Abstract
Eels do not mature under captive conditions. It is not uncommon for fish to begin and complete their final maturation under closed aquaculture conditions. In most cases, they proceed and successfully complete vitellogenesis and spermatogenesis. However, oocyte maturation and ovulation, and sperm maturation is not spontaneously triggered under aquaculture conditions, which is different from their natural spawning places. The difficulty of eel reproduction is that female and male eels will not proceed vitellogenesis and spermatogenesis, respectively. The Japanese eel undergoes vitellogenesis and spermatogenesis during long-distance migration toward the spawning site. Thus, because natural eels have not been captured during spawning migration in the ocean, the natural controls that promotes sexual maturation and final maturation are not known. Previous studies have provided the current known information on natural reproductive conditions by examining adult eels captured at their spawning ground, West Mariana Ridge; however all these eels had already completed ovulation or spawning (Tsukamoto et al. 2011; Shimizu et al. 2021). Therefore, reproductive physiological conditions can only be determined from artificially induced sexually maturing eels. In a previously published study, we described the control of ovarian vitellogenic growth with special focus on changes in the morphology of oocytes and steroid hormone production in the ovary of the Japanese eel (Adachi et al. 2003). In this chapter, we describe the latest insights, especially into natural sex differentiation and the mechanism of steroidogenic shift that promotes final oocyte maturation in the Japanese eel.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Eels do not mature under captive conditions. It is not uncommon for fish to begin and complete their final maturation under closed aquaculture conditions. In most cases, they proceed and successfully complete vitellogenesis and spermatogenesis. However, oocyte maturation and ovulation, and sperm maturation is not spontaneously triggered under aquaculture conditions, which is different from their natural spawning places. The difficulty of eel reproduction is that female and male eels will not proceed vitellogenesis and spermatogenesis, respectively. The Japanese eel undergoes vitellogenesis and spermatogenesis during long-distance migration toward the spawning site. Thus, because natural eels have not been captured during spawning migration in the ocean, the natural controls that promotes sexual maturation and final maturation are not known. Previous studies have provided the current known information on natural reproductive conditions by examining adult eels captured at their spawning ground, West Mariana Ridge; however all these eels had already completed ovulation or spawning (Tsukamoto et al. 2011; Shimizu et al. 2021). Therefore, reproductive physiological conditions can only be determined from artificially induced sexually maturing eels. In a previously published study, we described the control of ovarian vitellogenic growth with special focus on changes in the morphology of oocytes and steroid hormone production in the ovary of the Japanese eel (Adachi et al. 2003). In this chapter, we describe the latest insights, especially into natural sex differentiation and the mechanism of steroidogenic shift that promotes final oocyte maturation in the Japanese eel.
1 Gonadal Sex Differentiation
1.1 Morphological Gonadal Sex Differentiation
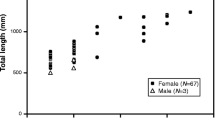
Most cultured eels differentiate into males, and although female eels are produced by oral estrogen administration, ovarian differentiation and development may not correspond to natural ovarian development. To understand natural gonadal sex differentiation in eels, we collected wild eels and investigated their gonadal sex differentiation status. A total of 189 eels ranging 20–40 cm in total length (TL) were collected from 10 rivers in Miyazaki and Oita prefectures. Age, which was determined by otolith analyses, was widely distributed between 2 and 8 years. Differentiated ovaries and testes were found as early as 3 and 4 years of age, respectively. Undifferentiated gonads were found until 7 years of age, indicating that the beginning of gonadal sex differentiation is not determined by aging. In relation to the growth assessed by TL, apparently differentiated ovaries and testes were observed after 32 and 30 cm, respectively, excluding 1 eel with an apparently differentiated ovary measuring 25 cm in TL. Undifferentiated gonads were not observed after 34 cm, suggesting that this size marked the completion of gonadal sex differentiation. Differentiating ovaries and testes emerged after 26 and 24 cm, respectively. Before the apparently differentiated ovary and testis emerged, early-stage differentiation of the ovary and testis could be distinguished by fine histological observation. These observations suggest that ovarian and testicular differentiation begin at 26–32 cm and 24–30 cm, respectively (Horiuchi et al. 2022).
1.2 Molecular Gonadal Sex Differentiation
Prior to morphological gonadal sex differentiation, it is well known that dimorphic gene expression pattern is observed between genetic male and female undifferentiated gonads (Ijiri et al. 2008). These genes are known to be related to sex differentiation. The period of the emergence of sexual dimorphic gene expression patterns in undifferentiated gonads before morphological gonadal differentiation begins is called the molecular sex differentiation period. In many fish species, the aromatase gene (cyp19) and a transcription factor involving cyp19 transcription ( foxl2) show higher expression in undifferentiated gonads of genetic females, and the resultant estrogen, which is produced by aromatase, promotes ovarian differentiation. Without estrogen production, testicle-specific genes such as gonadal-soma derived factor (gsdf) and/or anti-Müllerian hormone (amh) are expressed, and the gonad differentiates into the testis.
In wild Japanese eels, we investigated the gene expression patterns in the ovaries and testes shortly after morphological differentiation began. In addition, molecular sex differentiation was investigated in undifferentiated gonads. In differentiating ovaries, cyp19 and foxl2 typically showed higher expression than in differentiating testes, although the mRNA levels varied widely among individual eels. If ovaries were aligned in the order of detailed developmental stage according to morphological oocyte features, cyp19 expression tended to be high at the earliest stage of differentiating ovaries consisting of oogonial cysts, early meiosis, and postpachytene oocytes. Furthermore, cyp19 expression tended to decrease as oogonia growth progressed, suggesting that cyp19 mRNA is only briefly expressed after triggering ovarian differentiation. This assumption contradicts the general understanding that cyp19 expression lasts longer during ovarian differentiation and development than that during testicular differentiation and sexual maturation. Obtaining differentiating ovarian samples is difficult, probably due to the rapid differentiation period; however, molecular sex differentiation may occur during this limited period. Therefore, more gonadal samples within this narrow window should be investigated to understand the molecular control of sex differentiation in the eel.
Gsdf and amh mRNA levels in the early differentiated testis increased after reaching 33 cm in TL. In eels measuring <33 cm, gonads differentiated into testis did not show higher expression levels compared to ovaries. This suggests that gsdf and amh expression does not increase during the early stages of testis differentiation, further suggesting the existence of unknown genes that promote the early stage of testis differentiation.
In undifferentiated gonads, which are assumed to be found around the initial time of sex differentiation, distinct dimorphic gene expression patterns were not observed in 27 eels measuring from 30–33 cm in TL. The fact that molecular sex differentiation has not been detected in eel undifferentiated gonads may indicate that this window, in which gene expression shows distinct dimorphic patterns among undifferentiated gonads, is brief. Therefore, it would be difficult to obtain gonadal samples that may show dimorphic gene expression patterns. For further clarification, collecting gonadal samples at the beginning of sex differentiation is necessary in future studies (Fig. 12.1; Horiuchi et al. 2022).
2 Pre-vitellogenic Development, Oil Droplet Accumulation
After ovarian differentiation begins, gonia, which are essentially equivalent to primordial germ cells, gradually multiply by mitotic division. During mitotic division, cluster of gonium form cysts in which the cluster of gonium is surrounded by somatic cells (Fig. 12.2). This morphological feature is common at the beginning of ovarian differentiation. Simultaneously, the epithelium of the gonad begins to invaginate, which differentiates into a future ovarian lamella. After this typical morphological differentiation was observed, the gonia were termed oogonia. After gradual mitotic proliferation, oogonia enter meiotic division to create oocytes (Horiuchi et al. 2022). The meiotic division is halted during the first division prophase. The morphological classification of this stage of oocytes is called the chromatin nucleus stage. This arrest of meiotic division is not re-initiated until oocyte maturation is triggered. As oocyte size gradually increases, oocytes are transformed to the next developmental stage, termed the perinucleolus stage. Morphological differentiation of oocytes has been described in detail in our previous report (Adachi et al. 2003). The perinucleolus oocyte proceeds to the next developmental stage characterized by the accumulation of oil droplets in oocytes, which is called the oil droplet stage. Oil droplet accumulation occurs from summer to autumn in feminized eels (estradiol-administered eels) in the second year of the glass eel stage in captive conditions. During this period, oil droplet accumulation accelerates when the rearing water temperature gradually decreases in response to natural seasonal changes. Regarding changes in serum steroid hormone levels during this period, the 11-ketotestosterone (11KT) concentration tended to be high from October to February, in contrast to E2 levels that tended to be high in February. Because oil droplet accumulation tended to accelerate after September, 11KT was considered a candidate factor for oil droplet accumulation. To confirm this, we incorporated 11KT into silastic tubes that were subsequently implanted into the eel abdomen; concurrently, E2 implantation was conducted as a control. One month after implantation, 11KT implantation successfully facilitated oil droplet accumulation and increased oocyte diameter, in contrast to E2 implantation, which did not affect oocyte growth (Fig. 12.3; Matsubara 2003). We also demonstrated that 11KT facilitated VLDL incorporation into oocytes, which resulted in oil droplet accumulation and individual oocyte growth if ovarian fragments were incubated with 11KT and purified eel VLDL was added to the incubation (Endo et al. 2011). The results obtained from both in vivo and in vitro experiments clearly demonstrated the pivotal role of 11KT in oil droplet accumulation in oocytes at the perinucleolus stage. Furthermore, 11KT was implanted into first-year eels from the glass eel stage to induce oil droplet accumulation at a very small size. After oil droplet accumulation proceeded, the eels were able to enter vitellogenic growth if they received gonadotropic hormone administration in captive conditions (Fig. 12.4).
3 Vitellogenic Oocyte Growth
After oil droplet accumulation in oocytes proceeds, eels become ready for development to the next stage toward sexual maturation. The developmental stage of oocytes of natural silver eels, which migrate downstream and prepare for spawning migration, is the beginning of the vitellogenic growth stage, in which small yolk vesicles can be detected in the peripheral area of oocytes ranging from 200 to 330 μm in diameter. Cultivated feminized eels also accumulate oil droplets, and some begin vitellogenesis spontaneously if rearing temperatures gradually decrease after summer (Ijiri et al. 1998; Chai et al. 2010). These observations suggested that eels begin sexual maturation without exogenous hormone treatment. However, neither silver eels nor feminized eels that begin vitellogenic growth undergo further vitellogenic growth beyond the early stage in captive conditions. These eels maintain very early vitellogenic oocytes during winter, and oocytes degenerate after spring under captive conditions. Gonadotropin administration is commonly used for artificial eel maturation to induce further vitellogenic development. Morphological oocyte development during artificial maturation has been previously reported in detail (Adachi et al. 2003); thus, in this section, we describe how vitellogenic growth proceeds in response to weekly injections of salmon pituitary extract (SPE) based on gene expression related to oocyte development. Weekly injection of SPE into eels whose oocytes accumulate oil droplets induces vitellogenic growth. Before SPE injection, the eel pituitary contained follicle-stimulating hormone (FSH); however, luteinizing hormone (LH) was not detected by immunohistochemistry (Fig. 12.5). After SPE injection, fshβ subunit mRNA expression rapidly decreased and maintained a very low expression level. In contrast, lhβ subunit rapidly increased after SPE injection and was maintained at a high level until oocyte maturation (Fig. 12.6). However, eel FSH and LH do not seem necessary for artificial maturation because hypophysectomy eels can complete vitellogenesis and oocyte maturation if SPE is injected weekly and DHP is injected at the migratory nucleus stage (unpublished data). SPE contains salmon LH while FSH content is very low; therefore, salmon LH contained in SPE mainly proceeds via eel vitellogenesis until the migratory nuclear stage.
SPE was injected weekly from 8 to >15 weeks until the eels reached the migratory nucleus stage. During this period, serum estradiol-17β (E2) and 11KT levels increased linearly as the pulse mode was stimulated by every weekly SPE injection. However, DHP levels are consistently maintained at very low levels during oocyte development until the migratory nucleus stage before the exogenous DHP injection that induced oocyte maturation and ovulation (Adachi et al. 2003). The pivotal role of SPE injection in vitellogenic growth seems to be to stimulate E2 production in the ovary and simultaneously facilitating vitellogenin incorporation into oocytes. The mechanism of vitellogenin incorporation into oocytes remains unknown; however, evidence suggests that it is facilitated by gonadotropin; however, the synthesis of vitellogenin in the liver was facilitated by E2 stimulation. In fact, the expression of vitellogenin 1 and 2 mRNA levels in the liver increased synchronously with serum E2 elevation during artificial maturation induced by SEP injections (Adachi et al. 2003). To understand how E2 production is induced by SPE injection at the molecular level, cDNAs encoding steroidogenic enzymes responsible for E2 production were isolated, and changes in expression during artificial maturation were investigated. Cholesterol side-chain cleavage (P450scc) and steroid-17α-hydroxylase/C17–20 lyase (cyp17a1) mRNA levels were elevated during SPE injections and maintained at high levels during late-vitellogenic and migratory nucleus stages in the ovary. Furthermore, 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD) mRNA was expressed at a high level before SPE injection and did not show changes in expression during induced vitellogenic growth. Cyp19a1 encoding aromatase mRNA increased after SPE injection, reached a peak at the mid-vitellogenic stage, and these high expression levels were maintained until the migratory nucleus stage (Adachi et al. 2003). Taken together, the data suggests that weekly SPE injections stimulate the expression of almost all steroidogenic enzymes repeatedly, which facilitates incremental E2 production in the ovary as SPE injections proceed. SPE also likely facilitates the incorporation of vitellogenin into oocytes, and may also play a role in facilitating vitellogenin synthesis in the liver, although clear evidence has not been obtained. During artificial maturation, serum 11KT levels increase consistently. Oocytes continuously incorporate oil droplets inside during vitellogenic growth; therefore, consistent production of 11KT would facilitate the incorporation of oil droplets into oocytes during vitellogenic growth, as seen in pre-vitellogenic oocyte development.
After weekly SPE injections, the eel oocytes reached the migratory nucleus stage (prophase of the first meiotic division), and are still immature. Generally, to proceed with the oocyte at this stage to maturation, stimulation of maturation-inducing steroids (MIS) onto oocytes is required. In the next section, the control of MIS production in relation to LH surge is discussed.
4 Control of Maturation-Inducing Steroid Synthesis and Oocyte Maturation
After vitellogenic growth is completed, previously halted meiotic first division in oocytes is re-initiated by stimulation of the LH surge secreted from the pituitary until the second meiotic metaphase, which is called final oocyte maturation. After maturation, oocytes are stripped from follicular cells, which is called ovulation, and then become fertilizable eggs. During fertilization, the egg re-initiates and completes the meiotic second division and initiates ontogenetic development.
The environmental trigger causing the LH surge is unknown during natural eel maturation. In captive conditions, the LH surge does not occur spontaneously; thus, 17α, 20β-dihydroxy-4-pregnen-3-one (DHP) is routinely injected to obtain eel eggs. DHP was first identified in 1985 as a maturation-inducing steroid hormone in amago salmon (Oncorhynchus masou ishikawae) (Nagahama and Adachi 1985), and has since been confirmed in Japanese eels (Adachi et al. 2003). In series of in vivo and in vitro studies concerning DHP production in salmonids, it was demonstrated that LH stimulates DHP production through 20β-hydroxysteroid dehydrogenase (20β-HSD) which converts 17α-hydroxyprogesterone (17OHP) to DHP in granulosa cells, while LH simultaneously stimulates 17OHP production in thecal cells. Recently, we demonstrated for the first time that 17α-hydroxysteroid dehydrogenase type 12-like (hsd17b12L) encodes 20β-HSD, which is responsible for DHP production in granulosa cells during oocyte maturation in O. masou masou (Ijiri et al. 2017). The ortholog gene hsd17b12L in Nile tilapia (Oreochromis niloticus) and amur sturgeon (Acipenser schrenckii) were also shown to be responsible for DHP production (Aranyakanont et al. 2020; Hasegawa et al. 2022a, b). We also isolated orthologous genes and investigated their roles in DHP production in the Japanese eel.
The coding region of the Japanese eel hsd17b12L was inserted into the mammalian expression vector pSI harboring the SV40 promoter and was subsequently transfected into human embryonic kidney 293 T cells (HEK293T). Eel hsd17b12L transfected HEK293T cells in 24-well plate converted over 20% of exogenous 17OHP (100 ng/mL) to DHP, confirming that eel Hsd17b12L also possesses strong 20β-HSD activity against 17OHP. Unlike salmon, in which hsd17b12L mRNA expression was limited to the maturing ovarian follicle layer, eel hsd17b12L expression was ubiquitously detected in all examined tissues, such as the brain, pituitary, heart, muscle, liver, kidney, ovary, and testis; in contrast, DHP was not detected in the serum throughout all reproductive stages. Throughout vitellogenic growth, hsd17b12L expression in ovaries did not show any distinct changes. Before SPE injection at the late oil droplet stage of oocytes, hsd17b12L was detected and tended to increase until the early vitellogenic stage after SPE injection. Hsd17b12L expression was maintained until the late vitellogenic stage, and then slightly decreased until the migratory nucleus stage; however, the mRNA level was identical to that of the ovary before SPE injection. Despite injecting DHP after completion of vitellogenesis, hsd17b12L levels remained at a constant level compared with the late vitellogenic ovary (Fig. 12.7).
The reason that DHP is not detected in eel serum, although all tissues show constant hsd17b12L mRNA expression, may be due to the absence of 17OHP production as the precursor of DHP. Progesterone (P4) produces 17OHP via 17α-hydroxylase, or 17α-hydroxypregnenolone (17P5) via 3β-HSD. Many years ago, we identified Cyp17a1 in Japanese eel and demonstrated that this enzyme rapidly converts pregnenolone (P5) and P4 to dehydroepiandrosterone (DHEA) and androstenedione (A4), respectively. Intermediate metabolites, such as 17P5 or 17OHP, were only detected in a limited time span, when P5 or P4 were added to the incubation medium on COS7 cells transfected with eel cyp17a1 (Kazeto et al. 2000). This indicates that Cyp17a1 produces DHEA or A4 without accumulating 17P5 or 17OHP. Currently, the mechanisms of 17OHP production are completely unknown, although salmon thecal cells produce substantial amounts of 17OHP during oocyte maturation (Young et al. 1986); this was explained several years ago by the discovery of a novel enzyme, Cyp17a2. Cyp17a2 was first discovered in medaka, followed by Nile tilapia. In contrast to Cyp17a1, which possesses both 17α-hydroxylase and C17–20 lyase activities, Cyp17a2 possesses 17α-hydroxylase activity, but lacks C17–20 lyase activity, resulting in the production of 17P5 or 17OHP as terminal metabolites. In medaka and tilapia, cyp17a1 expression decreased instead of increasing cyp17a2 at the time of oocyte maturation, consequently resulting in 17OHP production (Zhou et al. 2007). In these species, hsd17b12L expression was also elevated and 17OHP was converted to DHP, which triggered oocyte maturation.
Based on these new discoveries, we isolated eel cyp17a2 from Japanese eels. HEK293T cells transfected with pSI-inserted eel cyp17a2 coding region converted P4 efficiently to 17OHP; however, it was not further converted to A4, confirming the lack of C17–20 lyase activity, similar to medaka and tilapia Cyp17a2. Simultaneously, eel cyp17a1 completely converted P4 to A4, which confirmed its strong C17–20 lyase activity (Fig. 12.8). In eel ovaries, cyp17a1 expression gradually increased after SPE injection began, reached a peak at the late vitellogenic stage, and was maintained at a high level even after DHP-induced oocyte maturation and ovulation. Cyp17a2 expression linearly increased in the ovaries after SPE injection until DHP-induced oocyte maturation and ovulation were completed (Fig. 12.7). The expression patterns of cyp17a1 and cyp17a2 were very different from those of medaka and tilapia. This is likely because DHP-injected oocyte maturation did not reflect the natural steroidogenic changes that would naturally occur at the beginning of oocyte maturation in the eel.
A decade ago, Dr. Hirohiko Kagawa developed a novel method to induce eel oocyte maturation without injecting DHP. Kagawa demonstrated that a high dose (tenfold) SPE injection (300 mg/kg BW) instead of DHP injection in eels whose oocytes reached the migratory nucleus stage (after vitellogenesis completion) could induce oocyte maturation and ovulation. This procedure implies that regular SPE injection (30 mg/kg BW) can induce vitellogenic growth; however, this injection dose is not sufficient to induce oocyte maturation and ovulation. High-dose SPE injections may mimic the natural LH surge to stimulate oocyte maturation and ovulation in eels. This indicates that it is possible to investigate natural steroidogenic shift to induce oocyte maturation in eels if we could obtain biological samples of eels that induced oocyte maturation and ovulation by high-dose SPE injection rather than DHP-induced oocyte maturation.
The steroidogenic shift during oocyte maturation and ovulation in eels induced by high-dose SPE injection was investigated. Eels whose oocytes reached the migratory nucleus stage, over 800 μm in diameter, received a high-dose SPE injection. In a representative case of eels in which oocyte maturation and ovulation were induced, ovulation was completed 18 h after injection. One hour after injection, cyp17a1 mRNA expression rapidly decreased, reached the lowest at 8 h, and consequently became undetectable at the time of ovulation at 18 h. Cyp17a2 expression was maintained before the high-dose SPE injection, and then increased linearly from 6 to 18 h. In contrast to these distinct changes in expression, hsd17b12L expression showed a gradual increase after injection until 18 h after ovulation was complete. In relation to these gene expression changes, the serum DHP concentration increased from 15 ng/mL to 50 ng/mL during induced oocyte maturation and ovulation (Fig. 12.9). In correlation with the changes in cyp17a1 and cyp17a2 mRNA expression, the expression of proteins observed by immunohistochemistry also showed drastic changes. Before injection, Cyp17a1 was detected in the thecal cells; however, immunohistochemically positive cells were not detected in the ovary after ovulation. Cyp17a2 positive cells were also detected in the thecal cells before the injection, and clusters of positive cells were widely observed in post-ovulatory follicular cells after ovulation (Fig. 12.10). These findings show for the first time that high-dose SPE injection mimics the physiological changes induced by the expected natural LH surge that occurs at the time of oocyte maturation. Furthermore, high-dose SPE injection stimulates downregulation of cyp17a1 expression at the transcriptional level, together with a linear increase in cyp17a2 expression. These gene expression changes likely enabled the production of 17OHP because of the disappearance of C17–20 lyase activity in the ovary. 17OHP enables the production of DHP via Hsd17b12L, which is constantly expressed in the eel ovary. A distinct difference in the mechanism behind DHP production at final maturation in salmon and tilapia is that eel DHP production seems to be regulated by complete downregulation of cyp17a1 gene expression rather than rapid upregulation of hsd17b12L (Fig. 12.11). These observations revealed, for the first time, the mechanism of DHP production stimulated by LH surge to induce oocyte maturation and ovulation in the eel. However, the molecular mechanisms that control the transcription of these key enzymes remain unknown. A complete understanding could be achieved if the action of LH surge is linked with transcriptional regulation of genes encoding steroidogenic enzymes at the molecular level.
5 Perspectives
Most cultured eels differentiate into males. Because of the limited availability of naturally differentiating females other than E2 induced females, investigations of morphological and molecular gonadal differentiation are not commonly conducted. Our investigation of wild eels reveals a part of natural eel gonadal sex differentiation. Natural morphological gonadal differentiation is clearly understood; however, molecular gonadal differentiation is not. The collection of wild eels is strictly regulated; therefore, wild eels are limited during the period of sex differentiation. The fact that male and female eels exist equally in the wild river indicates that cultivation conditions that lead to 50% female ratio must exist and should be disclosed. These rearing conditions would enable an in-depth investigation of the molecular control of gonadal sex differentiation. After ovarian differentiation, the overall process of oocyte development until oil droplet accumulation is largely understood. After oil droplet accumulation proceeded, the endocrine control to promote vitellogenic growth induced by SPE injection is also largely understood. However, the natural control of vitellogenic growth, which is probably stimulated by eel FSH action, should be investigated in the future. The biggest mystery is that FSH is not secreted under aquaculture conditions, which is the main reason why captive eels do not undergo vitellogenic growth. Unfortunately, this cannot be clarified until the mechanism for the control of synthesis and secretion of FSH is elucidated, which is also currently unknown throughout all fish species. After completion of vitellogenesis, an understanding of the initiation mechanisms of oocyte maturation was also largely developed in our recent study.
Throughout eel gonadal differentiation until completion of maturation, a shift in steroid hormone production, such as E2, 11KT, and DHP, plays a pivotal role in oocyte development. To further understand the gene expression levels involved in steroid production, the regulation of transcription governing steroid production is necessary to achieve a complete understanding of the total molecular controls of eel gonadal development preceding egg production.
Change history
13 February 2024
A correction has been published.
References
Adachi S, Ijiri S, Kazeto Y, Yamauchi K (2003) Oogenesis in the Japanese eel. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer, Tokyo, pp 301–317
Aranyakanont C, Ijiri S, Hasegawa Y, Adachi S (2020) 17β-Hydroxysteroid dehydrogenase type 12 is responsible for maturation-inducing steroid synthesis during oocyte maturation in Nile tilapia. Gen Comp Endocrinol 290:113399. https://doi.org/10.1016/j.ygcen.2020.113399
Chai Y, Tosaka R, Abe T, Sago K, Sago Y, Hatanaka E, Ijiri S, Adachi S (2010) The relationship between the developmental stage of oocytes in various seasons and the quality of the egg obtained by artificial maturation in the feminized Japanese eel Anguilla japonica. Aquaculture Sci 58:269–278. https://doi.org/10.11233/aquaculturesci.58.269; in Japanese with English abstract
Endo T, Todo T, Lokman PM, Kudo H, Ijiri S, Adachi S, Yamauchi K (2011) Androgens and very low density lipoprotein are essential for the growth of previtellogenic oocytes from Japanese eel, Anguilla japonica, in vitro. Biol Reprod 84:816–825. https://doi.org/10.1095/biolreprod.110.087163
Hasegawa Y, Ijiri S, Surugaya R, Sakai R, Adachi S (2022a) 17β-hydroxysteroid dehydrogenase type 12-like is associated with maturation-inducing steroid synthesis during induced oocyte maturation and ovulation in sturgeons. Aquaculture 564:737238. https://doi.org/10.1016/j.aquaculture.2021.737238
Hasegawa Y, Surugaya R, Adachi S, Ijiri S (2022b) Regulation of 17α-hydroxyprogesterone production during induced oocyte maturation and ovulation in Amur sturgeon (Acipenser schrenckii). J Mar Sci Eng 10:86. https://doi.org/10.3390/jmse10010086
Horiuchi M, Hagihara S, Kume M, Chushi D, Hasegawa Y, Itakura H, Yamashita Y, Adachi S, Ijiri S (2022) Morphological and molecular gonadal sex differentiation in the wild Japanese eel Anguilla japonica. Cell 11:1554. https://doi.org/10.3390/cells11091554
Ijiri S, Kayaba T, Takeda N, Tachiki H, Adachi S, Yamauchi K (1998) Pretreatment reproductive stage and oocyte development induced by salmon pituitary homogenate in the Japanese eel Anguilla japonica. Fish Sci 64:531–537. https://doi.org/10.2331/fishsci.64.531
Ijiri S, Kaneko H, Kobayashi T, Wang DS, Sakai F, Paul-Prasanth B, Nakamura M, Nagahama Y (2008) Sexual dimorphic expression of genes in gonads during early differentiation of a teleost fish, the Nile tilapia Oreochromis niloticus. Biol Reprod 78:333–341. https://doi.org/10.1095/biolreprod.107.064246
Ijiri S, Shibata Y, Takezawa N, Kazeto Y, Takatsuka N, Kato E, Hagihara S, Ozaki Y, Adachi S, Yamauchi K, Nagahama Y (2017) 17β-HSD type 12-like is responsible for maturation-inducing hormone synthesis during oocyte maturation in masu salmon. Endocrinology 158:627–639. https://doi.org/10.1210/en.2016-1349
Kazeto Y, Ijiri S, Todo T, Adachi S, Yamauchi K (2000) Molecular cloning and characterization of Japanese eel ovarian P450c17 (CYP17) cDNA. Gen Comp Endocrinol 118:123–133. https://doi.org/10.1006/gcen.1999.7449
Matsubara H (2003) Studies on ovarian steroidogenesis and artificial control of oocyte growth in eel. PhD thesis, 159 pp
Nagahama Y, Adachi S (1985) Identification of maturation-inducing steroid in a teleost, the amago salmon (Oncorhynchus rhodurus). Dev Biol 109:428–435. https://doi.org/10.1016/0012-1606(85)90469-5
Shimizu A, Ijiri S, Izumi H, Gen K, Kurogi H, Hashimoto H, Tanaka H, Jinbo T, Saito H, Chow S (2021) Histological evidence of multiple spawning in wild female Japanese eel Anguilla japonica. Zool Stud 60:61. https://doi.org/10.6620/zs.2021.60-61
Tsukamoto K, Chow S, Otake T, Kurogi H, Mochioka N, Miller MJ, Aoyama J, Kimura S, Watanabe S, Yoshinaga T, Shinoda A, Kuroki M, Oya M, Watanabe T, Hata K, Ijiri S, Kazeto Y, Nomura K, Tanaka H (2011) Oceanic spawning ecology of freshwater eels in the western North Pacific. Nat Commun 2:179. https://doi.org/10.1038/ncomms1174
Young G, Adachi S, Nagahama Y (1986) Role of ovarian thecal and granulosa layers in gonadotropin-induced synthesis of a salmonid maturation-inducing substance (17α, 20β-dihydroxy-4-pregnen-3-one). Dev Biol 118:1–8. https://doi.org/10.1016/0012-1606(86)90067-9
Zhou LY, Wang DS, Kobayashi T, Yano A, Paul-Prasanth B, Suzuki A, Sakai F, Nagahama Y (2007) A novel type of P450c17 lacking the lyase activity is responsible for C21-steroid biosynthesis in the fish ovary and head kidney. Endocrinology 148:4282–4291. https://doi.org/10.1210/en.2007-0487
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ijiri, S., Matsubara, H., Horiuchi, M., Kazeto, Y. (2023). Reproduction. In: Tsukamoto, K., Kuroki, M., Watanabe, S. (eds) Eel Science. Fisheries Science Series. Springer, Singapore. https://doi.org/10.1007/978-981-99-5692-0_12
Download citation
DOI: https://doi.org/10.1007/978-981-99-5692-0_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-5691-3
Online ISBN: 978-981-99-5692-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)