Abstract
Density functional theory (DFT) is used to investigate the N2O decomposition over Pd4−/0/+ clusters. The Eley–Rideal (ER) mechanism and the Langmuir–Hinshelwood (LH) mechanism are well established. The average binding energies show that the most stable structure of Pd4−/0/+ clusters is the tetrahedral configuration. For the Pd4− cluster, the activation energies indicate that the rate-limiting step in two mechanisms is the formation of O2, and the ER mechanism occurs more easily than the LH mechanism. While for the Pd40 and Pd4+ clusters, the rate-limiting step in two mechanisms is the N2O decomposition to N2, and the LH mechanism is more likely to process. Among all clusters, the Pd4− cluster exhibits better catalytic activity compared with the Pd40 and Pd4+ clusters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
N2O, as a greenhouse gas, not only severely damages the ozone layer in the atmosphere, but also has a higher warming potential than CO2 and CH4 [1,2,3]. Recently, Shakoor et al. reported that the emission of N2O is increasing due to anthropogenic activities [4]. Therefore, the elimination of N2O has become one of the measures to mitigate atmospheric pollution. Among the N2O treatment technologies, the direct catalytic decomposition of N2O into N2 and O2 is regarded as the most promising method. There are mainly three main steps for the N2O decomposition [5, 6]:
The ER mechanism consists of the decomposition of two N2O molecules. The first N2O adsorbs on the catalyst and furtherly decomposes to N2 and residual O*. Then, the residual O* binds to another N2O to transform into N2 and O2. However, some researchers find that the residual O* may react with another remaining O* to further form O2, which is known as the LH mechanism.
Small clusters are considered to be intermediates between the single atom and condensed matter. Many investigations have revealed that the reactivity of a cluster is dependent on the charge state. Francisco et al. [7] studied the charge effect on the N2O reduction by the Rh6− and Rh6+ clusters, they found that the N2O reduction process is influenced by the chosen charge of Rh particles. The Rh6− cluster has better catalytic activity compared with the Rh6+ cluster according to the activation energy results. Wu et al. [8] investigated the reaction mechanism of N2O decomposition on Au3+/0/− clusters, they point out that the Au3 neutral cluster exhibited the highest catalytic activity, which just needs an energy barrier of 11.60 kcal/mol in the decomposition of N2O. Lian et al. [9] explored the dissociation of water on neutral and charge-state Ni3M (M = Ni, Cr, Mn, Fe, and Co) clusters. The results indicate that the dissociation barriers of anionic clusters are relatively low, and the bimetallic Ni3Fe cluster exhibits the best performance.
Palladium catalysts have exhibited superior catalytic activity in many fields, especially in the control of automobile exhaust gas emissions [10, 11]. Its atoms, clusters, and compounds have been proven to be effective catalysts for N2O decomposition [12,13,14,15,16]. However, the charge state influence of Pd nanoparticles on the catalytic activity is uncertain, and there is lack of theoretical study for the catalytic mechanism in the dissociation of N2O on the charged Pd cluster.
Hence, in this work, the decomposition of N2O on the Pd4+/0/−cluster has been investigated by DFT calculation. Two reaction mechanisms for N2O decomposition on the Pd4+/0/− cluster have been well established. For two mechanisms, the effects of the charge state on catalytic activity are elaborated. The results obtained can provide some theoretical guidance for solving the N2O pollutant problem.
Computational methods
All DFT calculations were performed by Gaussian09 package [17]. The exchange–correlation interactions were treated by generalized gradient approximation (GGA) with Perdew-Burke-Ernzerhof (PBE) functional [18, 19]. The initial cluster structure optimization was performed at PBE/SDD level and the single point energy was calculated at PBE0/SDD level. The 6-311 + G(d,p) basis set was used for N and O atoms [20] and the SDD basis set was used for Pd atom [21]. The frequency analysis was supplemented by the same theoretical level to ensure that each TS has only one imaginary frequency.
The adsorption energy between cluster and molecules was calculated by Eq. 4:
Here the \({E}_{\mathrm{system}}\) was the energy of the molecule adsorbed on the cluster, the \({E}_{\mathrm{cluster}}\) was the energy of the cluster and the \({E}_{\mathrm{molecule}}\) was the energy of the molecule.
Results and discussions
Adsorption of N2O over the Pd4 −/0/+ clusters
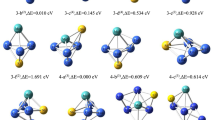
The overall possible configurations of Pd4−/0/+ clusters are optimized in various spin multiplicities, and the optimized structures are shown in Table S1. In all configurations of Pd4−/0/+ clusters, the tetrahedral structure is more stable and has higher binding energy than other configurations. Fig. S1 shows the most stable tetrahedral configuration of Pd4−/0/+ clusters. The Pd4+ cluster is a regular tetrahedral configuration with an average bonding length of 2.59 Å and binding angle of 60°, the Pd40 and Pd4− clusters are slightly distorted tetrahedral structures with average binding lengths of 2.61 Å and 2.66 Å. This is consistent with the theoretical data of 2.66 Å for the Pd40 cluster reported by Camacho [22].
Then the N-terminal and O-terminal adsorption of N2O at each possible site of Pd4−/0/+ clusters with various spin multiplicities are investigated, the full data are listed in Table S2. The adsorption energy is little affected by the different adsorption sites while it can be influenced availably by the adsorption pathway of N2O. The adsorption energy, bond lengths between the Pd4−/0/+ clusters and N2O in the N- and O-terminal adsorption are shown in Table 1. The adsorption energy of N2O on the Pd4− cluster in N-terminal adsorption mode is the highest among the three clusters. Generally, the adsorption energy in the N-terminal adsorption pathway of N2O is larger than that of the O-terminal, which suggests that the N2O prefers to adsorb on the cluster through the N-terminal.
Mechanisms of N2O decomposition on the Pd4 −/0/+ clusters
Considering the potential energy profiles and the structures along the dissociation pathways are very similar, the reaction pathways of the N-terminal adsorption over Pd4−/0/+ clusters are mainly described in detail as shown in Figs. 1, 2, 3. The potential energy profiles in the O-terminal adsorption are shown in Figs. S2–S4.
For the N2O reduction reaction, the initial step is the first N2O decomposition. Fig. 1 shows the potential energy profiles of the first N2O decomposition. For the Pd4− cluster, the first decomposition of N2O needs two reaction processes to realize, while Pd40 and Pd4+ clusters need just one step. For Pd40 and Pd4+ clusters, the N2O is slantly attached to the metal clusters with an ∠N–N–O angle of 180°, the energy of IM1* and IM** is − 0.52 eV and − 0.60 eV, respectively. Then, the IM1* and IM1** convert to the IM2* and IM2** through transition states of TS1* and TS1**. The angle of ∠N–N–O is bent from the original 180–118° and 115°. The Pd–O bond with lengths of 1.98 Å and 1.88 Å. Subsequently, the O–N2 bond is broken to form the N2 molecule, and the dissociated O atom locates at the two adjacent Pd atoms of Pd40 and Pd4+ clusters. The activation energies of this process are 1.32 eV and 2.24 eV.
For the Pd4− cluster, the N2O molecule is vertically adsorbed on the metal cluster to form IM1*** with an adsorption energy of − 1.03 eV. Then, the IM1*** transfers to IM2*** through a transition state of TS1*** with an activation energy of 0.27 eV. The ∠N–N–O angle of N2O is bent from 180 to 131° and the N and O atoms of N2O are connected to neighboring Pd atoms. The N–O bond lengthens from 1.29 to 1.67 Å and finally breaks. The activation energy of this process is 0.52 eV.
The potential energy profiles of ER mechanism are shown in Fig. 2, the Pd4O0, Pd4O+, and Pd4O− clusters combine with another N2O to further form IM1+, IM1++, and IM1+++ with energies of − 0.79 eV, − 0.94 eV, and − 0.81 eV. Then, IM1+, IM1++, and IM1+++ convert to IM2+, IM2++, and IM2+++ through transition states of TS1+, TS1++, TS1+++ with activation energies of 1.65 eV, 2.33 eV, and 0.68 eV. At this time, the N–O bond is broken and remains an O atom connecting to the Pd4O−/0/+ clusters. Finally, the two O atoms move close to each other by the lengthening of the Pd–O bond and generate the O2 (IM3+, IM3++, and IM3+++). The activation energies for this process are 0.22 eV, 0.01 eV, and 0.87 eV. Therefore, for the Pd4O0 and Pd4O+ clusters, the activation energy for the N2O decomposition to N2 is higher than the O2 formation, which indicates that the N2O decomposition to N2 is the rate-limiting step for the Pd4O0 and Pd4O+ clusters in the ER mechanism. However, for the Pd4O− cluster, the activation energy in the formation of O2 is higher than the N2O decomposition to N2, implying that the formation of O2 is the rate-limiting step.
The potential energy profiles of the LH mechanism are shown in Fig. 3. Different from the ER mechanism, the formation of O2 in the LH mechanism is mainly from the recombination of two residual O atoms. As shown in Fig. 3, the Pd4O0, Pd4O+, and Pd4O− clusters combine with another O atom to form IM1^, IM1^^, and IM1^^^ with energies of − 2.33 eV, − 1.99 eV, and − 3.24 eV. Then, the IM1^, IM1^^, IM1^^^ to further form IM2^, IM2^^, IM2^^^ through transition states TS1^, TS1^^, TS1^^^. The activation energies of this process are 0.58 eV, − 0.04 eV, and 1.45 eV, which indicates that the Pd4+ cluster has the best catalytic activity for the formation of O2 in LH mechanism. By comparing the ER mechanism and LH mechanism, it can be concluded that in the ER mechanism, the Pd40 cluster and the Pd4+ cluster tend to form O2, while the Pd4− cluster prefers to generate N2.
To further understand the effect of the charge state for the Pd4 clusters on the catalytic activity, we summarized all reaction energy barriers in Fig. 4. It is found that the charge state has significant effect on the catalytic activity. Among all investigated clusters, the Pd4− cluster exhibits superior catalytic activity for N2O decomposition, just needing 0.52 eV (the first N2O decomposition) and 0.68 eV (the second N2O decomposition) of the N-terminal pathway. It is much lower than the reported data of Au19Pd (1.12 eV) [23] and PdC23 (1.45 eV, 2.97 eV) [24] clusters. The Pd4+ cluster shows excellent catalytic activity for the O2 formation, which does not even need activation energy in the N-terminal pathway. As for the two mechanisms, it is noted that most of reactions are thermodynamically favorable. Furthermore, for the Pd4− cluster, the rate-limiting step is the formation of O2, while the decomposition of N2O to N2 is the rate-limiting step for the Pd40 and the Pd4+ clusters. Additionally, the energy barrier for the rate-limiting step in the LH mechanism is lower than the ER mechanism, which indicates the LH mechanism is more favorable.
Mulliken atomic charge analysis
The reactivity of the metal clusters derives from their electronic structure, thus we use the Mulliken atomic charge analysis to make a detailed investigation of the charge distribution between Pd4−/0/+ clusters and N2O. The Mulliken charges of N- and O-terminal for N2O molecule adsorbs on Pd4−/0/+ clusters as shown in Table 2. Electrons transfer from Pd4−/0/+ clusters to N2O, suggesting that the Pd4−/0/+ clusters donate electrons to the N2O as a Lewis base. Additionally, the Pd4− has the maximum amount of charge (1.425 e) transferred to the adsorbed N2O, indicating the Pd4− cluster has strong attraction for N2O molecule. This phenomenon is consistent with the results of the adsorption energies. By comparing the N2O decomposition barriers with the amounts of charge transfer from clusters to N2O, it is found that the more charge transfer, the lower dissociation barrier. This is due to the large number of electrons that accumulate on the N2O molecule, making it easy to activate. Therefore, of three different charged Pd clusters, Pd4− shows the best activity for N2O decomposition.
Molecular orbital analysis
The reaction activity can be determined by the energy values of the HOMO and LUMO of a molecule, as well as their energy difference ΔE. The HOMO represents the highest energy level of occupied electrons in a molecule and exhibits a high electron-donating capability. The LUMO represents the lowest energy level of unoccupied electrons in a molecule and possesses an electron-accepting ability. Thus, the smaller ΔE indicates that the electrons on the HOMO level are more likely to transfer to the LUMO level. The value of HOMO, LUMO and ΔE of Pd4−/0/+clusters are presented in Fig. 5. It can be observed that the HOMO value of Pd4− cluster is higher than Pd4+ and Pd40 clusters, suggesting that Pd4− cluster has a better electron donation ability. The value of LUMO is Pd4+ < Pd40 < Pd4−, which means that the Pd4+ cluster is prone to accepting electrons. The Pd4− cluster has the smallest difference (ΔE) of 2.18 eV. The small energy difference makes Pd4− easy to dissociate N2O, which is consistent with results of the N2O decomposition energy barrier.
Conclusion
The decomposition of N2O on Pd4−/0/+ clusters has been investigated by DFT calculation. The adsorption energies show that the N2O prefers to adsorb on Pd clusters through N-terminal than the O-terminal. The potential energy profiles show that the N- or O-terminal adsorption of N2O has little influence on the decomposition barriers of N2O. The Pd4− cluster exhibits excellent catalytic activity in the N2O decomposition to N2, which just needs 0.52 eV (the first N2O decomposition) and 0.68 eV (the ER mechanism) in the N- terminal pathway. The Pd4+ cluster shows the best catalytic activity in the formation of O2, and no activation energy is even required in the N-terminal pathway. Molecular orbital analysis indicates that the excellent catalytic activity of Pd4− cluster is due to the relatively small energy difference between the HOMO and LUMO. Besides, the Mulliken atomic charge analysis shows that the electron accumulation on the N2O can increase the interaction between the N2O molecule and Pd4−/0/+ clusters, and reduce the N2O decomposition activation energy. These conclusions provide theoretical support for designing potential catalytic materials by regulating charge states.
References
Itokawa H, Hanaki K, Matsuo T (2001) Nitrous oxide production in high-loading biological nitrogen removal process under low cod/n ratio condition. Water Res 35(3):657–664. https://doi.org/10.1016/S0043-1354(00)00309-2
Yan X, Zheng SK, Qiu DZ, Yang J, Han YP, Huo ZM, Su XF, Sun JH (2019) Characteristics of N2O generation within the internal micro-environment of activated sludge flocs under different dissolved oxygen concentrations. Bioresour Technol 291:121867. https://doi.org/10.1016/j.biortech.2019.121867
Tian HQ et al (2020) A comprehensive quantification of global nitrous oxide sources and sinks. Nature 586:248–256. https://doi.org/10.1038/s41586-020-2780-0
Shakoor A et al (2021) Effect of animal manure, crop type, climate zone, and soil attributes on greenhouse gas emissions from agricultural soils-A global meta-analysis. J Clean Prod 278:124019. https://doi.org/10.1016/j.jclepro.2020.124019
Tanaka S, Yuzaki K, Ito S, Kameoka S, Kunimori K (2001) Mechanism of O2 desorption during N2O decomposition on an oxidized Rh/USY catalyst. J Catal 200(2):203–208. https://doi.org/10.1006/jcat.2001.3197
Yamashita T, Vannice A (1996) N2O decomposition over manganese oxides. J Catal 161(1):254–262. https://doi.org/10.1006/jcat.1996.0183
Francisco H, Bertin V, Soto JR, Castro M (2016) Charge and geometrical effects on the catalytic N2O reduction by Rh6- and Rh6+ clusters. J Phys Chem C 120(41):23648–23659. https://doi.org/10.1021/acs.jpcc.6b08172
Wu LY, Chen C, Luo L, Wang YC, Yin B (2020) DFT Study of the reaction mechanism of N2O decomposition on Au3+/0/- clusters. ChemistrySelect 5:5391–5399. https://doi.org/10.1002/slct.202000752
Lian X, Guo WL, Nie Y, Xu P, Yi H, He B, Chen SK (2019) A density functional study of water dissociation on small cationic, neutral, and anionic Ni-based alloy clusters. Chem Phys 521:44–50. https://doi.org/10.1016/j.chemphys.2019.01.019
Omrani M, Goriaux M, Liu Y, Martinet S, Jean-Soro L, Ruban V (2020) Platinum group elements study in automobile catalysts and exhaust gas samples. Environ Pollut 257:113477. https://doi.org/10.1016/j.envpol.2019.113477
Cao YD, Ran R, Wu XD, Si ZC, Kang FY, Weng D (2022) Progress on metal-support interactions in Pd-based catalysts for automobile emission control. J Environ Sci 125:401–426. https://doi.org/10.1016/j.jes.2022.01.011
Kim K, Baek S, Kim JJ, Han JW (2020) Catalytic decomposition of N2O on PdxCuy alloy catalysts: a density functional theory study. Appl Surf Sci 510:145349. https://doi.org/10.1016/j.apsusc.2020.145349
Xing W, Yang XF, Wang AQ, Li L, Liu XY, Zhang T, Mou CY, Li J (2012) Bimetallic Au-Pd alloy catalysts for N2O dissociation: effects of surface structures on catalytic activity. J Phys Chem C 116(10):6222–6232. https://doi.org/10.1021/jp210555s
Kokalj A (2003) N2O interaction with Pd(110): cluster vs. slab model. Surf Sci 532–535:213–220. https://doi.org/10.1016/S0039-6028(03)00460-6
Hintz PA, Ervin KM (1995) Chemisorption and oxidation reactions of nickel group cluster anions with N2, O2, CO2, and N2O. J Chem Phys 103(18):7897–7906. https://doi.org/10.1063/1.470207
Parres-Esclapez S, Illán-Gómez MJ, Lecea SMD, Bueno-López A (2010) On the importance of the catalyst redox properties in the N2O decomposition over alumina and ceria supported Rh, Pd and Pt. Appl Catal B 96(3–4):370–378. https://doi.org/10.1016/j.apcatb.2010.02.034
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09. Revision B.01.01. Gaussian Inc., Wallingford
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46(11):6671–6687. https://doi.org/10.1103/physrevb.46.6671
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 78:1396. https://doi.org/10.1103/PhysRevLett.77.3865
Curtiss LA, McGrath MP, Blaudeau JP, Davis NE, Binning RC, Radom L (1995) Extension of Gaussian-2 theory to molecules containing third-row atoms Ga-Kr. J Chem Phys 103:6104–6113. https://doi.org/10.1063/1.470438
Cao XY, Dolg M (2002) Segmented contraction scheme for small-core lanthanide pseudopotential basis sets. J Mol Struct (Thoechem) 581:139–147. https://doi.org/10.1016/j.theochem.2003.12.015
Camacho-Mendoza RL, Cruz-Borbolla J (2020) Reaction mechanism for hydrogen production using the Pd4 cluster and formic acid by DFT. Chem Phys Lett 755:137794. https://doi.org/10.1016/j.cplett.2020.137794
Yu WL, Zuo HW, Lu CH, Li Y, Zhang YF, Chen WK (2015) Nitrous oxide decomposition catalyzed by Au19Pd and Au19Pt clusters. Acta Phys Chim Sin 31(3):425–434. https://doi.org/10.3866/PKU.WHXB201501191
Derdare M, Boudjahem AG, Boulbazine M (2022) Adsorption and decomposition mechanism of N2O molecule over MC23 (M=Ru, Mn, V, Pd, and Rh) nanoclusters: a comparative DFT investigation. Struct Chem 33:2043–2062. https://doi.org/10.1007/s11224-022-01984-2
Acknowledgements
This work is supported by the Science and Technology Research Program of Chongqing Municipal Education Commission (KJQN202101517).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, X., Zeng, W., Duan, H. et al. Density functional theory study of N2O decomposition catalyzed by Pd4−/0/+ clusters. Reac Kinet Mech Cat 136, 1933–1943 (2023). https://doi.org/10.1007/s11144-023-02456-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02456-2