K (2010) Vertical migrations may control maturation in migrating female Anguilla dieffenbachii. Mar Ecol Prog Ser 404:241–247
Jeng S-R, Yueh W-S, Chen G-R, Lee Y-H, Dufour S, Chang C-F (2007) Differential expression and regulation of gonadotropins and their receptors in the Japanese eel, Anguilla japonica. Gen Comp Endocrinol 154:161–173
Kagawa H (2003) Artificial induction of oocyte maturation and ovulation. In: Aida K, Tsukamoto K, Yamauchi Y (eds) Eel biology. Springer, Tokyo, pp 401–414
Kagawa H, Iinuma N, Tanaka H, Ohta H, Okuzawa K (1998) Effects of rearing period in seawater on induced maturation in female Japanese eel Anguilla japonica. Fish Sci 64:77–82
Kagawa H, Kasuga Y, Adachi J, Nishi A, Hashimoto H, Imaizumi H et al (2009) Effects of continuous administration of human chorionic gonadotropin, salmon pituitary extract, and gonadotropin-releasing hormone using osmotic pumps on induction of sexual maturation in male Japanese eel, Anguilla japonica. Aquaculture 296:117–122
Kagawa H, Fujie N, Imaizumi H, Masuda Y, Oda K, Adachi J et al (2013) Using osmotic pumps to deliver hormones to induce sexual maturation of female Japanese eels, Anguilla japonica. Aquaculture 388:30–34
Kazeto Y, Kohara M, Miura T, Miura C, Yamaguchi S, Trant JM et al (2008) Japanese eel follicle-stimulating hormone (Fsh) and luteinizing hormone (Lh): production of biologically active recombinant Fsh and Lh by Drosophila S2 cells and their differential actions on the reproductive biology. Biol Reprod 79:938–946
Li C, Zhou X (2015) Melatonin and male reproduction. Clin Chim Acta 446:175–180
Lokman PM, Young G (2000) Induced spawning and early ontogeny of New Zealand freshwater eels (Anguilla dieffenbachii and A. australis). N Z J Mar Freshw Res 34:135–145
Lokman PM, Vermeulen GJ, Lambert JGD, Young G (1998) Gonad histology and plasma steroid profiles in wild New Zealand freshwater eels (Anguilla dieffenbachii and A. australis) before and at the onset of the natural spawning migration. I. Females. Fish Physiol Biochem 19:325–338
Lokman PM, George KA, Divers SL, Algie M, Young G (2007) 11-Ketotestosterone and IGF-I increase the size of previtellogenic oocytes from shortfinned eel, Anguilla australis, in vitro. Reproduction 133:955–967
Lokman PM, Wylie MJ, Downes M, Di Biase A, Damsteegt EL (2015) Artificial induction of maturation in female silver eels, Anguilla australis: the benefits of androgen pre-treatment. Aquaculture 437:111–119
Lombardo F, Giorgini E, Gioacchini G, Maradonna F, Ferraris P, Carnevali O (2012) Melatonin effects on Fundulus heteroclitus reproduction. Rep Fertil Dev 24:794–803
Lombardo F, Gioacchini G, Fabbrocini A, Candelma M, D’Adamo R, Giorgini E et al (2014) Melatonin-mediated effects on killifish reproductive axis. Comp Biochem Physiol A 172:31–38
López-Olmeda JF, Oliveira C, Kalamarz H, Kulczykowska E, Delgado MJ, Sánchez-Vázquez FJ (2009) Effects of water salinity on melatonin levels in plasma and peripheral tissues and on melatonin binding sites in European sea bass (Dicentrarchus labrax). Comp Biochem Physiol A 152:486–490
Maitra SK, Chattoraj A, Bhattacharyya S (2005) Implication of melatonin in oocyte maturation in Indian major carp Catla catla. Fish Physiol Biochem 31:201–207
Malpaux B, Migaud M, Tricoire H, Chemineau P (2001) Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J Biol Rhythms 16:336–347
Malpaux B, Tricoire H, Maillet F, Daveau A, Migaud M, Skinner DC et al (2002) Melatonin and seasonal reproduction: understanding the neuroendocrine mechanisms using the sheep as a model. Reprod Suppl 59:167–179
Manabe R, Aoyama J, Watanabe K, Kawai M, Miller MJ, Tsukamoto K (2011) First observations of the oceanic migration of Japanese eel, from pop-up archival transmitting tags. Mar Ecol Prog Ser 437:229–240
Masuda Y, Imaizumi H, Oda K, Hashimoto H, Usuki H, Teruya K (2012) Artificial completion of the Japanese eel, Anguilla japonica, life cycle: challenge to mass production. Bull Fish Res Agency 35:111–117
Matsubara H, Lokman PM, Senaha S, Kazeto Y, Ijiri S, Kambegawa A et al (2003) Synthesis and possible function of 11-ketotestosterone during oogenesis in eel (Anguilla spp.). Fish Physiol Biochem 28:353–354
Mayer I, Bornestaf C, Borg B (1997) Melatonin in non-mammalian vertebrates: physiological role in reproduction? Comp Biochem Physiol 118A:515–531
Mazzeo I, Peñaranda DS, Gallego V, Baloche S, Nourizadeh-Lillabadi R, Tveiten H et al (2014) Temperature modulates the progression of vitellogenesis in the European eel. Aquaculture 434:38–47
McCleave JD (2003) Spawning areas of the Atlantic eels. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer, Tokyo, pp 141–155
Mes D, Dirks RP, Palstra AP (2016) Simulated migration under mimicked photothermal conditions enhances sexual maturation of farmed European eel (Anguilla anguilla). Aquaculture 452:367–372
Methling C, Tudorache C, Skov PV, Steffensen JF (2011) Pop up satellite tags impair swimming performance and energetics of the European eel (Anguilla anguilla). PLoS ONE 6:e20797
Migaud H, Davie A, Taylor JF (2010) Current knowledge on the photoneuroendocrine regulation of reproduction in temperate fish species. J Fish Biol 76:27–68
Mikawa N, Horie N, Yamada Y, Utoh T, Okamura A, Tanaka S et al. (2008) Maturation induction in female Japanese eel without treatment and improvement of fertilization method. World Aquaculture 2008, Busan (Korea) (book of abstracts)
Minegishi Y, Dirks RP, de Wijze DL, Brittijn SA, Burgerhout E, Spaink HP et al (2012) Quantitative bioassays for measuring biologically functional gonadotropins based on eel gonadotropic receptors. Gen Comp Endocrinol 178:145–152
Miura T, Yamauchi C, Nagahama Y, Takahashi H (1991) Induction of spermatogenesis in male Japanese eel, Anguilla japonica, by a single injection of human chorionic gonadotropin. Zool Sci 8:63–73
Mordenti M, Di Biase A, Sirri R, Modugno S, Tasselli A (2012) Induction of sexual maturation in wild female European eels (Anguilla anguilla) in darkness and light. Isr J Aquac-Bamidgeh 64:726
Mordenti O, Emmanuele P, Casalini A, Lokman PM, Zaccaroni A, Di Biase A, Parmeggiani A (2018) Effect of aromatable androgen (17-methyltestosterone) on induced maturation of silver European eels (Anguilla anguilla): oocyte performance and synchronization. Aquac Res 49:442–448
Neilson JD, Perry RI (1990) Diel vertical migrations of marine fishes: an obligate or facultative process? Adv Mar Biol 26:115–168
Nilsson L, Nyman L, Westin L, Ornhagen H (1981) Simulation of the reproduction migration of European eels (Anguilla anguilla (L)) through manipulation of some environmental factors under hydrostatic pressure. Specul Sci Technol 4:475–484
Ohta H, Kagawa H, Tanaka H, Okuzawa K, Hirose K (1996) Changes in fertilization and hatching rates with time after ovulation induced by 17,20β-dihydroxy-4-pregnen-3-one in the Japanese eel, Anguilla japonica. Aquaculture 139:291–301
Ohta H, Kagawa H, Tanaka H, Okuzawa K, Iinuma N, Hirose K (1997) Artificial induction of maturation and fertilization in the Japanese eel, Anguilla japonica. Fish Physiol Biochem 17:163–169
Okamura A, Yamada Y, Horie N, Utoh T, Mikawa N, Tanaka S et al (2008) Effects of silvering state on induced maturation and spawning in wild female Japanese eel Anguilla japonica. Fish Sci 74:642–648
Okamura A, Horie N, Mikawa N, Yamada Y, Tsukamoto K (2014) Recent advances in artificial production of glass eels for conservation of anguillid eel populations. Ecol Freshw Fish 23:95–110
Oliveira K, Hable WE (2010) Artificial maturation, fertilization, and early development of the American eel (Anguilla rostrata). Can J Zool 88:1121–1128
Palstra A, van den Thillart G (2009) Artificial maturation and reproduction of the European eel. In: van den Thillart G, Dufour S, Rankin JC (eds) Spawning migration of the European eel, reproduction index, a useful tool for conservation management. Springer, Dordrecht, pp 309–331
Palstra AP, Cohen EGH, Niemantsverdriet PRW, van Ginneken VJT, van den Thillart GEEJM (2005) Artificial maturation and reproduction of European silver eel: development of oocytes during final maturation. Aquaculture 249:533–547
Palstra AP, Schnabel D, Nieveen MC, Spaink HP, van den Thillart GEEJM (2008a) Male silver eels mature by swimming. BMC Physiol 8:14
Palstra A, van Ginneken V, van den Thillart G (2008b) Cost of transport and optimal swimming speed in farmed and wild European silver eels (Anguilla anguilla). Comp Biochem Physiol A 151:37–44
Palstra AP, Schnabel D, Nieveen MC, Spaink HP, van den Thillart G (2010) Swimming suppresses hepatic vitellogenesis in European female silver eel as shown by expression of the estrogen receptor 1, vitellogenin1 and vitellogenin2 in the liver. Reprod Biol Endocrinol 8:27
Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R (2006) Melatonin: nature’s most versatile biological signal? FEBS J 273:2813–2838
Pankhurst NW (1982) Relation of visual changes to the onset of sexual maturation in the European eel Anguilla anguilla L. J Fish Biol 21:127–140
Pankhurst NW, King HR (2010) Temperature and salmonid reproduction: implications for aquaculture. J Fish Biol 76:69–85
Pankhurst NW, Lythgoe JN (1983) Changes in vision and olfaction during sexual maturation in the European eel Anguill anguilla (L.). J Fish Biol 23:229–240
Pankhurst NW, Porter MJR (2003) Cold and dark or warm and light: variations on the theme of environmental control of reproduction. Fish Physiol Biochem 28:385–389
Park YJ, Park JG, Takeuchi Y, Hur SP, Lee YD, Kim SJ et al (2014) Influence of moonlight on mRNA expression patterns of melatonin receptor subtypes in the pineal organ of a tropical fish. Mar Genomics 14:67–70
Parmeggiani A, Govoni N, Zannoni A, Di Biase A, Sirri R, Forni M et al (2015) Effect of photoperiod on endocrine profiles and vitellogenin expression in European eels Anguilla anguilla during artificially induced ovarian development. Theriogenology 83:478–484
Pasquier J, Lafont A-G, Leprince J, Vaudry H, Rousseau K, Dufour S (2011) First evidence for a direct inhibitory effect of kisspeptins on LH expression in the eel, Anguilla anguilla. Gen Comp Endocrinol 173:216–225
Patiño R, Yoshizaki G, Thomas P, Kagawa H (2001) Gonadotropic control of ovarian follicle maturation: the two-stage concept and its mechanism. Comp Biochem Phyisol B 129:427–439
Pedersen BH (2003) Induced sexual maturation of the European eel Anguilla anguilla and fertilization of the eggs. Aquaculture 224:323–338
Pedersen BH (2004) Fertilisation of eggs, rate of embryonic development and hatching following induced maturation of the European eel Anguilla anguilla. Aquaculture 237:461–473
Peñaranda DS, Pérez L, Gallego V, Jover M, Tveiten H, Baloche S et al (2010) Molecular and physiological study of the artificial maturation process in European eel males: from brain to testis. Gen Comp Endocrinol 166:160–171
Pérez L, Peñaranda DS, Dufour S, Baloche S, Palstra AP, van den Thillart GEEJM et al (2011) Influence of temperature regime on endocrine parameters and vitellogenesis during experimental maturation of European eel (Anguilla anguilla) females. Gen Comp Endocrinol 174:51–59
Porter MJR, Duncan N, Mitchell D, Bromage NR (1998) The use of cage lighting to reduce plasma melatonin in Atlantic salmon (Salmo salar) and its effects on the inhibition of grilsing. Aquaculture 176:237–244
Porter MJR, Duncan N, Handeland SO, Stafansson SO, Bromage NR (2001) Temperature, light intensity and plasma melatonin levels in juvenile Atlantic salmon. J Fish Biol 58:431–438
Quérat B, Nahoul K, Hardy A, Fontaine YA, Leloup-Hatey J (1987) Plasma concentrations of ovarian steroids in the freshwater European silver eel (Anguilla anguilla L.): effects of hypophysectomy and transfer to sea water. J Endocrinol 114:289–294
Quérat B, Hardy A, Fontaine YA (1991) Regulation of the type-II gonadotrophin α and β subunit mRNAs by oestradiol and testosterone in the European eel. J Mol Endocrinol 7:81–86
Reiter RJ (1993) The melatonin rhythm: both a clock and a calendar. Experientia 49:654–664
Richkus WA, Whalen K (2000) Evidence for a decline in the abundance of the American eel, Anguilla rostrata (Lesueur), in North America since the early 1980s. Dana 12:83–97
Righton D, Aarestrup K, Jellyman D, Sébert P, van den Thillart G, Tsukamoto K (2012) The Anguilla spp. migration problem: 40 million years of evolution and two millennia of speculation. J Fish Biol 81:365–386
Rohr DH, Lokman PM, Davie PS, Young G (2001) 11-Ketotestosterone induces silvering-related changes in immature female short-finned eels, Anguilla australis. Comp Biochem Physiol A 130:701–714
Rojo-Bartolomé I, Martínez-Miguel L, Lafont AG, Vílchez MC, Asturiano JF, Pérez L, Cancio I (2017) Molecular markers of oocyte differentiation in European eel during hormonally induced oogenesis. Comp Biochem Physiol A Mol Integr Physiol 211:17–25
Rousseau K, Aroua S, Schmitz M, Elie P, Dufour S (2009) Silvering: metamorphosis or puberty? In: van den Thillart G, Dufour S, Rankin JC (eds) Spawning migration of the European eel: reproduction index, a useful tool for conservation management. Springer, Dordrecht, pp 39–63
Rousseau K, Lafont A-G, Pasquier J, Maugars G, Jolly C, Sébert M-E, Aroua S, Pasqualini C, Dufour S (2013) Advances in eel reproductive physiology and endocrinology. In: Trischitta F, Takei Y, Sébert P (eds) Eel physiology. CRC Press, Boca Raton, pp 1–43
Saha S, Singh KM, Gupta BBP (2018) Melatonin synthesis and clock gene regulation in the pineal organ of teleost fish compared to mammals: similarities and differences. Gen Comp Endocrinol. https://doi.org/10.1016/j.ygcen.2018.07.010
Saito K, Lokman PM, Young G, Ozaki Y, Matsubara H, Okumura H et al (2003) Follicle-stimulating hormone beta, luteinizing hormone beta and glycoprotein hormone alpha subunit mRNA levels in artificially maturing Japanese eel Anguilla japonica and naturally maturing New Zealand longfinned eel Anguilla dieffenbachii. Fish Sci 69:146–153
Sandlund OT, Diserud OH, Poole R, Bergesen K, Dillane M, Rogan G, Durif C, Thorstad EB, Vøllestad LA (2017) Timing and pattern of annual silver eel migration in two European watersheds are determined by similar cues. Ecol Evol. http://doi.org/10.1002/ece3.3099
Sato N, Kawazoe I, Suzuki Y, Aida K (2000) Time-course response of plasma testosterone and estradiol-17β to salmon gonadotropin treatment in the Japanese eel during ovarian development. Fish Sci 66:792–794
Sato N, Kawazoe I, Suzuki Y, Aida K (2003) Induction of vitellogenesis. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer, Tokyo, pp 387–399
Sato N, Kawazoe I, Suzuki Y, Aida K (2006) Effects of temperature on vitellogenesis in Japanese eel Anguilla japonica. Fish Sci 72:961–966
Schabetsberger R, Økland F, Aarestrup K, Kalfatak D, Sichrowsky U, Tambets M et al (2013) Oceanic migration behaviour of tropical Pacific eels from Vanuatu. Mar Ecol Prog Ser 475:177–190
Schabetsberger R, Økland F, Kalfatak D, Sichrowsky U, Tambets M, Aarestrup K et al (2015) Genetic and migratory evidence for sympatric spawning of tropical Pacific eels from Vanuatu. Mar Ecol Prog Ser 521:171–187
Schmidt J (1923) Breeding places of the eel. Nature 111:51–54
Schmitz M, Aroua S, Vidal B, Le Belle N, Elie P, Dufour S (2005) Differential regulation of luteinizing hormone and follicle-stimulating hormone expression during ovarian development and under sexual steroid feedback in the European eel. Neuroendocrinology 81:107–119
Schreck CB (2010) Stress and fish reproduction: the roles of allostasis and hormesis. Gen Comp Endocrinol 165:549–556
Sébert P, Barthélémy L, Caroff J, Hourmant A (1987) Effects of hydrostatic pressure per se (101 ATA) on energetic processes in fish. Comp Biochem Physiol A 86:491–495
Sébert P, Barthélémy L, Simon B (1990) Laboratory system enabling long-term exposure (≥ 30 d) to hydrostatic pressure (≤ 101 atm) of fishes or other animals breathing water. Mar Biol 104:165–168
Sébert M-E, Amérand A, Vettier A, Weltzien F-A, Pasqualini C, Sébert P et al (2007) Effects of hydrostatic pressure on the pituitary-gonad axis in the European eel, Anguilla anguilla (L.). Gen Comp Endocrinol 153:289–298
Sébert M-E, Legros C, Weltzien F-A, Malpaux B, Cheminaeu P, Dufour S (2008) Melatonin activates brain dopaminergic systems in the eel with an inhibitory impact on reproductive function. J Neuroendocrinol 20:917–929
Servili A, Herrera-Pérez P, del Carmen Rendón M, Muñoz-Cueto JA (2013) Melatonin inhibits GnRH-1, GnRH-3 and GnRH receptor expression in the brain of the European sea bass, Dicentrarchus labrax. Int J Mol Sci 14:7603–7616
Simon B, Sébert P, Cann-Moisan C, Barthélémy L (1992) Muscle energetics in yellow freshwater eels (Anguilla anguilla L.) exposed to high hydrostatic pressure (101 ATA) for 30 days. Comp Biochem Physiol B 102:205–208
Sørensen RI, Tomkiewicz J, Munk P, Butts IAE, Nielsen A, Lauesen P et al (2016) Ontogeny and growth of early life stages of captive-bred European eel. Aquaculture 456:50–61
Stone R (2003) Freshwater eels are slip sliding away. Science 302:221–222
Støttrup JG, Tomkiewicz J, Jacobsen C, Butts IAE, Holst LK, Krüger-Johnsen M et al (2016) Development of a broodstock diet to improve developmental competence of embryos in European eel, Anguilla anguilla. Aquac Nutr 22:725–737
Sudo R, Tosaka R, Ijiri S, Adachi S, Suetake H, Suzuki Y et al (2011a) Effect of temperature decrease on oocyte development, sex steroids, and gonadotropin b-subunit mRNA expression levels in female Japanese eel Anguilla japonica. Fish Sci 77:575–582
Sudo R, Suetake H, Suzuki Y, Utoh T, Tanaka S, Aoyama J et al (2011b) Dynamics of reproductive hormones during downstream migration in females of the Japanese eel, Anguilla japonica. Zool Sci 28:180–188
Sudo R, Okamura A, Fukuda N, Miller MJ, Tsukamoto K (2017) Environmental factors affecting the onset of spawning migrations of Japanese eels (Anguilla japonica) in Mikawa Bay Japan. Environ Biol Fish 100:237–249
Suetake H, Okubo K, Sato N, Yoshiura Y, Suzuki Y, Aida K (2002) Differential expression of two gonadotropin (GTH) b subunit genes during ovarian maturation induced by repeated injection of salmon GTH in the Japanese eel Anguilla japonica. Fish Sci 68:290–298
Suetake H, Okubo K, Yoshiura Y, Aida K (2003) GTH and GnRH molecules and their expression in the Japanese eel. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer, Tokyo, pp 351–372
Takemura A, Rahman MS, Park YJ (2010a) External and internal controls of lunar-related reproductive rhythms in fishes. J Fish Biol 76:7–26
Takemura A, Uchimura M, Shibata Y (2010b) Dopaminergic activity in the brain of a tropical wrasse in response to changes in light and hydrostatic pressure. Gen Comp Endocrinol 166:513–519
Takemura A, Shibata Y, Takeuchi Y, Hur SP, Sugama N, Badruzzaman M (2012) Effects of hydrostatic pressure on monoaminergic activity in the brain of a tropical wrasse, Halichoeres trimaculatus: possible implication for controlling tidal-related reproductive activity. Gen Comp Endocrinol 175:173–179
Tanaka H, Kagawa H, Ohta H (2001) Production of leptocephali of Japanese eel (Anguilla japonica) in captivity. Aquaculture 201:51–60
Tanaka H, Kagawa H, Ohta H, Unuma T, Nomura K (2003) The first production of glass eel in captivity: fish reproductive physiology facilitates great progress in aquaculture. Fish Physiol Biochem 28:493–497
Tang H, Liu Y, Luo D, Ogawa S, Yin Y, Li S, Zhang Y, Hu W, Parhar IS, Lin H, Liu X, Cheng CHK (2014) The kiss/kissr systems are dispensable for zebrafish reproduction: evidence from gene knockout studies. Endocrinology 156:589–599
Taranger GL, Haux C, Stefensson SO, Björnsson BT, Walther BTh, Hansen T (1998) Abrupt changes in photoperiod affect age at maturity, timing of ovulation and plasma testosterone and oestradiol-17β profiles in Atlantic salmon, Salmo salar. Aquaculture 162:85–98
Taranger GL, Carrillo M, Schultz RW, Fontaine P, Zanuy S, Felip A et al (2010) Control of puberty in farmed fish. Gen Comp Endocrinol 165:483–515
Tena-Sempere M, Felip A, Gómez A, Zanuy S, Carrillo M (2012) Comparative insights of the kisspeptin/kisspeptin receptor system: lessons from non-mammalian vertebrates. Gen Comp Endocrinol 175:234–243
Tesch F-W (2003) The eel, 5th edn. Blackwell, Oxford
Thomson-Laing G, Jasoni CL, Lokman PM (2018) The effects of migratory stage and 11-ketotestosterone on the expression of rod opsin genes in the shortfinned eel (Anguilla australis). Gen Comp Endocrinol 257:211–219
Todd PR (1981a) Morphometric changes, gonad histology, and fecundity estimates in migrating New Zealand freshwater eels (Anguilla spp). NZ J Mar Freshwat Res 15:155–170
Todd PR (1981b) Timing and periodicity of migrating New Zealand freshwater eels (Anguilla spp.). NZ J Mar Freshw Res 15:225–235
Tsukamoto K (2009) Oceanic migration and spawning of anguillid eels. J Fish Biol 74:1833–1852
Tsukamoto K, Lee T-W, Fricke H (2003) Spawning area of the Japanese eel. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer-Verlag, Tokyo, pp 121–140
Tsukamoto K, Chow S, Otake T, Kurogi H, Mochioka N, Miller MJ et al (2011) Oceanic spawning ecology of freshwater eels in the western North Pacific. Nat Commun 2:179
Tudorache C, Burgerhout E, Brittijn S, van den Thillart G (2014) The effect of drag and attachment site of external tags on swimming eels: experimental quantification and evaluation tool. PLoS ONE 9:e112280
Tudorache C, Burgerhout E, Brittijn S, van den Thillart G (2015) Comparison of swimming capacity and energetics of migratory European eel (Anguilla anguilla) and New Zealand short-finned eel (A. australis). Front Physiol 6:256
Underwood H (1989) The pineal and melatonin: regulator of circadian function in lower vertebrates. Experientia 45:914–922
van Ginneken VJT, Maes GE (2005) The European eel (Anguilla anguilla, Linneaus), its life cycle, evolution and reproduction: a literature review. Rev Fish Biol Fish 15:367–398
van Ginneken VJT, van den Thillart GEEJM (2000) Eel fat stores are enough to reach the Sargasso. Nature 403:156–157
van Ginneken V, Antonissen E, Müller UK, Booms R, Eding E, Verreth J et al (2005) Eel migration to the Sargasso: remarkably high swimming efficiency and low energy costs. J Exp Biol 208:1329–1335
van Ginneken V, Durif C, Balm SP, Boot R, Verstegen MW, Antonissen E et al (2007a) Silvering of European eel (Anguilla anguilla L.): seasonal changes of morphological and metabolic parameters. Anim Biol 57:63–77
van Ginneken V, Dufour S, Sbaihi M, Balm P, Noorlander K, de Bakker M et al (2007b) Does a 5500-km swim trial stimulate early sexual maturation in the European eel (Anguilla anguilla L.)? Comp Biochem Physiol A 147:1095–1103
Vera LM, De Oliveira C, López-Olmeda JF, Ramos J, Mañanós E, Madrid JA et al (2007) Seasonal and daily plasma melatonin rhythms and reproduction in Senegal sole kept under natural photoperiod and natural or controlled water temperature. J Pineal Res 43:50–55
Vidal B, Pasqualini C, Le Belle N, Claire M, Holland H, Sbaihi M et al (2004) Dopamine inhibits luteinizing hormone synthesis and release in the juvenile European eel: a neuroendocrine lock for the onset of puberty. Biol Reprod 71:1491–1500
Vøllestad LA, Jonsson B, Hvidsten NA, Næesje TF (1994) Experimental test of environmental factors influencing the seaward migration of European silver eels. J Fish Biol 45:641–651
Wang N, Teletchea F, Kestemont P, Milla S, Fontaine P (2010) Photothermal control of the reproductive cycle in temperate fishes. Rev Aquac 2:209–222
Watanabe H, Moku M, Kawaguchi K, Ishimaru K, Ohno A (1999) Diel vertical migration of myctophid fishes (Family Myctophidae) in the transitional waters of the western North Pacific. Fish Oceanogr 8:115–127
Weltzien F-A, Pasqualini C, Sébert M-E, Vidal B, Le Belle N, Kah O et al (2006) Androgen-dependent stimulation of brain dopaminergic systems in the female European eel (Anguilla anguilla). Endocrinology 147:2964–2973
Weltzien F-A, Sébert M-E, Vidal B, Pasqualini C, Dufour S (2009) Dopamine inhibition of eel reproduction. In: van den Thillart G, Dufour S, Rankin JC (eds) Spawning migration of the European eel: reproduction index, a useful tool for conservation management. Springer, Dordrecht, pp 279–307
Wysujack K, Westerberg H, Aarestrup K, Trautner J, Kurwie T, Nagel F et al (2015) The migration behaviour of European silver eels (Anguilla anguilla) released in open ocean conditions. Mar Freshw Res 66:145–157
Yamamoto K, Yamauchi K (1974) Sexual-maturation of Japanese eel and production of eel larvae in aquarium. Nature 251:220–221
Zachmann A, Ali MA, Falcon J (1992a) Melatonin and its effects in fishes: an overview. In: Ali MA (ed) Rhythms in fishes. Plenum Press, New York, pp 149–165
Zachmann A, Falcón J, Knijff SCM, Bolliet V, Ali MA (1992b) Effects of photoperiod and temperature on rhythmic melatonin secretion from the pineal organ of the white sucker (Catostomus commersoni) in vitro. Gen Comp Endocrinol 86:26–33
Zadmajid V, Falahatimarvast A, Damsteegt EL, Setiawan AN, Ozaki Y, Shoae A et al (2015) Effects of 11-ketotestosterone and temperature on inhibin subunit mRNA levels in the ovary of the shortfinned eel, Anguilla australis. Comp Biochem Physiol B 187:14–21
Zhang H, Futami K, Horie N, Okamura A, Utoh T, Mikawa N et al (2000) Molecular cloning of fresh water and deep-sea rod opsin genes from Japanese eel Anguilla japonica and expressional analyses during sexual maturation 1. FEBS Lett 469:39–43
Ziv L, Tovin A, Strasser D, Gothilf Y (2007) Spectral sensitivity of melatonin suppression in the zebrafish pineal gland. Exp Eye Res 84:92–99
Zohar Y, Munoz-Cueto JA, Elizur A, Kah O (2010) Neuroendocrinology of reproduction in teleost fish. Gen Comp Endocrinol 165:438–455
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burgerhout, E., Lokman, P.M., van den Thillart, G.E.E.J.M. et al. The time-keeping hormone melatonin: a possible key cue for puberty in freshwater eels (Anguilla spp.). Rev Fish Biol Fisheries 29, 1–21 (2019). https://doi.org/10.1007/s11160-018-9540-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-018-9540-3