Abstract
A series of heterocyclic compounds possessing imidazolo[1,2-a]pyridine moiety, namely, ethyl 7-methylimidazolo[1,2-a] pyridine-2-carboxylate L1; 2-(3-nitrophenyl)imidazo[1,2-a]pyridine L2; 3-(imidazo[1,2-a]pyridine-2-yl)aniline L3; 2-phenylimidazolo[1,2-a]pyridine-3carbaldehyde L4; and 2-phenylimidazo[1,2-a]pyridine L5 were synthesized. The in situ generated copper (II), iron (II), and zinc (II) complexes of these compounds (L1–L5) were examined for their catalytic activities and were found to be effective catalysts for the oxidation of catechol to o-quinone with the atmospheric oxygen. The present study reveals that the rate of oxidation depends on four parameters: the nature of the ligand, transition metals, ion salts, and the concentration of the complex. The combination L2(Cu(CH 3 COO) 2 ) gives the highest rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Catalysis is an interesting and ever-growing branch with useful applications in synthetic chemistry. We were interested in this work to discover the efficiency of copper (II)—imidazolo [1,2-a] pyridine complexes in the oxidation of catechol, which presents a major challenge both in biology and in medicine. The reason behind the interest in these complexes is their resemblance to biological systems, capable of activating the catalyst for many chemical reactions. The in situ preparation of complexes allowed us to easily vary the nature of the ligands and the anions.
We examined a series of five heterocyclic compounds based on imidazolo[1,2-a]pyridine moiety, namely, ethyl 7-methylimidazolo[1,2-a] pyridine-2-carboxylate L1 [1]; 2-(3-nitrophenyl)imidazo[1,2-a]pyridine L2 [2, 3]; 3-(imidazo[1,2-a]pyridine-2-yl)aniline L3 [4]; 2-phenylimidazolo[1,2-a]pyridine-3carbaldehyde L4 [5]; and 2-phenylimidazo[1,2-a]pyridine L5 [6].
Results and discussion
Experimental
In this study, kinetic measurements were made spectrophotometrically on a UV-Vis spectrophotometer (UV-1650 PC Shimadzu) at the COSTE (Centre de l’Oriental des Sciences and Technologies de l’Eau) following the appearance of o-quinone over time at 25 °C (390-nm absorbance maximum, ε = 1,600 M−1 cm−1 in methanol). The metal complex (prepared in situ [7–15] by mixing 0.15 mL of a solution of ligand (2 × 10−3 M) and 0.15 mL of a solution of metal salt (2 × 10−3 M) namely, Cu (CH3COO)2, CuSO4, Cu (NO3)2, CuCl2, FeCl2, and ZnCl2 and a 2-mL solution (10−1 M methanol solution) of catechol was added together in the spectrophotometric cell. We follow the evolution of absorbance at 390 nm versus time after setting to zero. So for each ligand, we varied the nature of the anion. The different kinetic parameters were determined by the method of the initial velocity (Table 1).
Chemistry
The synthesis and biological activities based on imidazolo[1,2-a]pyridine against many diseases have been reported [16–23]. The most potent scaffold was selected for further optimization leading to a series with potent Gram-positive antibacterial activity and a low resistance frequency (Scheme 1).
These ligands are known compounds [24]. For the synthesis of compound L1 [1]: the 7-methyl imidazo [1,2-a] pyridine-2-ethyl carboxylate was provided by Benchat and coworkers [24] by the following method: The synthetic strategy involves condensation of 7-methyl 2-amino pyridine (37.64 g; 0.4 mol) with ethylbromopyruvate (48.75 g; 0.25 mol) in boiling ethanol for 5 h, after cooling the solution the mixture was neutralized at 0 °C with Na2CO3; the product was extracted with dichloromethane. The organic layer was dried over sodium sulphate and the dichloromethane was removed under reduced pressure. The crude product was purified on a silica gel column and a colorless solid was obtained. The 7-methyl imidazo [1,2-a] pyridine-2-ethyl carboxylate compound was obtained with a good yield 70 % with mp = 144 °C. For the synthesis of compound L2 [2, 25]: Condensation of 2-aminopyridine with 1-(2-bromo-3-nitro-phenyl)-ethanone in boiling ethanol for 4 h, after cooling the solution the mixture was neutralized at 0 °C with Na2CO3, the product was extracted with dichloromethane. The organic layer was dried over sodium sulphate and the dichloromethane was removed under reduced pressure. The crude product was purified on a silica gel column and a colorless solid was obtained. The 2-(metanitrophenyl) imidazo [1,2-a] pyridine compound was obtained with a good yield 65 % with mp = 200 °C. For the synthesis of compound L3 [4]: The 2-(3-nitro-phenyl)-imidazo[1,2-a]pyridine was dissolved in methanol, a solution of tin in methanol was added drop-wise after the residue was agitated for 3 h, the 2-(metaaminophenyl)imidazopyridine was obtained with a good yield 65 % with mp = 212 °C.
For the synthesis of compound L4 [5, 26–30]: 1 mol of the 2-phenylimidazo[1,2-a]pyridine was dissolved in DMF, a solution of POCl3 (2 mol) was added slowly, after the addition, the solution was stirred for 7 h and the DMF was removed under reduced pressure; the was residue extracted with dichloromethane and purified on silica gel to give a white solid, 2-phenyl imidazo[1,2-a]pyridine-2-carbaldehyde was obtained in good yield with mp = 150 °C. For the synthesis of compound L5 [20, 31–37]: condensation of 2-aminopyridine with acetophenone-2-bromide in boiling ethanol for 4 h, after cooling the solution, the mixture was neutralized at 0 °C with Na2CO3, the product was extracted with dichloromethane. The organic layer was dried over sodium sulphate and the dichloromethane was removed under reduced pressure. The crude product was purified on a silica gel column and a colorless solid was obtained. The 2-phenyl imidazo[1,2-a]pyridine compound was obtained with a good yield 80 % with mp = 340 °C.
Scheme 2 depicts the oxidation of catechol to o-quinone:
Catechol oxidases: catechol in the absence of the catalyst
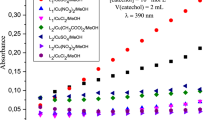
This study focuses on the catalytic activity of copper complexes to oxidize catechol along with a comparison of copper chloride with iron and zinc. Before starting the study, we verified that under the experimental conditions used, catechol does not undergo oxidation in the absence of copper catalyst. Figure 1 shows an almost zero absorbance with time in the absence of the catalyst under experimental conditions.
Figure 2 shows that the ligand alone does not allow oxidation.
Thus the ligands alone do not have a catalytic effect on the oxidation of catechol. Figure 3 shows the evolution in absorbance of o-quinone with time for the following metal salts: Cu(CH3COO)2, CuSO4, Cu (NO3)2, CuCl2, FeCl2 and ZnCl2.
From Fig. 3, it is clear that the absorbance is very low, when the metal salts alone are used for oxidation except Cu(CH3COO)2, which shows a remarkable catalytic activity.
Oxidation of catechol in the presence of complexes formed with L1–L5
The results obtained for compound L1 show that the oxidation rate for CH3COO− anion is much more important (55.7 nmol L−1 s−1) compared to other anions. The oxidation rates for compound L2 are low except CH3COO− anions, which presents a high rate of 68.5 nmol L−1 s−1.
The complex formed with CH3COO− and L3 shows a remarkable oxidation rate it is about (64.1 nmol L−1 s−1), whereas with SO4 2− and NO3 − with the same ligand shows average speeds (23.8 and 15.1 nmol L−1 s−1). These complexes with ligand L4 and the tested metals have not shown remarkable speeds, the best is the one with CH3COO− (26.4 nmol L−1 s−1). Regarding the complex of L5, the best rate is by acetate (CH3COO−) with a value of 65.0 nmol L−1 s−1, very low speeds shown in the case of other anions (Figs. 4, 5, 6, 7, 8).
The effect of the nature of the ligand on the kinetics of oxidation of catechol to o-quinone
The purpose of present study is to compare the catalytic effect of complexes of copper (II) formed by different ligands for the same anion. The following figure shows the oxidation of catechol by complexes of the ligands and copper (II) acetate (Figs. 9, 10, 11, 12, 13, 14).
We note that L2 has a higher catalytic effect, the ligands L1, L3, and L5 show a remarkable effect and almost identical, against L4, which gives a small effect. The labile bond between CH3COO− and the copper make this coordination very easy to displace, so the substrate has no problem to coordinate with the metal because it can easily replace the weakly bonded anion. It is found that the presence of an electron withdrawing group (–C=O) (such as in L4) decreases the electron density on the nitrogen (position 1) and does not allow it to co-ordinate well with the metal. In the case of SO4 2−, L3 and L4 dominate (Fig. 10). For NO3 −, L3 and L4 give a dominant low absorbance (Fig. 11). In both cases, the absorbance remains low. This is due to very strong links formed by these anions with copper. Chlorides do not show remarkable activities (Figs. 12, 13, 14), only the chlorides of zinc and iron revealed significant absorbance with L3, while those that bind chlorides strongly with the metal does not exhibit catalytic activity with this type of ligands.
In summary, as observed in the IR spectrum figures, the presence of catechol and the production of o-quinone caused significant changes in the IR spectrum with time in the case of the combination of L2(Cu(CH3COO)2). Other testes are in progress to understand exactly what is happening during this catalytic reaction. We tried to evaluate the catalytic activity of complexes prepared in situ from the ligands imidazo [1,2-a] pyridine, in the oxidation reaction of catechol to o-quinone by oxygen. The studied complexes have catalytic capabilities, which vary with the ligand and the anion. It is found that the presence of donor groups can enrich the coordination properties of the ligand and promotes stability of the complex, whereas the presence of an electron-withdrawing group is a disadvantage for complex formation due to a decrease in electron density on the sites of coordination. The anion used and its nature plays an important role in the catalytic efficiency of complex anions that bind tightly to the metal cannot be easily displaced by the substrate, thereby reducing the catalytic power. While the anions are less strongly attached with the metal ion, the substrate cannot find any difficulty to coordinate with the metal because it can easily replace the weakly bonded anions. Consequently, the oxidation reaction of catechol is favored and significant oxidation rates are observed.
Conclusions
While the preparation of these complexes in situ allowed us to easily vary the nature of the ligands and anions, the results show that the complexes exhibit catalytic activity for oxidation of catechol under very mild conditions at room temperature (25 °C) using air oxygen as an oxidant. Spectrophotometric monitoring of absorbance versus time shows that the different complexes catalyze the oxidation reaction of catechol with different speeds. The anions that are weakly attached with metal ion, the substrate finds no difficulty to coordinate with the metal because it can easily replace the weakly linked anions as in the case of acetate, while the anions that bind tightly to the metal cannot be easily moved by the substrate, thereby reducing the catalytic ability. The presence of donor groups enriches the coordination properties of the ligand and gives stability to the complex, whereas the presence of an electron-withdrawing group is a disadvantage for complex formation by decreasing the electron density on the sites of coordination (such as in L4).
References
G. Xia, J.P. Li, A. Peng, S. Lai, S. Zhang, J. Shen, Z. Liu, X. Chen, R. Ji, Bioorg. Med. Chem. Lett. 15, 2790 (2005)
C. Burkholder, W.R. Dolbier JR, M. Medebielle, S. Ait-Mohand, Tetrahedron Lett. 42, 3077 (2001)
M.A. Iradyan, N.S. Iradyan, F.G. Arsenyan, G.M. Steepanyan, Pharm. Chem. J. 43, 439 (2009)
T. Kercher, C. Rao, J.R. Bencsik, J.A. Josey, J. Comb. Chem. 9, 1177 (2007)
E. Öhler, M. El-badawi, E. Zbiral, Chem. Ber. 118, 4099 (1985)
N.P. Buu-Hoï, P. Jacquignon, N.D. Xuong, D. Lavit, J. Org. Chem. 19, 1370 (1954)
L. Calero, A. Vega, A.M. Garcia, E. Spodine, J. Manzur, J. Chil. Chem. Soc. 48, 2 (2003)
I. Bouabdallah, R. Touzani, I. Zidane, A. Ramdani, Catal. Commun. 8, 707 (2007)
I. Bouabdallah, R. Touzani, I. Zidane, A. Ramdani, J. Iran. Chem. Soc. 3, 299 (2007)
I. Bouabdallah, R. Touzani, I. Zidane, A. Ramdani, J. Mar. Chim. Heter. 6, 21 (2007)
M. El Kodadi, F. Malek, R. Touzani, A. Ramdani, Catal. Commun. 9, 966 (2008)
N. Boussalah, R. Touzani, I. Bouabdallah, S. El Kadiri, S. Ghalem, J. Mol. Catal. A 306, 113 (2009)
N. Boussalah, R. Touzani, I. Bouabdallah, S. El Kadiri, S. Ghalem, Int. J. Acad. Res. 2, 137 (2009)
A. Zerrouki, R. Touzani, S. El Kadiri, Arab. J. Chem. 4, 459 (2011)
Y. Toubi, R. Touzani, S. Radi, S. El Kadiri, J. Envir, Mater. Sci. 3, 328 (2012)
S.P. East, C.B. White, O. Barker, S. Barker, J. Bennett, D. Brown, E.A. Boyd, Bioorg. Med. Chem. Lett. 19, 894 (2009)
A.R. Katritzky, G. Qiu, Q.-H. Long, H.-Y. He, P.J. Steel, J. Org. Chem. 65, 9201 (2000)
E. Sulojeva, M. Yure, E. Gudriniece, Chem. Heter. Comp. 36, 885 (2000)
M. Velázquez, H. Salgado-Zamora, C. Pérez, M.E. Campos-A, P. Mendoza, H. Jiménez, R. Jiménez, J. Mol. Struct. 979, 56 (2010)
M. Aginagalde, Y. Vara, A. Arrieta, R. Zangi, V.L. Cebolla, A. Delgado-Camón, F.P. Cossío, J. Org. Chem. 75, 2776 (2010)
A. Herath, R. Dahl, N.D.P. Cosford, Org. Lett. 12, 412 (2010)
M.A.P. Martins, C.P. Frizzo, D.N. Moreira, N. Zanatta, H.G. Bonacorso, Chem. Rev. 108, 2015 (2008)
A. Arrault, F. Touzeau, G. Guillaumet, J.-M. Léger, C. Jarry, J.-Y. Mérour, Tetrahedron 58, 8145 (2002)
N. Benchat, Thesis for PhD in chemical sciences, Oujda 1999
P. Guerret, R. Jacquier, G. Maury, J. Heterocycl. Chem. 8, 643 (1971)
L. Yu, A. Lopez, A. Anaflous, B. El Bali, A. Hamal, E. Ericson, L.E. Heisler, A. Mcquibban, G. Giaever, C. Nislow, C. Boone, G.W. Brown, M. Bellaoui, PLoS Genet. 4, e1000284 (2008)
G. Jones, S.P. Stanforth, Organic reactions, vol 49, Hoboken, 1997
A. Anaflous, N. Benchat, M. Mimouni, S. Abouricha, T. Ben-Hadda, B. El-Bali, A. Hakkou, B. Hacht, Lett. Drug Des. Discov. 1, 224 (2004)
N. Benchat, B. El-Bali, S. Abouricha, M. Moneqqit, Med. Pharm. Chem., 0301001 (2003)
J. Koubachi, S. Berteina-Raboin, A. Mouaddib, G. Guillamet, Synthesis 2, 271 (2009)
M. Adib, A. Mohamadi, E. Sheikhi, S. Ansari, H.R. Bijanzadeh, Synlett 11, 1606 (2010)
D.-J. Zhu, J.-X. Chen, M.-C. Liu, J.-C. Ding, H.-Y. Wu, J. Braz. Chem. Soc. 20, 482 (2009)
S. Kumar, D.P. Sahu, Arkivoc 15, 88 (2008)
B.V. Yadav, Y. Subba Reddy, M. Gopal Rao, A.V. Srinivas, Narsaiah, Tetrahedron Lett. 48, 7717 (2007)
S.-Y. Takizawa, J.-I. Nishida, T. Tsuzuki, S. Tokito, Y. Yamashita, Inorg. Chem. 46, 4308 (2007)
T. De Paulis, K. Hemstapat, Y. Chen, Y. Zhang, S. Saleh, D. Alagille, R.M. Baldwin, G.D. Tamagnan, P. Jeffrey Conn, J. Med. Chem. 49, 3332 (2006)
J. Gerencsér, G. Panka, T. Nagy, O. Egyed, G. Dormán, L. Ürge, F. Darva, J. Comb. Chem. 7, 530 (2005)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saddik, R., Khoutoul, M., Benchat, N. et al. Evaluation of catalytic activity of imidazolo[1,2-a]pyridine derivatives: oxidation of catechol. Res Chem Intermed 38, 2457–2470 (2012). https://doi.org/10.1007/s11164-012-0561-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0561-6