Abstract
About ninety percent of all diabetic conditions account for T2D caused due to abnormal insulin secretion/ action or increased hepatic glucose production. Factors that contribute towards the aetiology of T2D could be well explained through biochemical, molecular, and cellular aspects. In this review, we attempt to explain the recent evolving molecular and cellular advancement associated with T2D pathophysiology. Current progress fabricated in T2D research concerning intracellular signaling cascade, inflammasome, autophagy, genetic and epigenetics changes is discretely explained in simple terms. Present available anti-diabetic therapeutic strategies commercialized and their limitations which are needed to be acknowledged are addressed in the current review. In particular, the pre-eminence of nanotechnology-based approaches to nullify the inadequacy of conventional anti-diabetic therapeutics and heterogeneous nanoparticulated systems exploited in diabetic researches are also discretely mentioned and are also listed in a tabular format in the review. Additionally, as a future prospect of nanotechnology, the review presents several strategic hypotheses to ameliorate the austerity of T2D by an engineered smart targeted nano-delivery system. In detail, an effort has been made to hypothesize novel nanotechnological based therapeutic strategies, which exploits previously described inflammasome, autophagic target points. Utilizing graphical description it is explained how a smart targeted nano-delivery system could promote β-cell growth and development by inducing the Wnt signaling pathway (inhibiting Gsk3β), inhibiting inflammasome (inhibiting NLRP3), and activating autophagic target points (protecting Atg3/Atg7 complex from oxidative stress) thereby might ameliorate the severity of T2D. Additionally, several targeting molecules associated with autophagic and epigenetic factors are also highlighted, which can be exploited in future diabetic research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
DM is a serious health disorder affecting millions of individuals worldwide and a further 629 million likely to be added to the tally by 2045 [1]. The disorder will be the 7th in the cause of global mortalities. DM can be categorized as T1D mainly caused due to deficit in the excretion of insulin and T2D which is a result of abnormal insulin secretion/ action or improved hepatic glucose production [2, 3]. There are copious facets that chip in towards aetiology of T2D and are well explained in biochemical, molecular, and cellular aspects. Chaudhury et al. have put forth that, T2D is modulated by eight main defects called ominous octet [4]. These eight core defects are dysfunction of the neurotransmitter, low uptake of glucose, amplified GNG in hepatocytes, decreased incretin effect, decreased insulin production, increased lipolysis, increased secretion of glucagon, and increased glucose reabsorption [5].
The disease is preliminarily detected through changes in hyperglycemia, macrovascular complications (cardiovascular disorders, stroke, and peripheral vascular complications), and consequential microvascular complications (retinopathy, neuropathy, and nephropathy) [6]. Current therapeutics are limited to drugs that can normalize defects starting with elevated insulin secretion by sulfonylureas and meglitinide. Use of Biguanide and thiazolidinedione to enhance insulin sensitivity through activation of AMPK and PPAR-γ receptor respectively are an effectual line of action but have some limitations. Amplified activation of the incretin pathway by DPP-4 inhibitors, SGLT inhibitors, and GLP-1 RAs are reported to be effective with limited or no side-effects [7]. Activation of insulin receptors by insulin bolus insulins, basal insulins, and premixed insulins though not cheap are efficient and available for the common man.
These limitations and side-effects can be neutralized if the cellular and molecular etiology of the T2D is properly comprehended. Moreover, a better understanding of the molecular pathogenesis of T2D can be achieved by dismantling the intricate pathophysiology of T2D in terms of emerging cellular and molecular cascades like inflammasome, autophagy, genetic and epigenetic modifications [8,9,10,11,12,13,14,15]. An in-depth investigation in T2D associated with these molecular and cellular cascades may lead to the development of novel diabetic markers and target points for future therapeutic interventions [16, 17]. Further, transforming these target points into viable therapeutic option requires a customizable, biocompatible delivery vehicle where nanotechnology appears to be the most formidable strategy [18, 19]. The developments made in the current decade and the expeditious evolution of nanotechnology, bridges currently developing applied branches of science and treatment of T2D. Formulating such cutting-edge nano-based products like nanocarriers, nanosensors and nanotools of heterogeneous utilities have radically transformed the height and depth of scientific innovation. Their inimitable and astonishing characteristics like nano-size, lighter weight, higher strength, and surface area, superior control of light spectrum convey their wide application in multidisciplinary areas [8]. Additionally, biocompatible properties like non-immunogenicity, non-toxicity, sustainability to physiological pH and temperature, control release, target-specific delivery, and action also make them a unique and potential candidate in biomedical application [20, 21]. Considering the benefits and competent medication of nanoparticles towards the mediators/ modulators of T2D, the current review highlights and presents the ventures of the nano-targeting system on finding the euphoria within molecular pandemonium represented in the form of the inflammasome, autophagic, genetic and epigenetic. Different types of nano-delivery systems currently exploited in diabetic research are introduced and some of these researches are listed in a tabular format within the review. Further, the paper describes the conventional commercially available antidiabetic drugs and emerging cellular pathways such as inflammasome, autophagy, genetic, and epigenetic changes at the molecular level and their association with T2D. Moreover, in the review, an effort has been made through hypothesizing novel nanotechnological based smart nuclear targeted nano-delivery systems as therapeutic strategies. The strategy exploits previously described inflammasome where inhibition of NLRP3 inflammasome by antisense oligonucleotide is described [9, 22,23,24]. Likewise, the nuclear targeted nano-delivery system is designed to induce autophagy by delivering antioxidant molecules for neutralizing ROS which might affect the Atg3/Atg7 complex [11, 12, 25, 26]. A strategy is also provided to promote β-cell growth and development by targeted nano delivery system which could induce the Wnt signaling pathway by inhibiting Gsk3β. Lastly, the paper provides several autophagic and epigenetic factors that can be further exploited for future diabetic researches. These hypothetical nanotechnological based therapeutic strategies may be helpful for the advancement in engineering future therapeutic approaches in T2D.
2 Factor inducing β-cell failure or loss in type-2 diabetes
2.1 Molecular aetiology
Pancreatic β-cell failure as a corollary of tangential IR, declining β-cell response, and compromised synchrony of hepatic GNG are the broad characteristics of T2D [31]. Adapter proteins transduce the signal from insulin receptors towards downstream cascade furnishing IR in T2D [32]. Under normal conditions, the binding of insulin to extracellular α-subunit of the heterotetrameric glycoprotein of insulin receptor and translocation of GLUT4 to the plasma membrane for glucose influx and triggering of glycogenesis is described in Fig. 1 [27, 28, 33]. Nuclear localization of β-catenin through the Wnt signaling pathway is another important factor as it interacts with TTCF/LEF TF regulating Pitx2 gene transcription which otherwise may result In mediation and degradation of β-catenin [29, 34, 35] (Fig. 1). Molecular alteration in any of the above mentioned intermediate signaling molecules or TFs may lead to failure and loss of β-cell.
Molecular aetiology of insulin resistance. Binding of insulin to its receptor results in their autophosphorylation of IRS-1/2 (insulin receptor substrates), which activates phosphoinositide 3-kinase (PI3K). This results in activation of P3 dependent kinase-1 (PDK-1) and AKT/Protein Kinase B which guides the translocation of GLUT4 transporter from glucose transport vesicles (GSV) to cell membrane. The activation of C-Jun N-Terminal Kinase (JNK) and Insulin Kappa B Kinase (IkK) by cytokines, TNFα, Endoplasmic reticulum stress (ER Stress) etc. also leads to negative regulation of insulin signalling pathway. Different Toll like receptors (TLR2, TLR4) are also indulged in insulin resistance by activating MYD88 (myeloid differentiation primary response gene) and TRIF (TIR domain containing adaptor inducing interferon-b ). ER stress activates AFT6 (activating transcription factor 6), IRE-1 (inositol requiring endonuclease-1) and PERK (pancreatic endoplasmic reticulum kinase-1) which leads to apoptosis. On other side, binding of Wnt ligand to its receptor called Frizzleds (Fz), triggers a signaling cascade that results in the stabilization and nuclear localization of β-catenin whose interactions with T cell-specific factor/lymphoid enhancer-binding factor (TCF/LEF) transcription factors control transcription of target genes like Pitx2. However, in the absence of Wnt signaling, cytoplasmic β-catenin in Wnt-responsive cells is processed to proteasomal degredation by a heteromeric protein complex containing Axin, glycogen synthase kinase 3 (GSK-3), and other proteins which together is known as β-catenin degradation complex. Wnt signalling regulates cell proliferation in pancreatic tissues. Inspired from [27,28,29,30]
2.2 Genetic aetiology
Predisposition at specific genes by default orchestrates an unambiguous consequence leading to malfunctioning of β-cell response or death. Some of the recent investigations suggest that TF like Pdx1 and FOXA2 genes are the chief regulator of β-cell maturation [36,37,38]. MODY, pancreas agenesis or diabetes susceptibility arises as a post mutation consequence in humans at cis-regulatory consensus binding sites of these genes [36]. Pdx1 along with PTF1A is fundamental for the growth and maturity of the pancreas [39]. Other factors like TFs like FOXA1/2, SOX9, HNF 1β, Ngn3, Hes1, Pax4/6, and GATA4/6 are also known to orchestrate an intense role in pancreatic development [40,41,42,43,44,45].
2.3 Environmental variables
Environmental factors specifically nutrients and the supply of oxygen plays an imperative role in pancreatic development. A decrease in β-cell mass and increase secretion of fetal corticosterone on the ingestion of low energy diet was evidenced in past investigations, where deprived development of β-cell mass was concurrent with a diminishing number of NGN3-and Pdx1-expressing cells [46]. At low pO2, the cell undergoes elevated angiogenesis and erythropoiesis with a simultaneous shift towards glycolysis. It is observed that under obese conditions chronic hypoxic state is amalgamated with suppressed NO bioavailability, progressing to T2D [47]. Data also suggests that alteration in any physiological concentrations of HIF1α may lead to inappropriate β-cell function and affect the release of insulin [30] (Fig. 1).
2.4 Biochemical erratic
Evaluation of GNG indicated that any form of compromised management in GNG may cause the failure of β-cell and ultimately malfunctioning of insulin action [48]. Besides pancreatic α-cells, multiple tissues and cell types such as adipose tissue, skeletal muscle, and brain are also involved in indirect gluconeogenic suppression. Transcriptional regulators like FOXO1- 3, 3a, 4, and 6, along with CREB, CREBH, PGC-1α, glucocorticoid receptor, sterol response element-binding protein (SREBP), STAT3, and DAX1 have also revealed to contribute towards the regulation of GNG [48] (Fig. 2). Additionally, prolonged exposure of glucose and free fatty acids are observed to cause β-cell dysfunction leading to cell apoptosis in T2D.
Role of genetic predisposition and environmental factors in declining β-cell response to insulin resistance. Different transcription factors (TF) and TFs like viz. Pancreatic Duodenal Homeobox 1 (Pdx1), Forkhead Box A2 (FOXA2), Pancreas Transcription Factor 1A (PTF1A), FOXA1/2, Sex Determining Region Y-box 9 (SOX9), Hepatocyte Nuclear Factor (HNF) 1β, GATA-binding protein (GATA4/6), Neuronal Differentiation 1 (NeuroD1), Nk class of Homeodomain-encoding genes 2.2 (Nkx2.2), Nkx6.1, V-Maf musculoaponeurotic fibrosarcoma oncogene family protein B (MafB) and Gli-Similar 3 (Glis3) are critical regulator of β-cell development, maturation and function in pancreas. Among nutrients, ingestion of low protein and low energy diet lead to reduction in number of Neurogenin-3 (NGN3)-and Pdx1-expressing cells, decrease in β-cell mass and increase secretion of fetal corticosterone. Hypoxia plays role in stabilization/destabilization of Hypoxia-inducible factor-1α (HIF-1α) by inhibiting Prolyl Hydroxylase Domain enzymes (PHD) & Factor-inhibiting hypoxia-Inducible Factor (FIH) which otherwise used to hydroxylate HIF-1α for their proteasomal degradation after its ubiquitination. Inactivation of PHD and FIH under hypoxia facilitates hetrodimerization of HIF-1α and HIF-1β which further with phosphorylated cAMP response element-binding protein- (CREB-) binding protein (CBP) and p300 binds to hypoxia-response element (HRE) in nucleus to up-/downregulate several genes including glucose transporters (GLUTs), adipokines, and cytokines for hypoxia adaptation. Inspired from [11, 48, 49]
3 Pharmacological treatments available for reversal of type-2 diabetes
Pharmacological treatments for T2D could be segregated into the following types sulfonylureas and meglitinides, biguanide, thiazolidinediones, α-glucosidase inhibitors, DPP-4 inhibitors, sodium-glucose transporter, inhibitors, GLP-1 receptor agnosists, and prandial, basal, and premixed insulin [7] (Fig. 3).
-
(i)
Sulfonylurea is a stimulator for pancreatic beta cells to secrete insulin by activation of SURs present at the β-cell membrane. The propinquity of SUs varies with different SURs present in potassium ATP dependent channels. Meglitinides is a drug with a similar mechanism which includes repaglinide and natelinide.
-
(ii)
Metformin a drug type of biguanide that inhibits hyperglycemia by reducing IR, triglycerides formation, hyperinsulinemia, and HbA1c. Further, boosts high-density lipoprotein level by simultaneously lowering low-density lipoproteins. Besides all these actions, it inhibits glycogenolysis, stimulating insulin-receptor substrate-2, and enhancing glucose transport through GLUT-1 translocation.
-
(iii)
TZD mainly reverses insulin sensitivity by mimicking ligand of PPARγ expressed on adipocyte, liver, heart, colon, activated macrophages, skeletal muscle, etc. The receptor regulates the metabolism of carbohydrate, lipid, cell proliferation, and glucose homeostasis through elevating fatty-acid uptake in blood maintains β-cell function and survival as well as increased insulin sensitivity [54]. Currently, two TZDs are marketed by the name of rosiglitazone and pioglitazone.
-
(iv)
PP4 is a cell surface protein expressed in many specialized cell types, functions as degradation of proteins, cell-cell interaction, inflammation, vascular function, immunity, and transduction pathways activation [50]. According to several recent studies, the reduced DPP4 expression and increased concentration of GLP-1 in circulation results in improved production of insulin, decreased GNG, less glucagon secretion, reduced HbA1c level, and improved glucose uptake. Therefore different forms of DPP4 inhibitors are marketed as drugs such as sitagliptin, saxagliptin, and linagliptin.
-
(v)
SGLT inhibitors are another novel therapy for T2D. These transporters regulate glucose homeostasis of SGLT1, T2, T4, and T5. SGLT mediates intestinal glucose absorption and re-absorption of kidney glucose. They are expressed in the small intestine, testes, brain, kidney, trachea, prostate, pancreatic α-cells, and heart. The inhibitors of SGLT2 namely dapagliflozin, canagliflozin, and empagliflozin are approved internationally for T2D patients. These novel antidiabetic drugs promote the lowering of blood glucose and HbA1c level by enhancing renal excretion.
-
(vi)
α-Glucosidase inhibitors like acarbose, voglibose, emiglitate, and miglitol act by competitive and reversibility inhibiting the enzyme and prevent the formation of monomeric α-glucose molecules. They are known to reduce postprandial hyperglycemia and hyperinsulinaemia, insulin sensitivity, and liberation β-cell stress.
-
(vii)
GLP-1 is an important antagonist that helps in restoring blood glucose regulation to normal levels by regulation of insulin and secretion of glucagon in Type-2 diabetic patients [55]. GLP-1 receptors have generated interest as they enhance incretin action and are segregated as short-acting (exenatide, lixisenatide) and long-acting (albiglutide, dulaglutide, exenatide, liraglutide, and semaglutide) GLP-1antagonists affecting both fasting and postprandial glucose [51].
-
(viii)
Insulin replacement therapy is a reliable therapeutic option for type-2 diabetic patients with insulin deficiency [56]. The therapy comprises basal or long-acting insulin such as aspart, glulisine, etc. or prandial or rapid-acting/ intermediate-acting commercialized insulin available as isophane insulin, biphasic insulin aspart, and insulin lispro [7]. Insulin replacement therapy encompassing the above-mentioned insulin or insulin analogs acts by activating insulin receptors to modulate the metabolism of carbohydrates, fats, and proteins.
Mechanisms involved in the development and pharmacological treatments of Type-2 Diabetes (T2D). Pharmacological treatments for T2D could be segregated into the following types such as sulfonylureas and meglitinides, metformine and thiazoldinediones, α-glucosidase inhibitors, DPP-4 inhibitors, sodium glucose transporter (SGLT) inhibitors, GLP-1 receptor agnosists. These drugs have specific site of action in pathway of insulin resistance mechanism under T2D. Inspired from [7, 50,51,52,53]
Though these medications have proved effective against T2D by targeting mostly post-symptomatic and certain molecular aspects specifically β-cell proliferation as well as apoptosis arrest, they have several side-effects. Some of the commonly associated ailments include low BP, skin rashes, weight gain, kidney complications, swelling of ankles, dizziness, diarrhea, anemia, etc. and henceforth needs serious consideration for T2D treatment. Additionally, investigation in the present era mostly focused combination therapy or different formulation therapy of these above-mentioned therapeutics or potent non- thiazolidinedione, selective PPAR-γ modulators like INT131 which are still in pipeline [57].
4 Targeted pathways in the development of future drugs against T2D
Cutting-edge advancements in diabetic researches encompass mostly omics studies, high throughput bioinformatic tools, and computational simulation-based biological systems. These dry lab generated sophisticated information to indulge one to venture into the world of miniature dynamic machinery where they unceasingly sustain life as we know it. Such dynamic machinery currently explore to understand T2D can be explained in accordance with inflammasome, autophagy, and epigenetics.
-
1
Inflammasome
Inflammasome can be described as intracellular sensors consisting of NLRP1, 3, 4, 6, 7, 12, AIM2, and NAIP. Association of inflammation in T2D is initiated after recognition of exogenous and endogenous factors like DAMPs by intracellular sensors. In metabolic disorder, ligand like ASC, accommodating a CARD upon interaction with inflammasome (NLRP1 and 3) through their respective common pyrin domain, is activated and recruited [58]. NAIP/NLRP4 then interacts with CARD domain of caspase-1 to regulate the enzyme and releases the activated forms of cytokines IL-1β and IL-18 after cleavage of pro-IL-1β and pro-IL-18 [22]. Other research has conceptualized that ER stress-mediated activation of IL-1β production by NLRP3 [9, 23]. These pro-inflammatory cytokines in a pleiotropic manner elicits a cellular immune response against DAMPs and also mediates β-cell death [9, 22, 23]. Activation of caspase-1 promotes maturation of GSDMD along with pro-IL-1β and pro-IL-18, where GSDMD causes pyroptosis via pore formation on the membrane through its N-terminal fragment. Li et al. [59] demonstrated the neuroinflammation as well as pyroptotic and apoptotic cell death of hippocampal neuronal cells mediated by NLRP3 inflammasome induction that further contributes to depression in diabetic patients. However, the involvement of GSDMD induced pyroptosis in the case of β-cell death is needed to be investigated.
There is a number of investigations suggesting the linkage of pro-inflammatory cytokines with T2D. It is evidenced that cytokines like aA-FABP, lipocalin-2, PEDF, resistin, fetuin A, IL-6, IL-8, MCP-1, TNF-α and IFNγ contributes to either IR or β-cell death [60]. The blocking of downstream insulin signalling pathway due to reduced phosphorylation of IRS1 represents the mechanism underlying IL-1β mediated induction of IR [61]. Solini and Novak [24] have reviewed the connection of P2X7R in insulin secretion from β-cell and diabetes induced micro- and macrovascular complications. Their review states that subjects with metabolic syndrome, P2X7R expression, and inflammasome activation are higher in adipose tissue often leading to chronic inflammation in WAT. In certain cases of T2D, adipose tissue is assailed by macrophages and other leukocytes, secretes, serine protease like GrB causing local and low-grade chronic inflammation. Therefore, it is understood that increased systemic GrB level could be allied with T2D diagnosis and seconded with elevated expression of IL-6, TNF-α, WISP1 in dysfunctional AT-linked systemic inflammation [62].
Experimental observation concludes that patients with T2D display subclinical inflammation, characterized by increased circulating levels of hs-CRP, TLRs, and other mentioned systemic inflammatory mediators [63]. Further reports also state that signal transduction cascade specifically MAP kinase and NFκB activity plays a discrete function in the secretion of the above-mentioned cytokines. Therefore it could possible to target inflammation to ameliorate T2D and the related micro- and macrovascular complications [64]. The recent data presented by Rai et al. [10] manifested the anti-inflammatory effect of metformin and resveratrol in the type-2 diabetic cells.
-
2.
Autophagy
The molecular degradation system within the cell known as autophagy, not only degrades specific proteins but also breaks down lipids, DNA, and RNA by delivering them to lysosome [65]. Like inflammation, association of autophagy with T2D through ER stress is vastly investigated [66]. Autophagy is a prominent player, amending degradation-regeneration inside cellular system by providing continuous flow of raw materials for anabolic process. The ambiguous function of autophagy in cellular homeostasis, or autophagic clearance of toxic cellular components through aggresomes is spectacular in itself.
In detailed, autophagosome construction marks the beginning of autophagy originating from ER membranes through four main steps namely initiation, nucleation, elongation, maturation and fusion which are synchronized by autophagy-related genes (Atgs) [25]. Instigation of autophagy begins with activation of Ulk1 complex which mediates phosphorylation of Atg13, Atg101 and FIP200 with consequential building up of pre-autophagosome [67, 68]. Ulk1 activates Beclin1 complex through phosphorelation resulting in vesicle nucleation [69]. Simultaneously elongation process initiates with enrollment of other essential machinery of the membrane such as p62 or SQSTM1 an ubiquitin cargo-binding protein and Autophagy-related NBR1 protein that acts as partial selective receptor for different substrate in cell. Two other important proteins namely, MAP1 and LC3-I are obligatory for elongation and maturation of autophagosome and Atg12-Atg5-Atg16L1 complex helps to target particular substrates and proteins to the developing autophagosome [70]. LC3-I the cleaved product of pro-LC3 is a cysteine-protease (Atg4), directed to autophagosome membrane, is later activated by E1-like enzyme Atg7 bound to E2-like enzyme Atg3 and gets conjugated to phosphatidylethanolamine forming LC3-II [71]. The mature autophagosome then amalgamates with lysosome by SNARE proteins and Rab7 forming autolyasosome that gets degraded in the acidic environment.
Autophagy is controlled through several regulated and inhibitory complexes like AMPK that regulate this complex cascade by activation of Ulk1, and mTORC1 which inhibits Ulk1 by phosphorylation [72]. In nucleation, Bcl2 bind and inhibit Beclin1, while, at lysosomal interphase, v-ATPase if inhibited could severely affect lysosomal biogenesis. mTORC1 are another sets of inhibitory enzymes that inhibits TFEB by phosphorylation and influencing biogenesis of lysosome [73]. TFEB also plays a prominent act in activation of autophagy, where if not phosphorylated, it may translocates into nucleus and clings to CLEAR sequence (a genetic up-regulation and expression of proteins crucial for lysosomal biogenesis) and involved in autophagic pathway. ZKSCAN3 a TF, which unlike TFEB, inhibiting autophaagy requiring proteins and severely affects lysosomal biogenesis.
According to current studies Atg7 is highly exploited in induced authophagic activation in animal cell-lines treated with saturated fatty acid. Atg7 induces a lysomal cysteine protease (cathepsin B) which contributes towards Atg7-induced NLRP3-dependent pro-inflammatory response and aggravates lipotoxicity [26]. Unlike chronic hypoxia, acute hypoxia activates autophagy induction of deacetylase SIRT1, either inhibition of mTORC1 or by deacetylation of multiple components of autophagy machinery such as Atg5, Atg7 and LC3 in T2D [11, 74]. Investigation shows that in prediabetes subjects, causing of T2D could be delayed by an “adaptive” increase in mitophagy which may result in preservation of β-cell function [12]. Therefore, Marasco and Linnemann [11] in their review stated that mutation in genes essential of mitophagy such as Clec 16a, Pdx1, Pink1 and Park2/Parkin may contribute towards progression of T2D . Zummo et al. [75] in their research have developed a therapeutic strategies intervention for T2D by modulating autophagy/lysomal homeostasis with GLP-1 signaling pathway. Likewise, there are heterogeneous experimentations designed to find novel natural biomolecules that could either autophagy/lysomal homeostasis or their restoration. Recently Xu et al. [76] found that trehalose a natural non-reducing disaccharide could restore hyperglycemia-impaired autophagy. Current reports suggest downregulation of macroautophagy in β-cells of T2D patients marked by reduced expression of LC3-II marker as well as increased level of p-62 substrate [77, 78]. It is evidenced by Rezabakhsh et al. [79] that quercetin is a plant flavonol, could endorse autophagy flux by inducing LC3-II, Beclin-1 and increased utility of p62. These investigation made are profound example where, novel therapeutic intervention for treatment of T2D by manipulating at the sub-cellular level.
-
3.
Epigenetics
Present investigations have reported that, more than 100 genetic alterations could be linked to progression of T2D, majority of which affects insulin secretion [13]. According to earlier interpretation, epigenetic modifications can be depicted variation inherited somatically within genome, without altering DNA sequence of an organism, whereas epigenomics regarded as complete set of these changes occurring in that particular organism [14]. These modifications of the genome could be represented as a tool that enables stable propagation of gene expression from parents to offspring through histone modification, DNA methylation and non-coding RNAs [15, 80,81,82].
4.1 Histone modification
Nuclear proteins in histone octamers could be post-translationally modified on any residue. These modifications are known as histone modification, or described as amino termini modification of histone by 18 different post-translational modifications [83,84,85]. In histone modification, acetylation and methylation in H3 and H4 molecules lysine residues at amino termini are the most studied processes [80, 84]. Unlike histone tail modifications (that influences nucleosome dynamics, chromatin compaction and transcription) histone core modifications were basically indeterminate until recent reports of its effect [86, 87]. Histone core could be modified by acetylation, methylation, citrullination and phosphorylation affecting chromatin structure by modulating histone-histone, histone-DNA and histone-chaperones [88, 89]. Histone modification regulates physiological processes through transcriptional activation, induced by increased acetylation, while repression through decreased acetylation of histone molecule [84]. Pathophysiology of T2D due to histone methylation is generally induced by transient hyperglycemia that changes NFκB-p65 expression in vascular epithelial cells [90, 91]. Therapeutics in the present era such as GIP and GLP-1 increases global acetylation of H3 at lysine residues 9 and 18, with increase phosphorylation at serine 10. This further increases its association with TFs, phosphor-CREB and with cAMP-response CREB coactivator 2, and targeting IL-1β [80, 92].
4.2 DNA methylation
DNA methylation is a different set of epigenetic regulator, where carbon-5 of cytosine (C) preceding guanine (G) is methylated by DNA methyltransferase. Majority of these C preceding Gs or CpGs are broadly scattered all over the genome [93]. Previously epigenomic studies had revealed that, there are approximately 450,000 CpG sites which is about 1.5% of all CpG sites in human genome [15]. But currently with Infinium MethylationEPIC BeadChip Kit more than 850,000 CpG sites at a single-nucleotide resolution could be studied which is about 2.833% of all the CpG sites in human genome [94]. Further, methylation status of CpG islands within promoter region immensely influences binding affinity of TF to DNA binding sites thereby regulating gene silencing, transcriptional regulation of cellular differentiation in specific tissue, promotes inactivation of X chromosome etc. [95,96,97]. T2D which was previously contemplated as a nightmare for geneticists due to weak genetic component, is gradually changing as increasing number of associated genes are being discovered lately. It is now accepted that genes like Ins, Pdx1, (PPARGC1-alpha), Glp1r and IL1R1 are hypermethylated in Type-2 diabetic donors [98, 99]. These genes orchestrate significant role in Type-2 diabetic pathophysiology like encoding insulin and are essential TFs both for development and functioning of mature β-cell (Pdx1), mitochondrial transcriptional coactivator (Ppargc1-α), receptors that stimulates insulin secretion and protects activated β-cell mass (Glp1R). Moreover, higher methylation level was associated with reduced mRNA expression in diabetic β-cells as well as higher HbA1c formation indicating critical role of methylation in T2D [100]. Studies involving methylation confirmed that out of 27000 CpG methylated sites in Type-2 diabetic donors, a total of 1649 CpG sites are altered, annotating 843 genes with 102 varied genes. Cdkn1A, Pde7b and Sept9 are examples of genes, whose increased expression and decreased DNA methylation in diabetic islets are observed [15, 81].
4.3 Non-coding RNAs
ncRNAs are emerging as novel multipotent regulators of T2D pathogenesis [101,102,103]. At the dawn of present decade, it is established that ncRNA like miRNAs are only fraction of ncRNA that displays its impact in multiple health disorders including diabetes [104,105,106]. Functionally there are two major groups of ncRNAs structural and regulatory ncRNAs [107]. Structural ncRNAs are classified as tRNAs and rRNAs whereas regulatory ncRNAs are categorized based on base pair length as short ncRNAs, mid-size ncRNAs and lncRNAs [104, 107, 108]. In T2D pathophysiology, there are three regulatory ncRNAs including two long ncRNAs (linear and circular lncRNAs) and one short ncRNAs i.e. miRNAs [102, 103, 106]. Major fraction of β-cell transcriptome constitute of ncRNAs where involvement of miRNAs are well established but the exemplified function of lncRNAs in modulating β-cell is gradually being unrevealed in the present scientific era [109,110,111]. Recently Reichelt-Wurm et al. [112] revealed differential expression pattern of 1746 lncRNAs that was noticeably different from miRNAs associated with diabetes in mouse model. lncRNAs like, Meg3 regulates and maintains β-cell’s identity by adjusting insulin production, programmed cell death and also promotes insulin production in hepatocytes. Zhu et al. [113] demonstrated that promotion of IR by Meg3 could possibly be elevated via expression of FoxO1 regulated by ATF4, which itself is suppressed by miRNA-214. Similarly, there are number of other lncRNAs like β-cell long intergenic noncoding RNA 1 (βlinc1) that is important for specification and function of insulin-producing β-cell which must be further studied for deeper insight into β-cell failure [114].
5 Nanotechnology based therapy for reversal of type-2 diabetes
Nanotechnology in present era is being orchestrated as an influential amalgamation of heterogeneous scientific fields. Expandability of these nano-systems to be engineered, in a customizable manner to address specific biological problems from multiple fronts is in itself a magnificent achievement. Execution of this idea to device a therapeutic strategy against diseases and disorders like T2D is slowly catching its drift in present decade. To understand in depth how nanotechnology could combat T2D, it is a basic necessity to know differential types of NPs or nanosystems presently available. For instance, inorganic, organic and hybrid NPs / nanosystems are the three basic categories of NPs or nanosystems. Inorganic NPs are simple metalic NPs and rigid nanosystem in the form of nanospheres, nanocapsule and nanocage. Whereas, organic NPs are extra flexible/ soft organic matrixes like liposomes, dendrimers, niosomes, micelles etc. [115]. Lastly, hybrid NPs / nanosystems which are most intricately customized nanodelivery systems, widely investigated in present era. These smart nanosystems in simple terms are standardized amalgamation of inorganic-organic, inorganic-inorganic (nanocomposites), and organic-organic nanoparticulated systems (lipid–polymer hybrid NPs) engineered according to the specific requirement or use at targeted site [116].
5.1 Inorganic nanoparticles (NPs)/ nanosystems
Inorganic NPs/ nanosystems are either simple metalic NPs like Ag, Au, ZnO, Se, CeO2 or rigid nanosystem including SiO2/TiO2 nanospheres or nanocapsule and carbon based nanotubes or nanocage. Though possibility to completely cure diabetes using these inorganic NPs/ nanosystems is questionable, but amelioration of the post diabetic effects without any side-effects is not out of reach (Table 1).
In detail when synthesized AgNPs subjected to STZ induced male albino rats a raise in the level of antioxydent enzymes like SOD, Catalase, GPx and GRD was observed [118]. This might indicate that AgNPs could alleviate oxidative stress under diabetic conditions. Kouame et al. [120] recently showed that Cinnamomum cassia aqueous extract mediated Green synthesized AgNPs subjected diabetes induced SD male rats, a reduced level of glucose, serum urea, creatinine, glutathione and malondialdehyde was observed. This suggests the possible role of AgNPs in modulating oxidative stress markers and renal function parameters. Green synthesized AuNPs also could alleviate certain post diabetic effects. Evidence suggests that when diabetic albino mice when injected with AuNPs an increases expression of reduced GSH, SOD, CAT, GPx produced [122]. Shilo et al. [124] demonstrated prevention rapid degradation of conjugated insulin, blood glucose concentration in diabetic male BALB/c mice after administration of insulin-coated AuNPs. The study confirmed that insulin-coated AuNPs possesses an adjustable and prolonged effect on hyperglycemia and are potential for diabetic treatment.
Other metallic NPs like ZnONPs synthesized by Hussein et al. [127] showed reduction in blood glucose, maintains in serum insulin and MDA in diabetic male albino rats after NPs ingestion. Nazarizadeh et al. [130] in consecutive year added that ZnONPs could also improves glucose disposal, insulin level, and zinc status and raise levels of lipid peroxidation but in diabetic male Wistar rats. Later, El-behery et al. [131] and Afify et al. [133] demonstrated the efficacy of ZnONPs in restoring architecture of semi-niferous epithelium and significant decrease in HbA1c respectively. Evidence suggesting anti-hyperglycemic activity of SeNPs is slowly coming up lately. Study conducted by Al-Quraishy et al. [134] suggests reduced blood glucose, lipid, cholesterol level, glucose-6-phosphatase activity and an enhanced serum insulin concentration, malic enzyme, hexokinase and glucose-6-phosphate dehydrogenase activity in diabetic adult male albino Wistar rats after SeNPs treatment. Additionally, alleviation of oxidative stress, improvement in pancreatic islet function and enhanced glucose utilization activity was also observed in diabetic SD rats and Goto Kakizaki rats after insulin-loaded SeNPs treatment [135]. Recently, Deng et al. [137] demonstrated that oral administration of mulberry leaf and Pueraria Lobata extracts loaded SeNPs to diabetic SD rats and Goto Kakizaki rats could effectively alleviates the oxidative stress, improve the pancreatic function, and promote glucose utilization by adipocytes. Similarly Abdulmalek and Balbaa [138] showed that HFD/STZ-induced adult male Wistar rats when administered with SeNPs and metformin improves in insulin signaling proteins like pIRS1/pAKT/pGSK-3β/pAMPK was observed.
Over 50% of diabetic patients are affected by neuropathy and presently the impact of CeO2NPs in limiting the worsening condition of diabetic neuropathy is promising, as per current research. Studies conducted by Najafi et al. [139] shows that diabetic male Wistar rats when subjected to CeO2NPs total thiol molecules, total antioxidant power, ADP/ATP ratio and body weight were recovered. Additionally, a decrease in lipid peroxidation and nociception latency was also observed. CeO2 nanocubes were efficient in angiogenesis under diabetic condition in Goto Kakizaki rats by interrupting TNF-α secretion at the transcription level, elevated EGF expression and in diminishing inflammatory reaction [141]. Recently, Pascual et al. [144] showed that CeO2NPs could also lessen the extracellular ROS and escalating expression of ADIPOQ and Il10 in diabetic mouse 3T3-L1 adipocytes. Antidiabetic drugs of protein origin are receiving mounting interest due to their specific functions and fewer side effects. The extensive application of protein drugs are stalled due their intrinsic properties such as structural and physiochemical sensitivity to temperature and pH from an entropic point of view [167]. Current evidence suggests that amphoteric NPs like SiO2 have limited therapeutic effect but serves as an excellent delivery system for protein based drugs [168]. In relation diabetes Hei et al. [150] designed an intelligent glucose-responsive insulin delivery system based on MSNP loaded with phenylboronic acids as glucose-responsive units and zinc oxide NPs as the capping agents. Hybrid MSNPs-Metformin developed by demonstrated by Patiño-Herrera et al. [151] prevented excessive drug ingest and increased release time of the drug. As per ongoing research, MSNPs are effectively exploited as a delivery system for either synthetic/ natural drug or natural antidiabetic proteins (Table 1).
The same is speculated for carbon based nanodelivery systems like fullerene, carbon nanotubes, carbon nanohorn, grapheme, carbon dots and comparatively related NPs for diagnosis like quantum dots. Recent investigations impact of amino-acid-functionalized GFNPs on db/db diabetic mice showed a promising improvement of their condition. The GFNPs could decrease hyperglycemia, reversed the pancreas islets dysfunctions and normalized the insulin secretory function, improved hepatic IR, inhibition of GNG and increasing glycogenesis in the liver [154]. As these carbon based nano-systems are an emerging platforms for both treatment and diagnosis therefore limited advancement are reported. Table 1 enlists carbon nanohorn, graphene and quantum dots in sensing glucose non-enzymetically, diabetic wound healing by boronic acid functionalized graphene in Wistar albino rats and efficient anti-diabetic drug release by carbon dots [157, 160, 161, 163, 165]. Current antidiabetic research involving inorganic NPs are mostly focused on sensing glucose or insulin [169,170,171,172]. Alghazzawi et al. [169], developed a non-enzymatic glucose detection sensor using rGO/NiO nanocomposite that exhibited good sensitivity, selectivity and was repeatable, stable, and economical. Similarly, Lavanya et al. [171] and Inyang et al. [172] also engineered non-enzymatic glucose detection sensors but with pure MgNi2O3 and MgNi2-xAxO3 (A = Co or Zn; x =2.5, 10, 25 wt.%) NPs and CuO NPs on a FTO respectively. Both of the detection systems were highly efficient in sensing glucose. Later, an aultra sensitive and highly selective immunochemical insulin detection system based on Ag/ZnIn2S4/RGO quenched by Au@SiO2 NPs was developed by Khan et al. [170].
5.2 Organic nanoparticles (NPs)/ nanosystems
Though inorganic NPs are efficient delivery systems but they have certain limitations such as rigidity, size dependent toxicity and nano-accumulation after chronic ingestion. To rule out such limitation, beneficiary properties of organic NPs like the ability to be metabolized after unloading their therapeutic contents are being recognized by researchers [95]. The mode of delivery of therapeutics could be modulated by selecting specific design features and chemical requirements for the synthesis purpose. Since, sustained release or burst release kinetics can directly influence therapeutic competence and toxicity of the nanosystem therefore, selection of appropriate component of the organic NPs based on their release properties is of concern [203]. Multiple form of organic NPs are exploited in field of nanomedicine but only certain specific organic NPs are presently being evaluated for their antidiabetic activity. These includes polymeric NPs, lipid based NPs, dendrimers, neosomes and nanomicelles (Table 2).
Heterogeneous verity of polymers (synthetic and natural) have been identified which greatly contributed towards drug delivery properties such as biodistribution/ bioavailability, biocompatibility, immunocompatibility etc. in biomedical sector [115]. Polymeric NP developed by Fang et al. [179] was efficient for pH based insulin delivery to diabetic SD rats. El-Naggar et al. [180] in a similar manner developed PLA-PEG copolymer NPs for the delivery of natural compound (Curcumin) to diabetic male white albino rats. Positively they found that the curcumin loaded nanosystem could trigger a remarkable decrease of serum glucose level, reduces the diabetic rate and attenuates liver inflammation, suppress NF-κB activation, COX-2 and TGF-β expression [180]. Even the simple form of polymeric NPs like chitosan NPs proves to be efficient in delivery of polydatin to diabetic male Wistar albino rats, which significantly improves body weight and hepatic glycogen contents [178]. Therefore, most of the investigation involving polymeric NPs are diverted towards controlled delivery of antidiabetic therapeutic agents [181,182,183]. Recently an effort was made by Laddha and Kshirsagar [181] to deliver pioglitazone-a a PPAR-γ agonist through topical administration by a surface modified PLGA NPs to posterior section of the eye. The entramp efficiency and release study was done in vitro which showed better entrampment efficiency and initial initial burst release followed by controlled release of pioglitazone-a. The in vivo delivery efficiency was also experimented on STZ-induced diabetic Wistar rats which showed controlled release of the PPAR-γ agonist which had an dose dependent impact on reduction of VEGF concentration in vitreous fluid [181]. Likewise, Arun et al. [182] developed Thyroscyphus ramosus derived chitosan nanodelivery system for the delivery of Metformin to STZ-induced diabetic albino Wistar rats, which was proved efficient in delivery. In virto delivery of insulin was attempted by Wong et al. [183] to skeletal muscle cells. The capability to induce glucose uptake in C2C12 and HT29 cell lines by in virto delivery insulin-loaded chitosan NPs showed promising results.
Ongoing advancement in solid-lipid NPs and outstanding efficiency of nanostructured lipid nanocarriers as a biocompatible vehicle in therapeutic delivery have stunned present researchers. Further, their well structured bio-absorbable and biocompatible properties have granted them a unique prospective to explore [204]. Limitations like transportation of therapeutics across major barriers such as BBB are now nullified. Preliminarily investigation on solid-lipid NPs by Sarmento et al. [184] devised a cetyl palmitate-based solid lipid NPs for insulin delivery to male diabetic Wistar rats. The lipid nanoteraputic could significantly reduce glucose levels, protects insulin and promote the insulin absorption in diabetic rat [184]. In vivo transdermal patches of metformin loaded solid lipid NPs in diabetic rats were significant in sustained decrease in bloog glucose level [185]. LMWP-fused growth factors (EGF, IGF-I), and PDGF-A complexed with HA, were encapsulated within cationic elastic liposomes to determine diabetic wound-healing efficiency in Human fibroblast (CCD-986sk) cells, female C57/BL6 mice. Modified growth factor loaded cationic elastic liposomes promoted fibroblast proliferation and the production of procollagen significantly, improves skin permeation, also significantly accelerates angiogenesis rate [186]. Currently lipid based NPs are also extensively experimented for evaluating their efficacy in delivering antidiabetic therapeutic agents [193, 194]. Recently in a research by Shaveta et al. [194], showed that, PGL conjugated SLNs loaded with pioglitazone an antidiabetic agent for the determination of in vitro and in vivo drug delivery efficacy via spectrophotometric analysis and in male albino Wistar rats induced with low dose STZ respectuively. Their result demonstrated that SLNs had low particle size, high encapsulation efficiency, high and prolonged release activity [194]. Nanostructured polymer-based cochleates [lipid-based polymer] are also being used for delivery purposes of insulin [193]. Khair et al. [193] demonstrates that insulin loaded polymer-based cochleates served as a promising delivery tool verified via in vivo delivery efficiency in SD rat model (Table 2).
Well-organized, hyperbranched organic compound such as dendrimers are receiving much attention in current scenario owing to their enhanced properties such as monodispersity, biocompatibility and biodegradability [205]. These compounds are also an extraordinary vehicle for gene, peptide or drugs (natural/ synthetic) delivery at the targeted site [206]. Despite the previous utility in biomedical science, their investigations as a carrier for antidiabetic drugs are now gradually gaining much appreciation. Some of their antidiabetic efficiencies include lessening of blood glucose concentration and reduction of diabetes-induced permeabilization of blood–brain barrier [195]. PAMAM dendrimer G3 could improve efficiency of impaired respiration of diabetic Wistar rat’s cardiac cell mitochondria [196]. Presently inhibition of EGFR-ERK1/2-ROCK, improved vascular remodeling and dysfunction were achieved in diabetic male Wistar rats by nano-sized PAMAM dendrimers [198] (Table 2).
Organic non-ionic surfactant-based vesicle like noisome, has emerges as the centre of attraction for current investigators. Though these nano-delivery systems have ample distributed applications in diseases management but their efficacy as an antidiabetic drug carrier is limited. The investigations made showed significant hypoglycemic effect, increases in SOD, CAT, and GSH, along with decreased lipid peroxidation level was obtained by embelin loaded nano-niosome in diabetic albino Wistar rats [199]. Recently it was observed that gymnemic acid loaded niosomes could substantially decreases antioxidant level, lipid levels, and significantly reduces pro-inflammatory cytokines like IL-6, TNF-α and fibronectin levels in Wistar rats [200].
Other organic NPs like nanomicelles which are described as supramolecular assemblage of surfactant molecule distributed in a colloidal liquid that gives rise to a globular micelle ranging in nano-size. Efficacy in their encapsulation, biocompatibility, colloidal stability and prolonged circulation time are some of the important properties of these polymeric micelles [207]. Specifically like PEG-PLA nanomicelles encapsulating curcumin are described to be significant in augmenting re-epithelialization of epidermis and collagen aggregation in the wound area consecutively accelerating angiogenesis, fibroblast accumulation in diabetic adult male BALB/C mice [201]. Again PVCL-PVA-PEG nanomicelle loaded with curcumin promotes corneal epithelial diabetic wound healing in diabetic male C57BL/6 mice [202].
5.3 Hybrid nanoparticles (NPs)/ nanosystems
Inorganic and organic NPs / Nanosystems are efficient but might be extra efficient in a combined form i.e. hybrid NPs, which is by far much sophisticated form of NPs/ Nanosystem. Starting from inorganic NPs such as Ag, Au, Se, SiO2, CNT, fullerene and graphene oxide towards organic NPs such as polymeric NPs, lipid NPs and niosomes all are been explored for their antidiabetic activity. Hybrid nanosystems can be considered as a trailblazing/emerging technology both diagnosis and therapy of T2D [208, 209].
Diagnoses of diabetes through hybrid nanosystem based therapy are successful only to specific test like detection of glucose in blood. The nano-based advancement in present prospective includes development of sensors like non-enzymatic glucose detection, nano-based glucose sensor and electrochemical glucose detection [211, 213] (Table 3). Dai et al. [214] developed a Cu2O–BSA core-shell nanoparticles modified glassy carbon electrode which exhibited good glucose detection with a lowest detection limit, with linear detection range and a high sensitivity along with reliable anti-interference property to uric acid, ascorbic acid, and acetaminophen. Recently Ni NPs-modified graphene–diamond hybrid electrodes was developed by Cui et al. [213] exhibits efficient glucose sensing capacity with higher resolution in detection. Porous NiMn2O4 nanosheet arrays on nickel foam a form of non-enzymatic glucose detection sensor exhibited high sensitivity and a fast response [211]. Further in-depth research is required to further broaden the detection of other bio-molecules associated with diabetes like advanced glycation end-products in blood. Over all almost every research conducted related to diabetes including inorganic NPs even at present is also predominantly directed towards development of nano-biosensors for detection of glucose and insulin concentration [215, 216].
Treatment of T2D with the help of hybrid nanosystems is much more efficient due to a specific property that is target specific delivery (Table 3). Curcumin-encapsulated PBLG-PEG-PBLG NPs could effectively alleviate pathological morphological damage of myocardium, increased H2S and [Ca2+] levels, and unregulated the expression of calcium-sensing receptor, endogenous cystathionine γ-lyase, and calmodulin in diabetic male SD rats [227]. Mukhopadhyay et al. [229] showed that succinylated chitosan-alginate core-shell-corona NPs encapsulating quercetin could maintain glucose homeostasis, significant reduction of blood cholesterol and triglyceride in diabetic male Wistar rats. Delivery of proteins like insulin by mucoadhesive NPs based on mucin-chitosan complexes and chitosan-coated cationic liposomes is gaining much attention lately [228, 230]. With the advancement of science, progresses made in the field of nanotechnology especially encompassing hybrid nanodelivery system in diabetic researches are lately being prioritized, essentially due to their unique properties like targeted delivery and tunable release efficiency etc. [18, 241, 243, 245]. Currently hybrid nanosystems like CA/Gel electrospun mat loaded with berberine and TA/Al3+ NPs systems loaded with liraglutide are being explored for verifying their effect on deibetic complications like diabetic foot ulcer and prediabetic glycemic condition respectively [241, 245]. The therapeutic efficiency of nanostructure loaded berberine L929 cell line in dult Wistar rats showed complete epithelialization and less infiltrated polymorphonuclear inflammatory cells deposition [241]. Whereas the iraglutide loded nanosystem showed exceptional delivery efficiency in T2D db/db mice [245]. Pouran et al. [242] have experimented insulin therapeutic efficacy of Green synthesized ZnO/Ag nanocomposites in alloxan-induced diabetic Wistar rats. They observed that nanocomposite was efficieni in enhancing of the insulin secretion and HDL-C plasma levels, while successfully reducing the FBG, T−CHOL and T-TG levels in diabetic rat model [242]. Lately, in an in vivo study was conducted where, the wound healing property of M-SeNPs-CCH complex nanohybrid system was verified on diabetic Wistar rats infected with Staphylococcus aureus [243]. The study confirmed that the nanohybrid system was effective in wound contraction, angiogenesis, fibroblastosis, collagenesis, and proliferation of hair follicle and epidermis [243]. In summation it will not be wrong to state that hybrid nano-delivery system holds immense potential in lessening the severity T2D and associated complications. But still advancements in nanotechnological research needs to progress, in refineing the current hybrid nano-based therapeutic systems for T2D treatment.
6 Future prospective of nanotechnology in reversal of type-2 diabetes
As previously described cutting-edge advancements made in understanding T2D, it clear that dynamic machineries starting with inflammasome, autophagy, and epigenetics could immensely impel the prospect of diabetic therapy. Numerous factors contribute towards atiology of T2D which are also described in detail previously. Therefore recent investigations must make efforts such as induction of β-cell proliferation and differentiation, regulation of inflammasome, autophagy, and epigenetics to alleviate T2D.
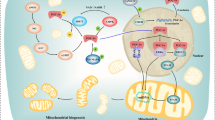
Inducing β-cell proliferation and differentiation by activation of Wnt signaling pathway is one such possibilities that can be achieved through a targeted nanodelivery system. In this signaling pathway, arresting the expression of one specific component within degradation complex i.e GSK-3, may result in intact β-catenin, which could then translocates into nucleus and promotes β-cell proliferation [246]. A targeted PNDS could be designed for in vivo for activation of the Wnt signalling pathway. The PNDS then could be loaded with NTP in conjugation with GSK-3β antisense oligonucleotide for gene silencing of GSK-3β. Later, this PNDS can be tagged with an agonist against pancreatic receptor specific to human β-cell for target-specific delivery. The peptide and antisense oligonucleotide conjugate could migrate into the cells through endocytosis and NTP could lead antisense oligonucleotide into the nucleus. Once inside the nucleus, antisense oligonucleotide could silence GSK-3β gene, which ultimately may inactivate β-catenin destruction complex ensuing in unhindered Wnt signaling pathway and promote β-cell proliferation and differentiation (Fig. 4).
In vivo activation of Wnt signalling pathway using polymeric nano-delivery system (PNDS). In absence of Wnt molecule, active β-catenin degradation complex degrades β-catenin and suppresses the proliferation of β-cells in Islets of Langerhans. The PNDS mediated targeting and release of antisense RNA (for example, Gsk3β) in the nucleus could destroy the Gsk3β which is required for stability of degradation complex. In absence of Gsk3β, β-catenin could be translocated to the nucleus for gene expression. Inspired from [246]
Acknowledging the role of inflammasomes in T2D, an effort can be made for targeted silencing of specific inflammasomes modulators that could effectively alienate metabolic stress. The present review provides an effective target motive to regulated T2D by nano-based targeting (Fig. 5). A number of investigations also suggest defensive mechanism of inflated autophagy against redox stress in β-cells as a preliminary action [7]. Further, it is also evidenced that under diabetic condition glucolipotoxicity based oxidative stress in β-cells may affect sensitive catalytic thiols of Atg3 and Atg7 which prevents LC3 lipidation, resulting in negative regulation of autophagy [247, 248]. Under such circumstances, natural antioxidants like bioactive phytocompounds could be encapsulated or linked to a β-cells targeted nano delivery system and evaluated for their antioxidant activity and revival of autophagy (Fig. 6).
The Schematic for the role of NLRP3 inflammasome in type-2 diabetes and its targeting by nanoparticles. Damage associated molecular pattern (DAMPs) such as islet amyloid polypeptide (IAPP), endoplasmic reticulum (ER) stress, reactive oxygen species (ROS), urate, extracellular ATP, fatty acids, ceramide can be recognized by NLRP3 inflammasome in myeloid cells. The assembly of NLRP3 with ASC and procaspase-1 activates inflammasome which cleave CARD domain to release active caspase-1. Caspase-1 converts pro IL-1β and pro IL-18 in to active IL-1β and IL-18. Such proinflamatory cytokines may induce insulin resistance or reduction in mass of pancreatic β-cells leading to development of diabetes mellitus. The different modulators of inflammasome can be carried by NPs to target the inflammasome in the myeloid cell. Inspired from [9, 22,23,24]
Role of inactivation of autophagy by Atg3 and Atg7 oxidation in Diabetes mellitus. The transfer of LC3 from inactive Atg3 & Atg7 to phosphatidyl ethanolamine (PE) results in maturation of autophagosome and activation of Atg3 and Atg7, which become vulnerable to glucolipotoxicity based oxidative stress under diabetic condition. Under oxidizing conditions, the catalytic thiol group of both Atg3 & Atg7 form intermolecular disulfide bond within same complex or if only a single catalytic thiol is present within the Atg3/ Atg7 complex, then oxidation will likely lead to stable S-glutathiolation (GSH). Use of targeted nanodelivery system carrying natural antioxidants like bioactive phytocompounds in β-cells can help in neutralization of the reactive oxygen species (ROS) and revival of autophagy for control of diabetes. Inspired from [25, 67, 69, 71]
Additionally, within secretory granules under low pH, insulin and proinsulin inhibits aggregation and maintains IAPP in soluble state. Further, in conjunction with glucose stimuli in β-cell, IAPP is coexpressed and co-secreted with insulin, thereby enhanced expression of IAPP aggregate formation can be a likely scenario under high insulin demand (IR) [249]. To remove these aggregation cells mainly deploy three mechanisms which includes ubiquitin protease system, autophagy, and aggresome formation. Out of three majorly investigated enzymes, Zn2+ metalloprotease named IDE which is generally involved in the clearance of insulin also degrades IAPP aggregations [250, 251]. THE second IAPP degrading enzyme, NEP is a type-2 Zn-containing metalloprotease expressed in diverse cell-type including β-cells could be an alternative target [252]. Lastly, UCH-L1 abundantly expressed in β-cells degrades IAPP by promoting its ubiquitination can also be targeted in therapeutic intervention purposes [253]. Recent research states that autophagy abnormalities specifically Atg 7 gene in β-cells may lead to the deposition of protein aggregates [254]. Sequestosome 1 (p62/SQSTM1) a multifunctional stress-inducible scaffold protein can form cytosolic protein inclusion with simultaneous degradation of specific protein clump by aggrephagy could be an example of such phenomenon [250, 255]. The possibilities of powering these enzymes and other protein degradation systems as a therapeutic measure against neural diseases show promising results, investigations in diabetes research might also lead to certain novel findings [251, 256, 257].
Disruption of epigenome homeostatic balance that is deregulation in DNA methylation, histone modifications, and RNA-mediated processes, may cause assorted pathologies impact, finally leading to T2D [15]. DNA methylation patterns within several loci such as Hnf4A, Irs1, Kcnq1, Pparg, Fto, and Tcf7L2 are some of the genetic variants persuading to T2D have been acknowledged recently [258]. Like DNA methylation, post-translational modifications to histone proteins such as variation in the levels of histone PTMs such as H3K9ac and H3K4me at key fibrotic, inflammatory, and cell cycle genes, are also concerned in the development of diabetes and its vascular complications [259]. Under the diabetic condition, oxidation results mostly from glucotoxicity and lipotoxicity, affecting the DNA methylation, post-translational modifications to histone proteins. These effects could be reversed preliminarily by countervailing the oxidative stress by implementing the effectiveness of natural antioxidant compounds, delivered through a targeted nano-delivery system. Recently, through wet and dry lab analyses of published reports, it was understood that Prkar1A gene is correlated to glucose metabolism, blood coagulation, and insulin signaling and targeted by miRNAs in T2DM. Later Pordzik et al. [260] observed that miR-30a-5p, miR-30d-5p, and miR-30c-5p are the most extensively regulated miRNAs across all specified ontologies and hence indulged as most promising biomarkers of T2DM to be confirmed in future clinical studies.
7 Conclusion
With a present death rate of 5 million per year and the future 7th cause of death, diabetes, alone holds a global expenditure of 760 billion US dollars per year. Despite the investment made for the treatment of diabetes, the possibility of a novel therapeutic intervention is questionable and the available commercialized therapeutics demonstrates limitations that need to be addressed. The conceptualized refinement of available commercialized anti-diabetic therapeutics has become the need of the present scientific community. This refinement will be abridged if the amalgamation of present cutting-edge science like nanotechnology is restricted. Additionally, if current evolving knowledge on intracellular modulating factors involved in inflammasome, autophagy, and gene regulating epigenetic changes remains unacknowledged in T2D. Targeting these governing mediators/ modulators with a nano-targeting system could be an effective means for the treatment of T2D. From the present review, advancement in nanoscience for the reversal of T2D has been highlighted, exploiting cellular and molecular modulating factors like auto-inflammatory, autophagic, genetic, and epigenetic factors. Therefore future research should concern in exploiting some of these strategies mentioned in this review to ensure the probability of devising a smart therapy for the reversal of T2D in near future.
Data availability
All data related to the manuscript are available in the form of tables and figures in the manuscript.
Abbreviations
- (TA)/Al3+:
-
Ternary liraglutide/tannic acid
- A-FABP:
-
adipocyte-specific fatty acid-binding protein
- AFT6:
-
activating transcription factor 6
- Ag:
-
Silver
- Ag/ZnIn2S4/RGO:
-
Silver/ZnIn2S4/reduced grapheme oxide composites
- Ag@AuNPs:
-
Core shell silver-gold nanoparticles
- AgNPs:
-
Sliver Nanoparticles
- AIM2:
-
Absent in melanoma 2
- AMPK:
-
AMP-activated protein kinase
- AMPK:
-
AMP-activated protein kinase
- ARG-EG:
-
Argpyrimidine-tagged rutin-encapsulated biocompatible (ethylene glycol dimers)
- ASC:
-
Apoptosis-associated speck-like protein
- ATF4:
-
Activating transcription factor 4
- Atg 7:
-
Autophagy Related 7
- Atgs:
-
autophagy-related genes
- Au:
-
Gold
- Au@SiO2 :
-
Gold decorated silicon dioxide nanoparticles
- AuNPs:
-
Gold Nanoparticles
- BINP:
-
Biomineralized insulin nanoparticle
- BSA:
-
Bovine serum albumin
- BSC-dots:
-
Biocompatible carbon dots derived from κ-carrageenan and phenyl boronic acid
- C:
-
Cytosine
- C60HyFn:
-
Hydrated C60 fullerene
- CA-Gel:
-
Cellulose Acetate/ Gelatin
- cAMP:
-
Adenosine 3',5'-cyclic monophosphate
- CARD:
-
Caspase activation and recruitment domain
- CAT:
-
Catalase
- CBP:
-
CREB-binding protein
- Cdkn1A:
-
Cyclin-dependent kinase inhibitor 1
- CeO2:
-
Cerium Oxide
- CeO2NPs:
-
Cerium Oxide Nanoparticles
- CNP-miR146a:
-
CeO2 NPs conjugated MicroRNA-146a
- COX-2:
-
Cyclooxygenase-2
- CPMNP:
-
Chitosan and pectin with Metformin nanoparticle
- CREB:
-
cAMP-response element binding
- CREBH:
-
cAMP-response element binding protein-H
- CS-NAc:
-
Thiolated Chitosan- Maleic Acid
- CS-ZnO-NC:
-
Chitosan loaded curcumin-metal zinc oxide complex
- Cu2O–BSA NPs:
-
Copper(I) oxide–bovine serum albumin core-shell nanoparticles
- CuNPs-graphene:
-
Cu Nanoparticles Modified as-grown CVD Graphene
- DAMPs:
-
Damage associated molecular pattern
- DM:
-
Diabetes mellitus
- DOPA:
-
3,4–dihydroxyl– l–phenylalanine nanoparticles
- DPP-4:
-
Dipeptidyl peptidase-4
- EDC·HCl:
-
N-Ethyl-N′-(3-(dimethylamino)propyl)carbodiimide hydrochloride- hydrochloric acid
- EGF:
-
Epidermal growth factor
- EGFR:
-
Epidermal growth factor receptor
- ER:
-
Endoplasmic reticulum
- ERK:
-
Extracellularly Regulated Kinase
- EXT:
-
Exenatide
- FBG:
-
Fasting blood glucose
- FIH:
-
Factor-inhibiting hypoxia-Inducible Factor
- FIP200:
-
Focal adhesion kinase family interacting protein of 200
- FOXA2:
-
Forkhead Box A2
- FoxO1:
-
Forkhead box protein O1
- FTO:
-
Fluorine doped tin oxide
- Fz:
-
Frizzleds
- G:
-
Guanine
- GATA4/6:
-
GATA-binding protein
- GCE:
-
Glassy carbon electrode
- GFNPs:
-
Amino-acid-functionalized gadofullerene nanoparticles
- GFNPs:
-
Gadofullerene nanoparticles
- GIP:
-
Glucose-dependent insulinotropic-peptide 1
- GK:
-
Glucokinase
- Glis3:
-
Gli-Similar 3
- GLP-1 Ras:
-
peptide-1 receptor agonists
- GLP-1:
-
Glucagon-Like Peptide 1
- GLP-1:
-
Glucagon-like peptide-1
- Glp1r:
-
Glucagon like peptide 1 receptor
- GLUT4:
-
Glucose transporter 4
- GNG:
-
Gluconeogenesis
- GNP-GOx:
-
Glucose oxidase complexed gold-graphene Nanocomposite
- GNP-PEG-BRXL:
-
Gold nanoparticle-Poly ethylene glycol-Benzoxaborole
- GO:
-
Graphene oxide
- GPx:
-
Glutathione Peroxidase
- GrB:
-
Granzyme B
- GRD:
-
Glutathione reductase
- GSH:
-
Glutathione
- GSK-3:
-
glycogen synthase kinase 3
- GSK-3β:
-
Glycogen synthase kinase 3-beta
- GSV:
-
glucose transport vesicles
- H3K4me:
-
H3K4 methylation
- H3K9ac:
-
H3K9 acetylation
- HA:
-
Hyaluronic acid
- HAuCl4 :
-
Tetrachloroauric acid
- HbA1c:
-
Glycated hemoglobin
- HbA1c:
-
Haemoglobin A1c
- HDL:
-
High density lipoprotein
- HDL-C:
-
High-density lipoprotein- cholesterol
- HFD/STZ:
-
High-fat diet/streptozotocin
- HHNs:
-
Hydrophilic hierarchically-porous nanoflowers
- HIF-1α:
-
Hypoxia-inducible factor-1α
- HNF:
-
Hepatocyte nuclear factor
- HPMCP:
-
Hydroxypropyl methylcellulose phthalate
- HRE:
-
Hypoxia-response element
- hs-CRP:
-
High-sensitive C-reactive proteins
- HY-CNT:
-
Carbon nanotubes functionalized with sodium hyaluronate
- IAPP:
-
Islet amyloid polypeptide
- IDE:
-
Insulin-degrading enzyme
- IFNγ:
-
Interferon gamma
- IGF-1:
-
Insulin-like growth factor-I
- IkK:
-
Insulin Kappa B Kinase
- IL-1β:
-
Interleukin-1 beta
- IL-6:
-
Interleukin
- Ins:
-
Insulin
- Ins/NGs-PEC:
-
Insulin and nanogels polyelectrolyte complex
- INS-SeNPs:
-
Insulin-loaded SeNPs
- insulin/CMCD-g-CS NPs:
-
Insulin-loaded carboxymethyl-b-cyclodextrin-grafted chitosan nanoparticles
- IR:
-
Insulin resistance
- IRA:
-
Insulin receptor A
- IRE-1:
-
Inositol requiring endonuclease-1
- IRS:
-
Insulin receptor substrates
- JNK:
-
C-Jun N-Terminal Kinase
- K2S2O8:
-
Potassium persulfate
- LC3:
-
Light chain 3
- LDL:
-
Low density lipoprotein
- LMWP:
-
Low-molecular-weight protamine
- LMWP:
-
Low-molecular-weight protamine
- lncRNAs:
-
Long ncRNAs
- LPO:
-
Lipid peroxidation
- MCP-1:
-
Monocyte chemoattractant protein-1
- MDA:
-
Malondialdehyde
- Meg3:
-
Maternally expressed gene 3
- Metf:
-
Metformin
- miRNAs:
-
microRNAs
- MODY:
-
Maturity-onset diabetes of the young
- MPE:
-
Mulberry leaf and Pueraria Lobata extracts
- mRNA:
-
Messenger RNA
- M-SeNPs-CC H:
-
Selenium-Chitosan-Mupirocin complex nanohybrid system
- M-SLN:
-
Solid lipid NPs
- MSNPs:
-
Mesoporous silica nanoparticles
- MYD:
-
Myeloid
- NaAuCl4 :
-
Sodium tetra chloroaurate (III) dehydrate
- NAIP:
-
Neuronal apoptosis inhibitory protein
- NA-MWCNTs:
-
Nicotinamide-functionalized multiwalled carbon Nanotubes
- Nano-EE:
-
Echinacea purpurea extract loaded Silica Nanoparticle
- Nano-PAC:
-
Petri dish-cultured Antrodia cinnamomea Nanoparticle
- NBR1:
-
Neighbor of BRCA1
- ncRNAs:
-
Non-coding RNAs
- NEP:
-
Neprilysin
- NFκB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NGN3:
-
Neurogenin-3
- NHS:
-
N-hydroxysuccinimide
- Ni3S2 :
-
Nickel subsulfide
- NiMn2O4 :
-
Nickel–manganese spinel oxide
- NiMn2O4NSs@NF:
-
Porous NiMn2O4 nanosheet arrays on nickel foam
- NL@Cas9-RNP:
-
Nano liposomal core Cas9-Ribonucleoprotein
- NLRP1:
-
Nuclear localization leucine-rich-repeat protein 1
- NO:
-
Nitric oxide
- NPs:
-
Nanoparticles
- NTP:
-
Nuclear targeting peptide
- p(AAPBA-b-NVCL):
-
Insulin-loaded poly (3-acrylamidophenylboronic acid-block-N-vinylcaprolactam) nanoparticles
- P2X7R:
-
Purinergic type 2X7 receptor
- Pa@AuNPs:
-
Propanoic acid gold nanoparticles
- PAMAM:
-
Poly (amido) amine
- PANC-1:
-
Primary Pancreatic Cancer
- PBLG-PEG-PBLG:
-
poly (gamma-benzyl l-glutamate)-poly (ethylene glycol)-poly (gammabenzyl l-glutamate)
- PBMCs:
-
Peripheral blood mononuclear cells
- PC:
-
Prohormone convertase
- Pde7b:
-
cAMP-specific 3', 5'-cyclic phosphodiesterase 7B
- PDGF-A:
-
Platelet-derived growth factor-A
- PDK-1:
-
P3 dependent kinase-1
- Pdx1:
-
Pancreatic duodenal homeobox 1
- PE:
-
Phosphatidyl ethanolamine
- PEDF:
-
Pigment epithelium-derived factor
- PEG-PLGA:
-
Polyethylene glycol-poly(lactic-co-glycolic acid)
- PERK:
-
Pancreatic endoplasmic reticulum kinase-1
- PGC-1α:
-
- Peroxisome proliferator-activated receptor gama co-activator-1α
- PGE:
-
Punica granatum leaf extract
- PGF2α:
-
Prostaglandin F2α
- PHD:
-
Prolyl Hydroxylase Domain enzymes
- phosphor-CREB:
-
Phosphorylated cAMP-response element-binding protein
- PI3K:
-
Phosphoinositide 3-kinase
- Pio:
-
Pioglitazone hydrochloride
- Pio-NLC-COL-CS:
-
Pioglitazone hydrochloride (Pio)-Nanostructured Lipid Carriers of Pioglitazone Loaded Collagen/Chitosan Composite Scaffold
- PLA-PEG:
-
Polylactide-poly(ethylene glycol)
- PLGA-SGNCs:
-
Gliclazide loaded poly D, L-lactide-coglycolide second-generation nanocrystals
- PNDS:
-
Polymeric nano-delivery system
- PPARGC1-alpha:
-
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PPAR-γ-:
-
Peroxisome proliferator-activated receptor gamma
- Pt/HCS:
-
Platinum Nanoparticles in Shell of Hollow Carbon Spheres
- PTF1A:
-
Pancreas transcription factor 1A
- PTMs:
-
Post-translational modification
- PVCL-PVA-PEG:
-
Polymer polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer
- PVCL-PVA-PEG:
-
Polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer
- rGO:
-
Reduced graphene oxide
- rGO/NiO:
-
Reduced graphene oxide and nickel oxide
- ROS:
-
Reactive oxygen species
- rRNAs:
-
Ribosomal RNAs
- SA/CPBA-MSN/INS:
-
SA-Functionalized 4-Carboxyphenylboronic Acid-Modified Mesoporous Silica Nanoparticles
- SD:
-
Sprague Dawley
- SDF:
-
Stromal cell-derived factor
- Se:
-
Selenium
- SeNPs:
-
Selenium Nanoparticles
- Sept9:
-
Septin-9 - Homo sapiens
- SGLT:
-
Sodium-linked glucose transporter
- SGLT:
-
Sodium-linked glucose transporter
- SiNPs:
-
Silica based nanoparticles
- SiO2:
-
Silicon Dioxide
- siRNA:
-
Short interfering RNA
- SIRT1:
-
NAD-dependent deacetylase sirtuin-1
- SLN:
-
Cetyl palmitate-based solid lipid nanoparticles
- SOD:
-
Superoxide Dismutase
- SOX9:
-
Sex determining region Y-box 9
- SQSTM1:
-
sequestosome 1
- SREBP:
-
Sterol response element-binding protein
- STAT3:
-
Signal transducer and activator of transcription 3
- STZ:
-
Streptozotocin
- SU:
-
Sulfonylurea
- SURs:
-
Sulfonylurea receptors
- T1D:
-
Type 1 diabetes
- T2D:
-
Type-2 diabetes
- TAC:
-
Total antioxidant capacity
- TCF/LEF:
-
T cell-specific factor/lymphoid enhancer-binding factor
- TCF/LEF:
-
T cell-specific factor/lymphoid enhancer-binding factor
- T-CHOL:
-
Total cholesterol
- TF:
-
Transcription factors
- TFEB:
-
Transcription factor EB
- TGF-β:
-
Transforming growth factor-ß
- TiO2:
-
Titanium Dioxide
- TLR:
-
Toll like receptors
- TLRs:
-
Toll-like receptors
- TNF-α:
-
Tumor necrosis factor-alpha
- TOS:
-
Total oxidative status
- TRIF:
-
TIR domain containing adaptor inducing interferon-b
- tRNAs:
-
Transfer RNAs
- T-TG:
-
Total triglyceride
- TZD:
-
Thiazoldinedione
- UCH-L1:
-
Ubiqutin carboxyl-terminal hydrolase L1
- Ulk1:
-
unc-51-like kinase 1
- v-ATPase:
-
Vacuolar ATPase
- WAT:
-
White adipose tissue
- WISP1:
-
Wnt1-inducible signalling pathway protein 1
- ZKSCAN3:
-
Zinc finger protein with KRAB and SCAN domains 3
- ZnO:
-
Zinc Oxide
- ZnO/Ag:
-
Zinc oxide and silver
- ZnO:Ag NR’s:
-
Silver (Ag) dopped ZnO nanorods
- ZnONPs:
-
Zinc Oxide Nanoparticles
- βlinc1:
-
β-cell long intergenic noncoding RNA 1
References
Aynalem SB, Zeleke AJ. Prevalence of diabetes mellitus and its risk factors among individuals aged 15 years and above in Mizan-Aman Town, Southwest Ethiopia, 2016: A Cross Sectional Study. Int J Endocrinol [Internet]. 2018 [cited 2020 Sep 14];2018. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5944196/
Morris AP. Progress in defining the genetic contribution to type 2 diabetes susceptibility. Curr Opin Genet Dev. 2018;50:41–51.
Matboli M, Shafei A, Ali M, Kamal KM, Noah M, Lewis P, et al. Emerging role of nutrition and the non-coding landscape in type 2 diabetes mellitus: a review of literature. Gene. 2018;675:54–61.
Chaudhury A, Duvoor C, Reddy Dendi VS, Kraleti S, Chada A, Ravilla R, et al. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol. 2017;8:6.
Blaslov K, Naranđa FS, Kruljac I, Renar IP. Treatment approach to type 2 diabetes: past, present and future. World J Diabetes. 2018;9:209–19.
Kumar A, Bharti SK, Kumar A. Therapeutic molecules against type 2 diabetes: what we have and what are we expecting? Pharmacol Rep. 2017;69:959–70.
Yang J-S, Lu C-C, Kuo S-C, Hsu Y-M, Tsai S-C, Chen S-Y, et al. Autophagy and its link to type II diabetes mellitus. Biomedicine. 2017;7:8.
Keçili R, Büyüktiryaki S, Hussain CM. Advancement in bioanalytical science through nanotechnology: past, present and future. TrAC Trends Analyt Chem. 2019;110:259–76.
Oslowski CM, Hara T, O’Sullivan-Murphy B, Kanekura K, Lu S, Hara M, et al. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 2012;16:265–73.
Rai RC, Bagul PK, Banerjee SK. NLRP3 inflammasome drives inflammation in high fructose fed diabetic rat liver: effect of resveratrol and metformin. Life Sci. 2020;253:117727.
Marasco MR, Linnemann AK. β-cell autophagy in diabetes pathogenesis. Endocrinology. 2018;159:2127–41.
Bhansali S, Bhansali A, Walia R, Saikia UN, Dhawan V. Alterations in mitochondrial oxidative stress and mitophagy in subjects with prediabetes and type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2017;8:347.
Sikhayeva N, Iskakova A, Saigi-Morgui N, Zholdybaeva E, Eap C-B, Ramanculov E. Association between 28 single nucleotide polymorphisms and type 2 diabetes mellitus in the Kazakh population: a case-control study. BMC Med Genet. 2017;18:76.
Rosen ED, Kaestner KH, Natarajan R, Patti M-E, Sallari R, Sander M, et al. Epigenetics and epigenomics: implications for diabetes and obesity. Diabetes. 2018;67:1923–31.
Ling C, Rönn T. Epigenetics in human obesity and type 2 diabetes. Cell Metab. 2019;29:1028–44.
Goldfine AB, Shoelson SE. Therapeutic approaches targeting inflammation for diabetes and associated cardiovascular risk. J Clin Invest. American Society for Clinical Investigation; 2017;127:83–93.
Cao Q, Chen X-M, Huang C, Pollock CA. MicroRNA as novel biomarkers and therapeutic targets in diabetic kidney disease: an update. FASEB Bioadv. 2019;1:375–88.
Simos YV, Spyrou K, Patila M, Karouta N, Stamatis H, Gournis D, et al. Trends of nanotechnology in type 2 diabetes mellitus treatment. Asian J Pharm Sci [Internet]. 2020 [cited 2020 Sep 18]; Available from: http://www.sciencedirect.com/science/article/pii/S1818087619310098
Zhao R, Lu Z, Yang J, Zhang L, Li Y, Zhang X. Drug delivery system in the treatment of diabetes mellitus. Front Bioeng Biotechnol [Internet]. Frontiers; 2020 [cited 2020 Sep 19];8. Available from: https://www.frontiersin.org/articles/10.3389/fbioe.2020.00880/full
Evans ER, Bugga P, Asthana V, Drezek R. Metallic nanoparticles for cancer immunotherapy. Mater Today (Kidlington). 2018;21:673–85.
Li H, Wu X, Yang B, Li J, Xu L, Liu H, et al. Evaluation of biomimetically synthesized mesoporous silica nanoparticles as drug carriers: structure, wettability, degradation, biocompatibility and brain distribution. Mater Sci Eng C Mater Biol Appl. 2019;94:453–64.
Sepehri Z, Kiani Z, Afshari M, Kohan F, Dalvand A, Ghavami S. Inflammasomes and type 2 diabetes: an updated systematic review. Immunol Lett. 2017;192:97–103.
Han CY, Rho HS, Kim A, Kim TH, Jang K, Jun DW, et al. FXR inhibits endoplasmic reticulum stress-induced NLRP3 inflammasome in hepatocytes and ameliorates liver injury. Cell Rep. 2018;24:2985–99.
Solini A, Novak I. Role of the P2X7 receptor in the pathogenesis of type 2 diabetes and its microvascular complications. Curr Opin Pharmacol. 2019;47:75–81.
Bhattacharya D, Mukhopadhyay M, Bhattacharyya M, Karmakar P. Is autophagy associated with diabetes mellitus and its complications? A review. EXCLI J. 2018;17:709–20.
Li S, Du L, Zhang L, Hu Y, Xia W, Wu J, et al. Cathepsin B contributes to autophagy-related 7 (Atg7)-induced nod-like receptor 3 (NLRP3)-dependent proinflammatory response and aggravates lipotoxicity in rat insulinoma cell line. J Biol Chem. 2013;288:30094–104.
Xu W-Q, Wang Y-S. The role of Toll-like receptors in retinal ischemic diseases. Int J Ophthalmol. 2016;9:1343–51.
Griffin C, Eter L, Lanzetta N, Abrishami S, Varghese M, McKernan K, et al. TLR4, TRIF, and MyD88 are essential for myelopoiesis and CD11c+ adipose tissue macrophage production in obese mice. J Biol Chem. 2018;293:8775–86.
Rulifson IC, Karnik SK, Heiser PW, ten Berge D, Chen H, Gu X, et al. Wnt signaling regulates pancreatic beta cell proliferation. Proc Natl Acad Sci USA. 2007;104:6247–52.
Duvillié B. Vascularization of the pancreas: an evolving role from embryogenesis to adulthood. Diabetes. 2013;62:4004–5.
Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr: Clin Res Rev. 2019;13:1165–72.
Chen Z. Adapter proteins regulate insulin resistance and lipid metabolism in obesity. Sci Bull. 2016;61:1489–97.
Sah SP, Singh B, Choudhary S, Kumar A. Animal models of insulin resistance: A review. Pharmacol Rep. 2016;68:1165–77.
Vethe H, Ghila L, Berle M, Hoareau L, Haaland ØA, Scholz H, et al. The effect of Wnt pathway modulators on human iPSC-derived pancreatic beta cell maturation. Front Endocrinol (Lausanne). 2019;10:293.
Scheibner K, Bakhti M, Bastidas-Ponce A, Lickert H. Wnt signaling: implications in endoderm development and pancreas organogenesis. Curr Opin Cell Biol. 2019;61:48–55.
Bastidas-Ponce A, Roscioni SS, Burtscher I, Bader E, Sterr M, Bakhti M, et al. Foxa2 and Pdx1 cooperatively regulate postnatal maturation of pancreatic β-cells. Mol Metab. 2017;6:524–34.
Fujimoto K, Polonsky KS. Pdx1 and other factors that regulate pancreatic β-cell survival. Diabetes Obes Metab. 2009;11:30–7.
Tran R, Moraes C, Hoesli CA. Controlled clustering enhances PDX1 and NKX6.1 expression in pancreatic endoderm cells derived from pluripotent stem cells. Sci Rep. Nature Publishing Group; 2020;10:1190.
Noguchi H. Pancreatic stem/progenitor cells for the treatment of diabetes. Rev Diabet Stud. 2010;7:105–11.
Alejandro EU, Gregg B, Blandino-Rosano M, Cras-Méneur C, Bernal-Mizrachi E. Natural history of β-cell adaptation and failure in type 2 diabetes. Mol Aspects Med. 2015;42:19–41.
Balakrishnan S, Dhavamani S, Prahalathan C. β-Cell specific transcription factors in the context of diabetes mellitus and β-cell regeneration. Mech Dev. 2020;163:103634.
Kropp PA, Zhu X, Gannon M. Regulation of the pancreatic exocrine differentiation program and morphogenesis by onecut 1/Hnf6. Cell Mol Gastroenterol Hepatol. 2019;7:841–56.
Sheets TP, Park K-E, Park C-H, Swift SM, Powell A, Donovan DM, et al. Targeted mutation of NGN3 gene disrupts pancreatic endocrine cell development in pigs. Sci Rep. 2018;8:3582.
Jørgensen MC, de Lichtenberg KH, Collin CA, Klinck R, Ekberg JH, Engelstoft MS, et al. Neurog3-dependent pancreas dysgenesis causes ectopic pancreas in Hes1 mutant mice. Development. 2018;145.
Napolitano T, Avolio F, Courtney M, Vieira A, Druelle N, Ben-Othman N, et al. Pax4 acts as a key player in pancreas development and plasticity. Semin Cell Dev Biol. 2015;44:107–14.
Heinis M, Simon MT, Duvillié B. New insights into endocrine pancreatic development: the role of environmental factors. Horm Res Paediatr. 2010;74:77–82.
Norouzirad R, González-Muniesa P, Ghasemi A. Hypoxia in obesity and diabetes: potential therapeutic effects of hyperoxia and nitrate. Oxid Med Cell Longev. 2017;2017:5350267.
Hatting M, Tavares CDJ, Sharabi K, Rines AK, Puigserver P. Insulin regulation of gluconeogenesis. Ann N Y Acad Sci. 2018;1411:21–35.
Edgerton DS, Kraft G, Smith M, Farmer B, Williams PE, Coate KC, et al. Insulin’s direct hepatic effect explains the inhibition of glucose production caused by insulin secretion. JCI Insight. 2017;2:e91863.
Shi S, Koya D, Kanasaki K. Dipeptidyl peptidase-4 and kidney fibrosis in diabetes. Fibrogenesis Tissue Repair. 2016;9:1.
Gentilella R, Pechtner V, Corcos A, Consoli A. Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: are they all the same? Diabetes Metab Res Rev. 2019;35:e3070.
Kanasaki K. The role of renal dipeptidyl peptidase-4 in kidney disease: Renal effects of dipeptidyl peptidase-4 inhibitors with a focus on linagliptin. Clin Sci. 2018;132:489–507.
Scheen AJ. Is there a role for alpha-glucosidase inhibitors in the prevention of type 2 diabetes mellitus? Drugs. 2003;63:933–51.
Gupta D, Kono T, Evans-Molina C. The role of peroxisome proliferator-activated receptor γ in pancreatic β cell function and survival: therapeutic implications for the treatment of type 2 diabetes mellitus. Diabetes Obes Metab. NIH Public Access; 2010;12:1036.
Holst JJ, Vilsbøll T, Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol. 2009;297:127–36.
Hodish I. Insulin therapy for type 2 diabetes - are we there yet? The d-Nav® story. Clin Diabetes Endocrinol. 2018;4:8.
Upadhyay J, Polyzos SA, Perakakis N, Thakkar B, Paschou SA, Katsiki N, et al. Pharmacotherapy of type 2 diabetes: an update. Metab Clin Exp. 2018;78:13–42.
Ciążyńska M, Bednarski IA, Wódz K, Narbutt J, Lesiak A. NLRP1 and NLRP3 inflammasomes as a new approach to skin carcinogenesis. Oncol Lett. 2020;19:1649–56.
Li D-X, Wang C-N, Wang Y, Ye C-L, Jiang L, Zhu X-Y, et al. NLRP3 inflammasome-dependent pyroptosis and apoptosis in hippocampus neurons mediates depressive-like behavior in diabetic mice. Behav Brain Res. 2020;391:112684.
Reinehr T. Inflammatory markers in children and adolescents with type 2 diabetes mellitus. Clin Chim Acta. 2019;496:100–7.
Pirzada RH, Javaid N, Choi S. The roles of the NLRP3 inflammasome in neurodegenerative and metabolic diseases and in relevant advanced therapeutic interventions. Genes (Basel) [Internet]. 2020 [cited 2020 Sep 19];11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7074480/
Cimini FA, D’Eliseo D, Barchetta I, Bertoccini L, Velotti F, Cavallo MG. Increased circulating granzyme B in type 2 diabetes patients with low-grade systemic inflammation. Cytokine. 2019;115:104–8.
Elimam H, Abdulla AM, Taha IM. Inflammatory markers and control of type 2 diabetes mellitus. Diabetes Metab Syndr. 2019;13:800–4.
Jialal I, Chaudhuri A. Targeting inflammation to reduce ASCVD in type 2 diabetes. J Diabetes Complicat. 2019;33:1–3.
Xie Y, Li J, Kang R, Tang D. Interplay between lipid metabolism and autophagy. Front Cell Dev Biol [Internet]. 2020 [cited 2020 Sep 19];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7283384/
Liu H, Cao M, Wang Y, Li L, Zhu L, Xie G, et al. Endoplasmic reticulum stress is involved in the connection between inflammation and autophagy in type 2 diabetes. Gen Comp Endocrinol. 2015;210:124–9.
Zachari M, Ganley IG. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017;61:585–96.
Lee CH, Ingrole RSJ, Gill HS. Generation of induced pluripotent stem cells using elastin like polypeptides as a non-viral gene delivery system. Biochim Biophys Acta Mol Basis Dis. 1866;2020:165405.
Galluzzi L, Green DR. Autophagy-independent functions of the autophagy machinery. Cell. 2019;177:1682–99.
Yao D, GangYi Y, QiNan W. Autophagic dysfunction of β cell dysfunction in type 2 diabetes, a double-edged sword. Genes Dis [Internet]. 2020 [cited 2020 Sep 19]; Available from: http://www.sciencedirect.com/science/article/pii/S2352304220300453
Chun Y, Kim J. Autophagy: An essential degradation program for cellular homeostasis and life. Cells [Internet]. 2018 [cited 2020 Sep 15];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6315530/
Rocha M, Apostolova N, Diaz-Rua R, Muntane J, Victor VM. Mitochondria and T2D: role of autophagy, ER stress, and inflammasome. Trends Endocrinol Metab. 2020.
Fedele AO, Proud CG. Chloroquine and bafilomycin A mimic lysosomal storage disorders and impair mTORC1 signalling. Biosci Rep. 2020;40.
Packer M. Interplay of adenosine monophosphate-activated protein kinase/sirtuin-1 activation and sodium influx inhibition mediates the renal benefits of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes: a novel conceptual framework. Diabetes Obes Metab. 2020;22:734–42.
Zummo FP, Cullen KS, Honkanen-Scott M, Shaw JAM, Lovat PE, Arden C. Glucagon-like peptide 1 protects pancreatic β-cells from death by increasing autophagic flux and restoring lysosomal function. Diabetes. 2017;66:1272–85.
Xu C, Chen X, Sheng W-B, Yang P. Trehalose restores functional autophagy suppressed by high glucose. Reprod Toxicol. 2019;85:51–8.
Vivot K, Pasquier A, Goginashvili A, Ricci R. Breaking bad and breaking good: β-Cell autophagy pathways in diabetes. J Mol Biol. 2020;432:1494–513.
Li J, Ye W, Xu W, Chang T, Zhang L, Ma J, et al. Activation of autophagy inhibits epithelial to mesenchymal transition process of human lens epithelial cells induced by high glucose conditions. Cell Signal. 2020;75:109768.
Rezabakhsh A, Rahbarghazi R, Malekinejad H, Fathi F, Montaseri A, Garjani A. Quercetin alleviates high glucose-induced damage on human umbilical vein endothelial cells by promoting autophagy. Phytomedicine. 2019;56:183–93.
Pinney SE, Simmons RA. Epigenetic mechanisms in the development of type 2 diabetes. Trends Endocrinol Metab. 2010;21:223–9.
Ling C. Epigenetic regulation of insulin action and secretion - role in the pathogenesis of type 2 diabetes. J Intern Med. 2020;288:158–67.
Ouni M, Saussenthaler S, Eichelmann F, Jähnert M, Stadion M, Wittenbecher C, et al. Epigenetic changes in islets of Langerhans preceding the onset of diabetes. Diabetes. American Diabetes Association; 2020;db200204.
Celano M, Mio C, Sponziello M, Verrienti A, Bulotta S, Durante C, et al. Targeting post-translational histone modifications for the treatment of non-medullary thyroid cancer. Mol Cell Endocrinol. 2018;469:38–47.
Demetriadou C, Koufaris C, Kirmizis A. Histone N-alpha terminal modifications: genome regulation at the tip of the tail. Epigenetics Chromatin. 2020;13:29.
Fernandes MT, Almeida-Lousada H, Castelo-Branco P. Chapter 1 - Histone modifications in diseases. In: Castelo-Branco P, Jeronimo C, editors. Histone Modifications in Therapy [Internet]. Academic Press; 2020 [cited 2020 Sep 21]. p. 1–15. Available from: http://www.sciencedirect.com/science/article/pii/B9780128164228000015
Lawrence M, Daujat S, Schneider R. Lateral thinking: how histone modifications regulate gene expression. Trends Genet. 2016;32:42–56.
Ludwig CH, Bintu L. Mapping chromatin modifications at the single cell level. Development [Internet]. Oxford University Press for The Company of Biologists Limited; 2019 [cited 2020 Sep 21];146. Available from: https://dev.biologists.org/content/146/12/dev170217
Tessarz P, Kouzarides T. Histone core modifications regulating nucleosome structure and dynamics. Nat Rev Mol Cell Biol. 2014;15:703–8.
Zhou K, Gaullier G, Luger K. Nucleosome structure and dynamics are coming of age. Nat Struct Mol Biol. Nature Publishing Group; 2019;26:3–13.
Khullar M, Cheema BS, Raut SK. Emerging evidence of epigenetic modifications in vascular complication of diabetes. Front Endocrinol (Lausanne). 2017;8:237.
Wei X, Yi X, Zhu X-H, Jiang D-S. Histone methylation and vascular biology. Clinical Epigenetics. 2020;12:30.
Donath MY, Dinarello CA, Mandrup-Poulsen T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat Rev Immunol. Nature Publishing Group. 2019;19:734–46.
Bernstein D, Golson ML, Kaestner KH. Epigenetic control of β-cell function and failure. Diabetes Res Clin Pract. 2017;123:24–36.
Gonzalez-Jaramillo V, Portilla-Fernandez E, Glisic M, Voortman T, Ghanbari M, Bramer W, et al. Epigenetics and inflammatory markers: a systematic review of the current evidence. Int J Inflam. 2019;2019:6273680.
Zhu H, Wang G, Qian J. Transcription factors as readers and effectors of DNA methylation. Nat Rev Genet. 2016;17:551–65.
Greenberg MVC, Bourc’his D. The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol. Nature Publishing Group. 2019;20:590–607.
de Mendoza A, Lister R, Bogdanovic O. Evolution of DNA methylome diversity in Eukaryotes. J Mol Biol. 2020;432:1687–705.
Davegårdh C, García-Calzón S, Bacos K, Ling C. DNA methylation in the pathogenesis of type 2 diabetes in humans. Mol Metab. 2018;14:12–25.
Roshanzamir N, Hassan-Zadeh V. Methylation of specific CpG sites in IL-1β and IL1R1 genes is affected by hyperglycaemia in type 2 diabetic patients. Immunol Invest. Taylor & Francis; 2020;49:287–298.
Willmer T, Johnson R, Louw J, Pheiffer C. Blood-based DNA methylation biomarkers for type 2 diabetes: Potential for clinical applications. Front Endocrinol (Lausanne). 2018;9:744.
Lekka E, Hall J. Noncoding RNAs in disease. FEBS Lett. 2018;592:2884–900.
An T, Zhang J, Ma Y, Lian J, Wu Y-X, Lv B-H, et al. Relationships of Non-coding RNA with diabetes and depression. Sci Rep. Nature Publishing Group; 2019;9:10707.
Zhang J-R, Sun H-J. Roles of circular RNAs in diabetic complications: from molecular mechanisms to therapeutic potential. Gene. 2020;763:145066.
Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–74.
Li Y, Shan G, Teng Z-Q, Wingo TS. Editorial: Non-coding RNAs and human diseases. Front Genet [Internet]. Frontiers; 2020 [cited 2020 Sep 21];11. Available from: https://www.frontiersin.org/articles/10.3389/fgene.2020.00523/full
Eliasson L, Esguerra JLS. MicroRNA networks in pancreatic islet cells: normal function and type 2 diabetes. Diabetes. American Diabetes Association. 2020;69:804–12.
Dahariya S, Paddibhatla I, Kumar S, Raghuwanshi S, Pallepati A, Gutti RK. Long non-coding RNA: classification, biogenesis and functions in blood cells. Mol Immunol. 2019;112:82–92.
Hombach S, Kretz M. Non-coding RNAs: classification, biology and functioning. Adv Exp Med Biol. 2016;937:3–17.
Motterle A, Gattesco S, Peyot M-L, Esguerra JLS, Gomez-Ruiz A, Laybutt DR, et al. Identification of islet-enriched long non-coding RNAs contributing to β-cell failure in type 2 diabetes. Mol Metab. 2017;6:1407–18.
Lopez-Noriega L, Callingham R, Martinez-Sánchez A, Pizza G, Haberman N, Cvetesic N, et al. The long non-coding RNA Pax6os1/PAX6-AS1 modulates pancreatic β-cell identity and function. bioRxiv. Cold Spring Harbor Laboratory; 2020;2020.07.17.209015.
Ji E, Kim C, Kim W, Lee EK. Role of long non-coding RNAs in metabolic control. Biochim Biophys Acta Gene Regul Mech. 1863;2020:194348.
Reichelt-Wurm S, Wirtz T, Chittka D, Lindenmeyer M, Reichelt RM, Beck S, et al. Glomerular expression pattern of long non-coding RNAs in the type 2 diabetes mellitus BTBR mouse model. Sci Rep. Nature Publishing Group; 2019;9:9765.
Zhu X, Li H, Wu Y, Zhou J, Yang G, Wang W. lncRNA MEG3 promotes hepatic insulin resistance by serving as a competing endogenous RNA of miR-214 to regulate ATF4 expression. Int J Mol Med. 2019;43:345–57.
Arnes L, Akerman I, Balderes DA, Ferrer J, Sussel L. βlinc1 encodes a long noncoding RNA that regulates islet β-cell formation and function. Genes Dev. 2016;30:502–7.
Kerry RG, Sahoo S, Das G, Patra JK. Theranostic application of nanoparticulated system: Present and future prospects. In: Inamuddin, Rangreez TA, Ahamed MI, Asiri AM, editors. Materials Research Forum [Internet]. Millersville, USA: Materials Research Foundation; 2019 [cited 2020 Sep 15]. p. 241–88. Available from: https://www.mrforum.com/product/9781644900130-7/
Mandal B, Bhattacharjee H, Mittal N, Sah H, Balabathula P, Thoma LA, et al. Core-shell-type lipid-polymer hybrid nanoparticles as a drug delivery platform. Nanomedicine. 2013;9:474–91.
Sengottaiyan A, Aravinthan A, Sudhakar C, Selvam K, Srinivasan P, Govarthanan M, et al. Synthesis and characterization of Solanum nigrum-mediated silver nanoparticles and its protective effect on alloxan-induced diabetic rats. J Nanostruct Chem. 2016;6:41–8.
Afifi M, Abdelazim AM. Ameliorative effect of zinc oxide and silver nanoparticles on antioxidant system in the brain of diabetic rats. Asian Pac J Trop Biomed. 2015;5:874–7.
Saratale RG, Shin HS, Kumar G, Benelli G, Kim D-S, Saratale GD. Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2). Artif Cells Nanomed Biotechnol. 2018;46:211–22.
Kouame K, Peter AI, Akang EN, Moodley R, Naidu EC, Azu OO. Histological and biochemical effects of Cinnamomum cassia nanoparticles in kidneys of diabetic Sprague-Dawley rats. Bosn J Basic Med Sci. 2019;19:138–45.
Das G, Patra JK, Debnath T, Ansari A, Shin H-S. Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.). PLoS ONE. 2019;14:e0220950.
Barathmanikanth S, Kalishwaralal K, Sriram M, Pandian SRK, Youn H-S, Eom S, et al. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J Nanobiotechnol. 2010;8:16.
Daisy P, Saipriya K. Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int J Nanomedicine. 2012;7:1189–202.
Shilo M, Berenstein P, Dreifuss T, Nash Y, Goldsmith G, Kazimirsky G, et al. Insulin-coated gold nanoparticles as a new concept for personalized and adjustable glucose regulation. Nanoscale. 2015;7:20489–96.
Ponnanikajamideen M, Rajeshkumar S, Vanaja M, Annadurai G. In vivo type 2 diabetes and wound-healing effects of antioxidant gold nanoparticles synthesized using the insulin plant Chamaecostus cuspidatus in albino rats. Can J Diabetes. 2019;43:82–89.e6.
Manna K, Mishra S, Saha M, Mahapatra S, Saha C, Yenge G, et al. Amelioration of diabetic nephropathy using pomegranate peel extract-stabilized gold nanoparticles: assessment of NF-κB and Nrf2 signaling system. Int J Nanomedicine. 2019;14:1753–77.
Hussein S, El-Senosi YA, El-Dawy K, Baz H. Protective effect of zinc oxide nanoparticles on oxidative stress in experimental-induced diabetes in rats. Benha Vet Med J. 2014;27:405–14.
Alkaladi A, Abdelazim AM, Afifi M. Antidiabetic activity of zinc oxide and silver nanoparticles on streptozotocin-induced diabetic rats. Int J Mol Sci. 2014;15:2015–23.
Afifi M, Almaghrabi OA, Kadasa NM. Ameliorative effect of zinc oxide nanoparticles on antioxidants and sperm characteristics in streptozotocin-induced diabetic rat testes. Biomed Res Int. 2015;2015:153573.
Nazarizadeh A, Asri-Rezaie S. Comparative study of antidiabetic activity and oxidative stress induced by zinc oxide nanoparticles and zinc sulfate in diabetic rats. AAPS PharmSciTech. 2016;17:834–43.
El-Behery EI, El-Naseery NI, El-Ghazali HM, Elewa YHA, Mahdy EAA, El-Hady E, et al. The efficacy of chronic zinc oxide nanoparticles using on testicular damage in the streptozotocin-induced diabetic rat model. Acta Histochem. 2019;121:84–93.
Kamel M, Khairy M, ELSadek N, Hussein M. Therapeutic efficacy of zinc oxide nanoparticles in diabetic rats. Slov Vet Res. 2019;56:187–94.
Afify M, Samy N, Hafez NA, Alazzouni AS, Mahdy ES, El Mezayen HAE-M, et al. Evaluation of zinc-oxide nanoparticles effect on treatment of diabetes in streptozotocin-induced diabetic rats. Egypt J Chem. National Information and Documentation Centre (NIDOC), Academy of Scientific Research and Technology, ASRT; 2019;62:1771–83.
Al-Quraishy S, Dkhil MA, Abdel Moneim AE. Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int J Nanomedicine. 2015;10:6741–56.
Deng W, Xie Q, Wang H, Ma Z, Wu B, Zhang X. Selenium nanoparticles as versatile carriers for oral delivery of insulin: Insight into the synergic antidiabetic effect and mechanism. Nanomedicine. 2017;13:1965–74.
Lin Y, Ren Y, Zhang Y, Zhou J, Zhou F, Zhao Q, et al. Protective role of nano-selenium-enriched Bifidobacterium longum in delaying the onset of streptozotocin-induced diabetes. R Soc Open Sci. 2018;5:181156.
Deng W, Wang H, Wu B, Zhang X. Selenium-layered nanoparticles serving for oral delivery of phytomedicines with hypoglycemic activity to synergistically potentiate the antidiabetic effect. Acta Pharm Sin B. 2019;9:74–86.
Abdulmalek SA, Balbaa M. Synergistic effect of nano-selenium and metformin on type 2 diabetic rat model: Diabetic complications alleviation through insulin sensitivity, oxidative mediators and inflammatory markers. PLoS ONE. 2019;14:e0220779.
Najafi R, Hosseini A, Ghaznavi H, Mehrzadi S, Sharifi AM. Neuroprotective effect of cerium oxide nanoparticles in a rat model of experimental diabetic neuropathy. Brain Res Bull. 2017;131:117–22.
Shanker K, Naradala J, Mohan GK, Kumar GS, Pravallika PL. A sub-acute oral toxicity analysis and comparative in vivo anti-diabetic activity of zinc oxide, cerium oxide, silver nanoparticles, and Momordica charantia in streptozotocin-induced diabetic Wistar rats. RSC Adv. The Royal Society of Chemistry; 2017;7:37158–37167.
Han Y, Li L, Liu Y, Wang Y, Yan C, Sun L. Study on the effect of cerium oxide nanocubes on full-thickness cutaneous wound healing of type 2 diabetic rats. Diabetes [Internet]. American Diabetes Association; 2018 [cited 2020 Sep 15];67. Available from: https://diabetes.diabetesjournals.org/content/67/Supplement_1/643-P
Zgheib C, Hilton SA, Dewberry LC, Hodges MM, Ghatak S, Xu J, et al. Use of cerium oxide nanoparticles conjugated with MicroRNA-146a to correct the diabetic wound healing impairment. J Am Coll Surg. 2019;228:107–15.
Zgheib C, Hilton SA, Dewberry LC, Hodges MM, Ghatak S, Xu J, et al. Amelioration of diabetes-induced testicular and sperm damage in rats by cerium oxide nanoparticle treatment. J Am Coll Surg. 2019;228:107–15.
Lopez-Pascual A, Urrutia-Sarratea A, Lorente-Cebrián S, Martinez JA, González-Muniesa P. Cerium oxide nanoparticles regulate insulin sensitivity and oxidative markers in 3T3-L1 adipocytes and C2C12 myotubes. Oxid Med Cell Longev. 2019;2019:2695289.
Andreani T, Miziara L, Lorenzón EN, de Souza ALR, Kiill CP, Fangueiro JF, et al. Effect of mucoadhesive polymers on the in vitro performance of insulin-loaded silica nanoparticles: Interactions with mucin and biomembrane models. Eur J Pharm Biopharm. 2015;93:118–26.
Chen C, Zheng H, Xu J, Shi X, Li F, Wang X. Sustained-release study on Exenatide loaded into mesoporous silica nanoparticles: In vitro characterization and in vivo evaluation. Daru. 2017;25:20.
Mao C-F, Zhang X-R, Johnson A, He J-L, Kong Z-L. Modulation of diabetes mellitus-induced male rat reproductive dysfunction with micro-nanoencapsulated Echinacea purpurea ethanol extract. Biomed Res Int. 2018;2018:4237354.
Sudirman S, Hsu Y-H, Johnson A, Tsou D, Kong Z-L. Amelioration effects of nanoencapsulated triterpenoids from petri dish-cultured Antrodia cinnamomea on reproductive function of diabetic male rats. Int J Nanomedicine. 2018;13:5059–73.
Hou L, Zheng Y, Wang Y, Hu Y, Shi J, Liu Q, et al. Self-regulated carboxyphenylboronic acid-modified mesoporous silica nanoparticles with “Touch Switch” releasing property for insulin delivery. ACS Appl Mater Interfaces. 2018;10:21927–38.
Hei M, Wu H, Fu Y, Xu Y, Zhu W. Phenylboronic acid functionalized silica nanoparticles with enlarged ordered mesopores for efficient insulin loading and controlled release. J Drug Deliv Sci Tec. 2019;51:320–6.
Patiño-Herrera R, Louvier-Hernández JF, Escamilla-Silva EM, Chaumel J, Escobedo AGP, Pérez E. Prolonged release of metformin by SiO2 nanoparticles pellets for type II diabetes control. Eur J Pharm Sci. 2019;131:1–8.
Hosseini A, Sharifzadeh M, Rezayat SM, Hassanzadeh G, Hassani S, Baeeri M, et al. Benefit of magnesium-25 carrying porphyrin-fullerene nanoparticles in experimental diabetic neuropathy. Int J Nanomedicine. 2010;5:517–23.
Bal R, Türk G, Tuzcu M, Yilmaz O, Ozercan I, Kuloglu T, et al. Protective effects of nanostructures of hydrated C(60) fullerene on reproductive function in streptozotocin-diabetic male rats. Toxicology. 2011;282:69–81.
Li X, Zhen M, Zhou C, Deng R, Yu T, Wu Y, et al. Gadofullerene nanoparticles reverse dysfunctions of pancreas and improve hepatic insulin resistance for type 2 diabetes mellitus treatment. ACS Nano. 2019;13:8597–608.
Sá MA, Andrade VB, Mendes RM, Caliari MV, Ladeira LO, Silva EE, et al. Carbon nanotubes functionalized with sodium hyaluronate restore bone repair in diabetic rat sockets. Oral Dis. 2013;19:484–93.
Ilie I, Ilie R, Mocan T, Tabaran F, Iancu C, Mocan L. Nicotinamide-functionalized multiwalled carbon nanotubes increase insulin production in pancreatic beta cells via MIF pathway. Int J Nanomedicine. 2013;8:3345–53.
Chen J, He P, Bai H, Lei H, Liu K, Dong F, et al. Novel phosphomolybdic acid/single-walled carbon nanohorn-based modified electrode for non-enzyme glucose sensing. J Electroanal Chem. 2017;784:41–6.
Luan F, Zhang S, Chen D, Wei F, Zhuang X. Ni3S2/ionic liquid-functionalized graphene as an enhanced material for the nonenzymatic detection of glucose. Microchem J. 2018;143:450–6.
Thangavel P, Kannan R, Ramachandran B, Moorthy G, Suguna L, Muthuvijayan V. Development of reduced graphene oxide (rGO)-isabgol nanocomposite dressings for enhanced vascularization and accelerated wound healing in normal and diabetic rats. J Colloid Interface Sci. 2018;517:251–64.
Wang S, Zhao L, Xu R, Ma Y, Ma L. Facile fabrication of biosensors based on Cu nanoparticles modified as-grown CVD graphene for non-enzymatic glucose sensing. J Electroanal Chem. 2019;853:113527.
Beyranvand S, Pourghobadi Z, Sattari S, Soleymani K, Donskyi I, Gharabaghi M, et al. Boronic acid functionalized graphene platforms for diabetic wound healing. Carbon. 2020;158:327–36.
Balasubramani K, Sivarajasekar N, Naushad M. Effective adsorption of antidiabetic pharmaceutical (metformin) from aqueous medium using graphene oxide nanoparticles: Equilibrium and statistical modelling. J Mol Liq. 2020;301:112426.
Das P, Maity PP, Ganguly S, Ghosh S, Baral J, Bose M, et al. Biocompatible carbon dots derived from κ-carrageenan and phenyl boronic acid for dual modality sensing platform of sugar and its anti-diabetic drug release behavior. Int J Biol Macromol. 2019;132:316–29.
Ngo Y-LT, Choi WM, Chung JS, Hur SH. Highly biocompatible phenylboronic acid-functionalized graphitic carbon nitride quantum dots for the selective glucose sensor. Sensors Actuators B Chem. 2019;282:36–44.
Du L, Li Z, Yao J, Wen G, Dong C, Li H-W. Enzyme free glucose sensing by amino-functionalized silicon quantum dot. Spectrochim Acta A Mol Biomol Spectrosc. 2019;216:303–9.
Abazar F, Noorbakhsh A. Chitosan-carbon quantum dots as a new platform for highly sensitive insulin impedimetric aptasensor. Sensors Actuators B Chem. 2020;304:127281.
Lee AC-L, Harris JL, Khanna KK, Hong J-H. A comprehensive review on current advances in peptide drug development and design. Int J Mol Sci. 2019;20.
Xu C, Lei C, Yu C. Mesoporous silica nanoparticles for protein protection and delivery. Front Chem. 2019;7:290.
Alghazzawi W, Danish E, Alnahdi H, Salam MA. Rapid microwave-assisted hydrothermal green synthesis of rGO/NiO nanocomposite for glucose detection in diabetes. Synthetic Metals. 2020;267:116401.
Khan MS, Ameer H, Ali A, Li Y, Yang L, Ren X, et al. Electrochemiluminescence behaviour of silver/ZnIn2S4/reduced graphene oxide composites quenched by Au@SiO2 nanoparticles for ultrasensitive insulin detection. Biosens Bioelectron. 2020;162:112235.
Lavanya N, Leonardi SG, Marini S, Espro C, Kanagaraj M, Reddy SL, et al. MgNi2O3 nanoparticles as novel and versatile sensing material for non-enzymatic electrochemical sensing of glucose and conductometric determination of acetone. J Alloys Compd. 2020;817:152787.
Inyang A, Kibambo G, Palmer M, Cummings F, Masikini M, Sunday C, et al. One step copper oxide (CuO) thin film deposition for non-enzymatic electrochemical glucose detection. Thin Solid Films. 2020;709:138244.
Damgé C, Socha M, Ubrich N, Maincent P. Poly(epsilon-caprolactone)/eudragit nanoparticles for oral delivery of aspart-insulin in the treatment of diabetes. J Pharm Sci. 2010;99:879–89.
Oh KS, Kim JY, Yoon BD, Lee M, Kim H, Kim M, et al. Sol-gel transition of nanoparticles/polymer mixtures for sustained delivery of exenatide to treat type 2 diabetes mellitus. Eur J Pharm Biopharm. 2014;88:664–9.
Song M, Wang H, Chen K, Zhang S, Yu L, Elshazly EH, et al. Oral insulin delivery by carboxymethyl-β-cyclodextrin-grafted chitosan nanoparticles for improving diabetic treatment. Artif Cells Nanomed Biotechnol. 2018;46:S774–82.
Jamshidi M, Ziamajidi N, Khodadadi I, Dehghan A, Kalantarian G, Abbasalipourkabir R. The effect of insulin-loaded trimethylchitosan nanoparticles on rats with diabetes type I. Biomed Pharmacother. 2018;97:729–35.
Shi Y, Sun X, Zhang L, Sun K, Li K, Li Y, et al. Fc-modified exenatide-loaded nanoparticles for oral delivery to improve hypoglycemic effects in mice. Sci Rep. 2018;8:726.
Abdel-Moneim A, El-Shahawy A, Yousef AI, Abd El-Twab SM, Elden ZE, Taha M. Novel polydatin-loaded chitosan nanoparticles for safe and efficient type 2 diabetes therapy: In silico, in vitro and in vivo approaches. Int J Biol Macromol. 2020;154:1496–504.
Fang Y, Wang Q, Lin X, Jin X, Yang D, Gao S, et al. Gastrointestinal responsive polymeric nanoparticles for oral delivery of insulin: optimized preparation, characterization and in vivo evaluation. J Pharm Sci. 2019;108:2994–3002.
El-Naggar ME, Al-Joufi F, Anwar M, Attia MF, El-Bana MA. Curcumin-loaded PLA-PEG copolymer nanoparticles for treatment of liver inflammation in streptozotocin-induced diabetic rats. Colloids Surf B Biointerfaces. 2019;177:389–98.
Laddha UD, Kshirsagar SJ. Formulation of PPAR-gamma agonist as surface modified PLGA nanoparticles for non-invasive treatment of diabetic retinopathy: in vitro and in vivo evidences. Heliyon. 2020;6:e04589.
Arun G, Rajaram R, Kaleshkumar K, Gayathri N, Sivasudha T, Kandasamy S. Synergistic effect of novel chitosan combined metformin drug on streptozotocin-induced diabetes mellitus rat. Int J Biol Macromol. 2020;153:1335–49.
Wong CY, Al-Salami H, Dass CR. Formulation and characterisation of insulin-loaded chitosan nanoparticles capable of inducing glucose uptake in skeletal muscle cells in vitro. J Drug Deliv Sci Technol. 2020;57:101738.
Sarmento B, Martins S, Ferreira D, Souto EB. Oral insulin delivery by means of solid lipid nanoparticles. Int J Nanomedicine. 2007;2:743–9.
Sharma N, Rana S, Shivkumar HG, Sharma RK. Solid lipid nanoparticles as a carrier of metformin for transdermal delivery. Int J Drug Deliv. 2013;5:137–45.
Choi JU, Lee SW, Pangeni R, Byun Y, Yoon I-S, Park JW. Preparation and in vivo evaluation of cationic elastic liposomes comprising highly skin-permeable growth factors combined with hyaluronic acid for enhanced diabetic wound-healing therapy. Acta Biomater. 2017;57:197–215.
Pellegrino M, Ceccacci F, Petrini S, Scipioni A, De Santis S, Cappa M, et al. Exploiting novel tailored immunotherapies of type 1 diabetes: Short interfering RNA delivered by cationic liposomes enables efficient down-regulation of variant PTPN22 gene in T lymphocytes. Nanomedicine. 2019;18:371–9.
Suzuki K, Kim KS, Bae YH. Long-term oral administration of Exendin-4 to control type 2 diabetes in a rat model. J Control Release. 2019;294:259–67.
Ahad A, Raish M, Ahmad A, Al-Jenoobi FI, Al-Mohizea AM. Eprosartan mesylate loaded bilosomes as potential nano-carriers against diabetic nephropathy in streptozotocin-induced diabetic rats. Eur J Pharm Sci. 2018;111:409–17.
Yücel Ç, Karatoprak GŞ, Aktaş Y. Nanoliposomal resveratrol as a novel approach to treatment of diabetes mellitus. J Nanosci Nanotechnol. 2018;18:3856–64.
Ahangarpour A, Oroojan AA, Khorsandi L, Kouchak M, Badavi M. Solid lipid nanoparticles of myricitrin have antioxidant and antidiabetic effects on streptozotocin-nicotinamide-induced diabetic model and myotube cell of male mouse. Oxid Med Cell Longev. 2018;2018:7496936.
Cho EY, Ryu J-Y, Lee HAR, Hong SH, Park HS, Hong KS, et al. Lecithin nano-liposomal particle as a CRISPR/Cas9 complex delivery system for treating type 2 diabetes. J Nanobiotechnol. 2019;17:19.
Khair R, Shende P, Kulkarni YA. Nanostructured polymer-based cochleates for effective transportation of insulin. J Mol Liq. 2020;311:113352.
Shaveta S, Singh J, Afzal M, Kaur R, Imam SS, Alruwaili NK, et al. Development of solid lipid nanoparticle as carrier of pioglitazone for amplification of oral efficacy: formulation design optimization, in-vitro characterization and in-vivo biological evaluation. J Drug Deliv Sci Technol. 2020;57:101674.
Karolczak K, Rozalska S, Wieczorek M, Labieniec-Watala M, Watala C. Poly(amido)amine dendrimers generation 4.0 (PAMAM G4) reduce blood hyperglycaemia and restore impaired blood-brain barrier permeability in streptozotocin diabetes in rats. Int J Pharm. 2012;436:508–18.
Siewiera K, Labieniec-Watala M. Ambiguous effect of dendrimer PAMAM G3 on rat heart respiration in a model of an experimental diabetes - Objective causes of laboratory misfortune or unpredictable G3 activity? Int J Pharm. 2012;430:258–65.
Labieniec-Watala M, Przygodzki T, Sebekova K, Watala C. Can metabolic impairments in experimental diabetes be cured with poly(amido)amine (PAMAM) G4 dendrimers? In the search for minimizing of the adverse effects of PAMAM administration. Int J Pharm. 2014;464:152–67.
Akhtar S, Chandrasekhar B, Yousif MH, Renno W, Benter IF, El-Hashim AZ. Chronic administration of nano-sized PAMAM dendrimers in vivo inhibits EGFR-ERK1/2-ROCK signaling pathway and attenuates diabetes-induced vascular remodeling and dysfunction. Nanomedicine. 2019;18:78–89.
Alam MS, Ahad A, Abidin L, Aqil M, Mir SR, Mujeeb M. Embelin-loaded oral niosomes ameliorate streptozotocin-induced diabetes in Wistar rats. Biomed Pharmacother. 2018;97:1514–20.
Singhal T, Mohd M, Ahad A, Mohd A, Rahman SO, Najmi AK, et al. Preparation, optimization and biological evaluation of gymnemic acid loaded niosomes against streptozotocin-nicotinamide induced diabetic-nephropathy in Wistar rats. J Drug Deliv Sci Tec. 2019;54:101328.
Alibolandi M, Mohammadi M, Taghdisi SM, Abnous K, Ramezani M. Synthesis and preparation of biodegradable hybrid dextran hydrogel incorporated with biodegradable curcumin nanomicelles for full thickness wound healing. Int J Pharm. 2017;532:466–77.
Guo C, Li M, Qi X, Lin G, Cui F, Li F, et al. Intranasal delivery of nanomicelle curcumin promotes corneal epithelial wound healing in streptozotocin-induced diabetic mice. Sci Rep. 2016;6:29753.
Ravindran S, Suthar JK, Rokade R, Deshpande P, Singh P, Pratinidhi A, et al. Pharmacokinetics, metabolism, distribution and permeability of nanomedicine. Curr Drug Metab. 2018;19:327–34.
Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12:62–76.
Sepúlveda-Crespo D, Ceña-Díez R, Jiménez JL, Ángeles M-FM. Mechanistic studies of viral entry: an overview of dendrimer-based microbicides as entry inhibitors against both HIV and HSV-2 overlapped infections. Med Res Rev. 2017;37:149–79.
Xu B, Li A, Hao X, Guo R, Shi X, Cao X. PEGylated dendrimer-entrapped gold nanoparticles with low immunogenicity for targeted gene delivery. RSC Adv. The Royal Society of Chemistry; 2018;8:1265–1273.
Talelli M, Barz M, Rijcken CJ, Kiessling F, Hennink WE, Lammers T. Core-crosslinked polymeric micelles: principles, preparation, biomedical applications and clinical translation. Nano Today. 2015;10:93–117.
Sun F, Wang S, Wang Y, Zhang J, Yu X, Zhou Y, et al. Synthesis of Ni-Co hydroxide nanosheets constructed hollow cubes for electrochemical glucose determination. Sensors (Basel). 2019;19.
Mukherjee A, Waters AK, Kalyan P, Achrol AS, Kesari S, Yenugonda VM. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: state of the art, emerging technologies, and perspectives. Int J Nanomedicine. 2019;14:1937–52.
Zhang C, Zhang R, Gao X, Cheng C, Hou L, Li X, et al. Small naked Pt nanoparticles confined in mesoporous shell of hollow carbon spheres for high-performance nonenzymatic sensing of H2O2 and glucose. ACS Omega. 2018;3:96–105.
Zhang J, Sun Y, Li X, Xu J. Fabrication of porous NiMn2O4 nanosheet arrays on nickel foam as an advanced sensor material for non-enzymatic glucose detection. Sci Rep. 2019;9:18121.
Ge Y, Lakshmipriya T, Gopinath SC, Anbu P, Chen Y, Hariri F, et al. Glucose oxidase complexed gold-graphene nanocomposite on a dielectric surface for glucose detection: a strategy for gestational diabetes mellitus. Int J Nanomedicine. 2019;14:7851–60.
Cui N, Guo P, Yuan Q, Ye C, Yang M, Yang M, et al. Single-step formation of Ni nanoparticle-modified graphene–diamond hybrid electrodes for electrochemical glucose detection. Sensors (Basel). 2019;19.
Dai Z, Yang A, Bao X, Yang R. Facile non-enzymatic electrochemical sensing for glucose based on Cu2O–BSA nanoparticles modified GCE. Sensors (Basel). 2019;19.
Zhu Q, Hu S, Zhang L, Li Y, Carraro C, Maboudian R, et al. Reconstructing hydrophobic ZIF-8 crystal into hydrophilic hierarchically-porous nanoflowers as catalyst carrier for nonenzymatic glucose sensing. Sensors Actuators B Chem. 2020;313:128031.
Azharudeen AM, Karthiga R, Rajarajan M, Suganthi A. Fabrication, characterization of polyaniline intercalated NiO nanocomposites and application in the development of non-enzymatic glucose biosensor. Arab J Chem. 2020;13:4053–64.
Ahmed HH, Abd El-Maksoud MD, Abdel Moneim AE, Aglan HA. Pre-clinical study for the antidiabetic potential of selenium nanoparticles. Biol Trace Elem Res. 2017;177:267–80.
Bhattacharyya S, Kattel K, Kim F. Modulating the glucose transport by engineering gold nanoparticles. J Nanomedicine Biotherapeutic Discov. Longdom Publishing SL; 2016;6:141.
Shaheen TI, El-Naggar ME, Hussein JS, El-Bana M, Emara E, El-Khayat Z, et al. Antidiabetic assessment; in vivo study of gold and core-shell silver-gold nanoparticles on streptozotocin-induced diabetic rats. Biomed Pharmacother. 2016;83:865–75.
Singla R, Soni S, Patial V, Kulurkar PM, Kumari A, S M, et al. Cytocompatible anti-microbial dressings of Syzygium cumini cellulose nanocrystals decorated with silver nanoparticles accelerate acute and diabetic wound healing. Sci Rep. 2017;7:10457.
Xiao Y, Wang X, Wang B, Liu X, Xu X, Tang R. Long-term effect of biomineralized insulin nanoparticles on type 2 diabetes treatment. Theranostics. 2017;7:4301–12.
Bhattacherjee A, Chakraborti AS. Argpyrimidine-tagged rutin-encapsulated biocompatible (ethylene glycol dimers) nanoparticles: Application for targeted drug delivery in experimental diabetes (Part 2). Int J Pharm. 2017;528:8–17.
Maestrelli F, Mura P, González-Rodríguez ML, Cózar-Bernal MJ, Rabasco AM, Di Cesare ML, et al. Calcium alginate microspheres containing metformin hydrochloride niosomes and chitosomes aimed for oral therapy of type 2 diabetes mellitus. Int J Pharm. 2017;530:430–9.
Shi G, Chen W, Zhang Y, Dai X, Zhang X, Wu Z. An antifouling hydrogel contained silver nanoparticles for modulating therapeutic immune response in chronic wound healing. Langmuir. 2019;35:1837–45.
Pham TT, Nguyen TT, Pathak S, Regmi S, Nguyen HT, Tran TH, et al. Tissue adhesive FK506-loaded polymeric nanoparticles for multi-layered nano-shielding of pancreatic islets to enhance xenograft survival in a diabetic mouse model. Biomaterials. 2018;154:182–96.
Chinnaiyan SK, Deivasigamani K, Gadela VR. Combined synergetic potential of metformin loaded pectin-chitosan biohybrids nanoparticle for NIDDM. Int J Biol Macromol. 2019;125:278–89.
Tong F, Chai R, Jiang H, Dong B. In vitro/vivo drug release and anti-diabetic cardiomyopathy properties of curcumin/PBLG-PEG-PBLG nanoparticles. Int J Nanomed. 2018;13:1945–62.
Shalaby TI, El-Refaie WM. Bioadhesive chitosan-coated cationic nanoliposomes with improved insulin encapsulation and prolonged oral hypoglycemic effect in diabetic mice. J Pharm Sci. 2018;107:2136–43.
Mukhopadhyay P, Maity S, Mandal S, Chakraborti AS, Prajapati AK, Kundu PP. Preparation, characterization and in vivo evaluation of pH sensitive, safe quercetin-succinylated chitosan-alginate core-shell-corona nanoparticle for diabetes treatment. Carbohydr Polym. 2018;182:42–51.
Mumuni MA, Kenechukwu FC, Ofokansi KC, Attama AA, Díaz DD. Insulin-loaded mucoadhesive nanoparticles based on mucin-chitosan complexes for oral delivery and diabetes treatment. Carbohydr Polym. 2020;229:115506.
Uma Suganya KS, Govindaraju K, Veena Vani C, Premanathan M, Ganesh Kumar VK. In vitro biological evaluation of anti-diabetic activity of organic-inorganic hybrid gold nanoparticles. IET Nanobiotechnol. 2019;13:226–9.
Ansari S, Bari A, Ullah R, Mathanmohun M, Veeraraghavan VP, Sun Z. Gold nanoparticles synthesized with Smilax glabra rhizome modulates the anti-obesity parameters in high-fat diet and streptozotocin induced obese diabetes rat model. J Photochem Photobiol B Biol. 2019;201:111643.
Chauhan P, Mahajan S, Prasad GBKS. Preparation and characterization of CS-ZnO-NC nanoparticles for imparting anti-diabetic activities in experimental diabetes. J Drug Deliv Sci Tec. 2019;52:738–47.
He Z, Hu Y, Gui Z, Zhou Y, Nie T, Zhu J, et al. Sustained release of exendin-4 from tannic acid/Fe (III) nanoparticles prolongs blood glycemic control in a mouse model of type II diabetes. J Control Release. 2019;301:119–28.
Panda BP, Krishnamoorthy R, Bhattamisra SK, Shivashekaregowda NKH, Seng LB, Patnaik S. Fabrication of second generation smarter PLGA based nanocrystal carriers for improvement of drug delivery and therapeutic efficacy of gliclazide in type-2 diabetes rat model. Sci Rep. 2019;9:17331.
Yang B-Y, Hu C-H, Huang W-C, Ho C-Y, Yao C-H, Huang C-H. Effects of bilayer nanofibrous scaffolds containing curcumin/lithospermi radix extract on wound healing in streptozotocin-induced diabetic rats. Polymers (Basel). 2019;11.
Wu J-Z, Yang Y, Li S, Shi A, Song B, Niu S, et al. Glucose-sensitive nanoparticles based on poly (3-acrylamidophenylboronic acid-block-nvinylcaprolactam) for insulin delivery. Int J Nanomedicine. 2019;14:8059–72.
Nikpasand A, Parvizi MR. Evaluation of the effect of titatnium dioxide nanoparticles/gelatin composite on infected skin wound healing; an animal model study. Bull Emerg Trauma. 2019;7:366–72.
Natarajan J, Sanapalli BKR, Bano M, Singh SK, Gulati M, Karri VVSR. Nanostructured lipid carriers of pioglitazone loaded collagen/chitosan composite scaffold for diabetic wound healing. Adv Wound Care (New Rochelle). 2019;8:499–513.
Mudassir J, Darwis Y, Muhamad S, Khan AA. Self-assembled insulin and nanogels polyelectrolyte complex (Ins/NGs-PEC) for oral insulin delivery: characterization, lyophilization and in-vivo evaluation. Int J Nanomedicine. 2019;14:4895–909.
Samadian H, Zamiri S, Ehterami A, Farzamfar S, Vaez A, Khastar H, et al. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: In vitro and in vivo studies. Sci Rep. Nature Publishing Group; 2020;10:8312.
Pouran SR, Bayrami A, Mohammadi Arvanag F, Habibi-Yangjeh A, Darvishi Cheshmeh Soltani R, Singh R, et al. Biogenic integrated ZnO/Ag nanocomposite: surface analysis and in vivo practices for the management of type 1 diabetes complications. Colloids Surf B Biointerfaces. 2020;189:110878.
Golmohammadi R, Najar-Peerayeh S, Tohidi Moghadam T, Hosseini SMJ. Synergistic antibacterial activity and wound healing properties of selenium-chitosan-mupirocin nanohybrid system: An in vivo study on rat diabetic Staphylococcus aureus wound infection model. Sci Rep. Nature Publishing Group; 2020;10:2854.
Robkhob P, Ghosh S, Bellare J, Jamdade D, Tang I-M, Thongmee S. Effect of silver doping on antidiabetic and antioxidant potential of ZnO nanorods. J Trace Elem Med Biol. 2020;58:126448.
He Z, Nie T, Hu Y, Zhou Y, Zhu J, Liu Z, et al. A polyphenol-metal nanoparticle platform for tunable release of liraglutide to improve blood glycemic control and reduce cardiovascular complications in a mouse model of type II diabetes. J Control Release. 2020;318:86–97.
Mussmann R, Geese M, Harder F, Kegel S, Andag U, Lomow A, et al. Inhibition of GSK3 promotes replication and survival of pancreatic beta cells. J Biol Chem. 2007;282:12030–7.
Frudd K, Burgoyne T, Burgoyne JR. Oxidation of Atg3 and Atg7 mediates inhibition of autophagy. Nat Commun. 2018;9:95.
Ji J, Petropavlovskaia M, Khatchadourian A, Patapas J, Makhlin J, Rosenberg L, et al. Type 2 diabetes is associated with suppression of autophagy and lipid accumulation in β-cells. J Cell Mol Med. 2019;23:2890–900.
Akter R, Cao P, Noor H, Ridgway Z, Tu L-H, Wang H, et al. Islet amyloid polypeptide: structure, function, and pathophysiology. J Diabetes Res. 2016;2016:2798269.
Mukherjee A, Morales-Scheihing D, Butler PC, Soto C. Type 2 diabetes as a protein misfolding disease. Trends Mol Med. 2015;21:439–49.
Kulas JA, Franklin WF, Smith NA, Manocha GD, Puig KL, Nagamoto-Combs K, et al. Ablation of amyloid precursor protein increases insulin-degrading enzyme levels and activity in brain and peripheral tissues. Am J Physiol Endocrinol Metab. 2019;316:E106–20.
Grimm MOW, Mett J, Stahlmann CP, Haupenthal VJ, Zimmer VC, Hartmann T. Neprilysin and Aβ clearance: impact of the APP intracellular domain in NEP regulation and implications in Alzheimer’s disease. Front Aging Neurosci. 2013;5:98.
Zhang M, Cai F, Zhang S, Zhang S, Song W. Overexpression of ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) delays Alzheimer’s progression in vivo. Sci Rep. 2014;4:7298.
Kim J, Lim Y-M, Lee M-S. The role of autophagy in systemic metabolism and human-type diabetes. Mol Cells. 2018;41:11–7.
Matsumoto G, Inobe T, Amano T, Murai K, Nukina N, Mori N. N-Acyldopamine induces aggresome formation without proteasome inhibition and enhances protein aggregation via p62/SQSTM1 expression. Sci Rep. 2018;8:9585.
Sikanyika NL, Parkington HC, Smith AI, Kuruppu S. Powering amyloid beta degrading enzymes: a possible therapy for Alzheimer’s Disease. Neurochem Res. 2019;44:1289–96.
Pierzynowska K, Podlacha M, Gaffke L, Majkutewicz I, Mantej J, Węgrzyn A, et al. Autophagy-dependent mechanism of genistein-mediated elimination of behavioral and biochemical defects in the rat model of sporadic Alzheimer’s disease. Neuropharmacol. 2019;148:332–46.
Elliott HR, Shihab HA, Lockett GA, Holloway JW, McRae AF, Smith GD, et al. Role of DNA Methylation in type 2 diabetes etiology: using genotype as a causal anchor. Diabetes. 2017;66:1713–22.
Smail HO. The epigenetics of diabetes, obesity, overweight and cardiovascular disease. AIMS Genet. 2019;6:36–45.
Pordzik J, Jakubik D, Jarosz-Popek J, Wicik Z, Eyileten C, De Rosa S, et al. Significance of circulating microRNAs in diabetes mellitus type 2 and platelet reactivity: bioinformatic analysis and review. Cardiovasc Diabetol. 2019;18:113.
Acknowledgments
Authors are grateful to their respective institutions for support. RG Kerry would like to thank the Department of Science & Technology, Government of Odisha, India, for Biju Patnaik Research Fellowship (Ref. No. ST-BT-MISC-0008-2018-1933/ST), for perusing Ph.D. in Biotechnology (with registration ID:- 05-Biotechnology-2017-18), at Department of Biotechnology, Utkal University, Bhubaneswar, Odisha, India. JK Patra and G Das are grateful to Dongguk University, the Republic of Korea for support. JK Patra acknowledge the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1G1A1004667) for support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
RG Kerry, GP Mahapatra, GK Maurya, S Mahari, S Patra, G Das, JK Patra, S Sahoo declare they have no conflict of interest with the manuscript.
Code availability
Not applicable
Ethics approval
Not applicable
Consent to participate
All authors gave their consent to participate in the review.
Consent for publication
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Major Objectives
• Cellular and molecular pathophysiology of T2D
• Commercialized antidiabetic drugs and their limitations
• Types of nanoparticulated systems exploited in diabetic researches
• Emerging intracellular signaling cascade that includes inflammasome, autophagy, genetic and epigenetic changes and their associations with T2D
• Hypothetical smart targeted nano-delivery system modulating the Wnt signaling pathway for promoting β-cell growth and development
• Hypothetical smart targeted nano-delivery systems exploiting inflammasome and autophagic target points for T2D treatment
Rights and permissions
About this article
Cite this article
Kerry, R.G., Mahapatra, G.P., Maurya, G.K. et al. Molecular prospect of type-2 diabetes: Nanotechnology based diagnostics and therapeutic intervention. Rev Endocr Metab Disord 22, 421–451 (2021). https://doi.org/10.1007/s11154-020-09606-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-020-09606-0