Abstract
Eight tridentate bipyrazole derivatives with different side arms have been prepared in one step and with good yields. The products were screened for their cytotoxic activity against three tumor cell lines—human breast cancer cell line MDA-MB231, human prostate cancer cell line PC3, and human colorectal cell line LoVo, by use of colorimetric MTT assay. Structure–activity relationships reflected the effect of substituted drugs. Among this series, two compounds had remarkable in-vitro antiproliferative activity against the LoVo cell line with IC50 values ranging from 2.6 to 2.7 μg ml−1. All the compounds had suitable drug-like characteristics according to Lipinski’s rule.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1959, the first natural pyrazole, 1-pyrazolylalanine, was isolated from watermelon seeds. The term pyrazole was given to this class of compound by Ludwig Knorr in 1883. Since the discovery of this natural structure, its derivatives have been important in medicinal chemistry because of their wide range of bioactivity, for example anti-inflammatory [1], anti-anxiety [2, 3], antipyretic [4], antimicrobial [5], antiviral [6], antitumor [7, 8], anticonvulsant [9], antihistaminic [10], antidepressant [11], and antihyperglycemic [12], and as insecticides [13] and fungicides [13]. The pyrazole structure is also found in a variety of kinase inhibitors and it has also been found that these compounds have hypoglycemic activity; they are also known to be inhibitors and deactivators of liver alcohol dehydrogenase and oxidoreductases [14]. It has been shown in vivo that some pyrazole derivatives have appreciable antihypertensive activity [15]. These compounds also have properties such as cannabinoid hCB1 and hCB2 receptor activity, inhibition of p38 kinase, and activity as CB1 receptor antagonists [16]. These characteristics suggest pyrazoles would make good templates for lead-generation libraries.
In our recent work, a series of pyrazole compounds were prepared and shown to have antitumor, antibacterial and antifungal activity [17–20]. In continuation of our work in this field, this study was performed to investigate antitumor inhibition by several bipyrazole units, with a lariat side, of breast cancer cell line MDA-MB231, human prostate cancer cell line PC3, and human colorectal cell line LoVo, by use of colorimetric MTT assay.
Results and discussion
Chemistry
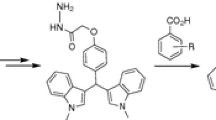
Synthesis of the desired bipyrazoles 1–8 was accomplished as illustrated in Scheme 1. The key starting materials, N-hydroxymethylpyrazoles, have already been reported in old and recent papers [21–24]. Indeed, many heterocycles with a ring NH group, including pyrazole, react with formaldehyde to yield addition products of type N(CH2OH).
The target bipyrazoles were prepared, in one pot, by condensation of two equivalents of N-hydroxymethylpyrazoles with one equivalent of amine derivatives, under gentle conditions (room temperature, atmospheric pressure, 4–5 days), in anhydrous acetonitrile as solvent. The reaction is very slow but is selective at room temperature [25].
The new bipyrazole structures were characterized by spectroscopic methods (1H NMR, IR, and MS) before the tests. Analytical and spectroscopic data for the known compounds were in agreement with those reported previously by our laboratory [26–31].
Biological assays
Compounds 1–8 described in this manuscript were tested for their activity against three human cancer cell lines by use of MTT tests. These cancer cell lines cover three histological cancer types—breast (MDA-MB231), prostate (PC3), and colorectal (LoVo).
The results are shown in Table 1.
As shown in our recent previous work, several of the monopyrazole derivatives tested had no interesting effect against any of the three cancer cell lines examined [17]. However, products herein containing two pyrazole rings had different anti-proliferative activity against a variety of cancer cell lines, as evident from the range of IC50 values (Table 1). Among the bipyrazoles, compound 6 and 7 consistently had higher anti-proliferative effects against the colorectal cell line (LoVo). The IC50 values were 2.6 and 2.7 μg ml−1 respectively.
The bipyrazole derivatives have two regions for SAR evaluation; the greater potency of compounds 6 and 7 could be attributed most probably to the side arm with the alcohol donor atom; it is known that a particular substituent may modify activity relative to the parent compound by virtue of resulting changes in hydrophobic, electronic, and steric effects [32]. As another characteristic, hydrogen-bonding substituent properties were determined by a simple approach taking into account the topological distances of the hydrogen bond donor and acceptor centers from the substituent root [33].
In another variation, we alternated methyl and carboxylate groups at the 3-position of the pyrazole region. The result indicated the importance of the methyl group in the 3-position for the three cancer cell lines examined; indicating methyl might be a good substituent for modification. These results are in agreement with previous reports [17].
The results obtained clearly indicate that the series of bipyrazoles discussed here are moderately active toward the three cancer cell lines. Products 6 and 7 were most active against the colorectal cell line (LoVo). We conclude that introduction of different side arms can contribute favorably to antitumor activity; the activity could also be related to different phenomena, for example complexation capacity, which is easy in tripodal compounds.
The properties formulated in Lipinski’s rule of five were also considered. Both active compounds 6 and 7 fulfil criteria for becoming orally active drugs (Table 2).The rule states that, in general, an orally active drug violates no more than one of the following criteria [34]:
-
no more than five hydrogen-bond donors (nitrogen or oxygen atoms with one or more hydrogen atoms)
-
no more than ten hydrogen-bond acceptors (nitrogen or oxygen atoms)
-
a molecular mass <500 Da
-
an octanol–water partition coefficient log P not >5.
Experimental
Chemistry
Melting points were determined by using a Buchï 510 m.p. apparatus. NMR spectra were obtained with a Bruker AC 300 spectrometer. Infrared spectra were recorded on a Perkin–Elmer 1310 spectrophotometer. Molecular weights were determined on a Jeol JMS DX-300 mass spectrometer.
General procedure
The bipyrazole products were prepared by addition of primary amine derivatives (5 mmol) to a solution of the substituted hydroxymethylpyrazole (10 mmol) in acetonitrile (25 ml). The mixture was stirred at room temperature for 4–5 days. The crude material was evaporated, washed with water and CH2Cl2, and purified by silica gel column flash-chromatography to give the target product.
1-{Bis[(3-ethylcarboxylate-5-methyl-1H-pyrazol-1-yl)methyl]amino}propan-2-ol (8)
Yield 88 %. IR (KBr, cm−1): 3340 (ν O–H), 2880 (ν =C–H), 2800 (ν C–H), 1710 (ν C=O), 1440 (ν C=C), 1H NMR (300 MHz, CDCl3) δ ppm: 1.12 (d, 3H, CH 3 –CHOH–CH2N, J = 6.0 Hz), 1.40 (t, 6H, –O–CH2–CH 3 , J = 6.9 Hz), 2.25 (m, 2H, –CHOH–CH 2 N), 2.30 (s, 6H, pyrazole–CH3), 3.40 (br s, 1H, –CHOH–), 3.76 (m, 1H, –CHOH–). 4.48 (q, 4H, –O–CH 2 –CH3, J = 6.9 Hz), 5.40 (s, 4H, N–CH2–N), 5.68 (s, 2H, PzH). m/z (M+): 407.46.
MTT cell-viability assay
Prepared compounds were screened against three tumor cell lines at the Angiogenesis and Cancer Research Laboratory, Institute of Experimental and Clinical Research (UCL, Brussels, Belgium).
MTT tests were performed to rapidly, i.e. within 3 days, measure the effect of compounds on overall cell growth. The test measures the number of metabolically active living cells that are able to transform the yellow product 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (herein referred to as MTT) into the blue product formazan dye by mitochondrial reduction. The amount of formazan obtained at the end of the experiment, measured by means of a spectrophotometer (Plate Reader Victor ×4 Microplate reader (Perkin Elmer), is directly proportional to the number of living cells.
To perform the assay, cells were grown in 96-well microplates with a flat bottom after addition of 100 μl cell suspension per well with 4,500 cells/well. Cell lines were seeded in Glutamax-containing RPMI supplemented with 10 % foetal bovine serum (PAA) and 1 % penicillin + streptomycin mix (Invitrogen).
The detailed experimental procedure was as follows. After incubation for 24 h at 37 °C, the culture medium was replaced with 100 μl fresh medium in which the tested compounds had previously been dissolved at concentrations of 10−8, 5 × 10−8, 10−7, 5 × 10−7, 10−6, 5 × 10−6, 10−5, 5 × 10−5, and 10−4 g ml−1. Each experiment was performed in sextuplicate. Cells were then incubated at 37 °C in a Binder incubator under a 5 % CO2-containing humidified atmosphere.
After incubation for 72 h, with or without the compound being tested, the medium was replaced with 100 μl HBSS (without phenol red) containing MTT at a concentration of 1 mg/ml. The micro-wells were subsequently incubated for 3 h at 37 °C then centrifuged at 1,300 rpm for 10 min. Medium was removed and formazan crystals formed were dissolved in 100 μl DMSO. The micro-wells were shaken for 5 min and read on a spectrophotometer at a wavelength of 570 nm (maximum absorbance of formazan).
For each experimental condition, the mean optical density was calculated, enabling determination of the percentage of living cells in comparison with the control.
Calculation of lipophilicity
Log P, the logarithm of the n-octanol–water partition coefficient, was calculated by use of the software ACD/ChemSketch ver. 12.01 (ACD/log P) and a variety of approaches available online.
Conclusion
Eight bipyrazole-derivative drugs were successfully prepared in one step from readily accessible starting materials. In-vitro test results against three human cancer cell lines, breast cancer MDA-MB231, prostate cancer PC3, and colorectal cancer LoVo, were indicative of substantial antitumor activity, especially for products 6 and 7 against the colorectal cancer cell line (LoVo). Based on Lipinski’s rule of five these products may be suitable candidates for orally active drugs.
References
A.K. Tewari, A. Mishra, Bioorg. Med. Chem. 9, 715 (2001)
J. Haufel, E. Breitmaier, Angew. Chem. 13, 604 (1974)
D.J. Wustrow, T. Capiris, R. Rubin, J.A. Knobelsdorf, H. Akunne, M.D. Davis, R. MacKenzie, T.A. Pugsley, K.T. Zoski, T.G. Heffner, L.D. Wise, Bioorg. Med. Chem. Lett. 8, 2067 (1998)
R.H. Wiley, P. Wiley, Pyrazolones, Pyrazolidones and Derivatives (Wiley, New York, 1964), p. 102
E.V. Pimerova, E.V. Voronina, Pharm. Chem. J. 35, 18 (2001)
S.L. Janus, A.Z. Magdif, B.P. Erik, N. Claus, Monatsh. Chem. 130, 1167 (1999)
H.J. Park, K. Lee, S. Park, B. Ahn, J.C. Lee, H.Y. Cho, K.I. Lee, Bioorg. Med. Chem. Lett. 15, 3307 (2005)
I. Bouabdallah, L.A. M’barek, A. Zyad, A. Ramadani, I. Zidane, A. Melhaoui, Nat. Prod. Res. 20, 1024 (2006)
V. Michon, C.H. Du Penhoat, F. Tombret, J.M. Gillardin, F. Lepage, L. Berthon, Eur. J. Med. Chem. 30, 147 (1995)
I. Yildirim, N. Ozdemir, Y. Akçamur, M. Dinçer, O. Andaç, Acta Cryst. E61, 256 (2005)
D.M. Bailey, P.E. Hansen, A.G. Hlavac, E.R. Baizman, J. Pearl, A.F. Defelice, M.E. Feigenson, J. Med. Chem. 28, 256 (1985)
K.L. Kees, J.J. Fitzgerald, K.E. Steiner, J.F. Mattes, B. Mihan, T. Tosi, D. Mondoro, M.L. McCaleb, J. Med. Chem. 39, 3920 (1996)
C.K. Chu, J. Cutler, J. Heterocycl Chem. 23, 289 (1986)
A.R. Katritzky, C.W. Rees, E.F.V. Scriven, in Comprehensive Heterocyclic Chemistry, ed. by T. Potts Kevin, vol 5 (Pergamon, Oxford, 1984), p. 291
S. Demirayak, A.C. Karaburum, R. Beis, Eur. J. Med. Chem. 39, 1089 (2004)
R. Silvestri, M.G. Cascio, G.L. Regina, F. Piscitelli, A. Lavecchia, A. Brizzi, S. Pasquini, M. Botta, E. Novellino, V.D. Marzo, F. Corelli, J. Med. Chem. 51, 1560 (2008)
S. Radi, Y. Toubi, N. Draoui, O. Feron, O. Riant, Lett. Drug Des. Discov. 9, 305 (2012)
S. Radi, Y. Toubi, I. Hamdani, A. Hakkou, F. Souna, I. Himri, M. Bouakka, Res. J. Chem. Sci. 2, 40 (2012)
S. Radi, S. Salhi, A. Radi, Lett. Drug Des. Discov. 7, 27 (2010)
A. Yahyi, A. Ettouhami, S. Radi, I. Zidane, A. Hakkou, M. Bouakka, Lett. Drug Des. Discov. 4, 382 (2007)
I.R. Evans, K.M. Szécsényi, V.M. Leovac, Acta Cryst. E61, 625 (2005)
W.L. Driessen, Recl. Trav. Chim. Pays-Bas 101, 441 (1982)
R. Huttel, P. Jochum, Chem. Ber. 85, 820 (1952)
I. Dvoretzky, G.H. Richter, J. Org. Chem. 15, 1285 (1950)
M. Daoudi, N. Ben Larbi, A. Kerbal, B. Bennani, J.-P. Launay, J. Bonvoisin, T. Ben Hadda, P.H. Dixneuf, Tetrahedron 62, 3123 (2006)
R. Touzani, A. Ramdani, T. Ben-Hadda, S. El Kadiri, O. Maury, H. Le Bozec, P.H. Dixneuf, Synth. Commun. 31, 1315 (2001)
A. Attayibat, R. Touzani, S. Radi, A. Ramdani, B. Hacht, S. El Kadiri, J. Mar. Chim. Heterocycl. 9, 15 (2010)
M. El Kodadi, F. Malek, R. Touzani, A. Ramdani, S. El Kadiri, D. Eddike, Molecules 8, 780 (2003)
M. El Ayyachy, M. El Kodadi, A. Aouinti, A. Ramdani, B. Hammouti, F. Malek, A. El Idrissi, Mater. Chem. Phys., 93, 281 (2005)
M. Elkodadi, M. Benamar, I. Bouabdellah, A. Zyad, F. Malek, R. Touzani, A. Ramdani, A. Melhaoui, Nat. Prod. Res. 21, 947 (2007)
T. Harit, M. Cherfi, J. Isaad, A. Riahi, F. Malek, Tetrahedron 68, 4037 (2012)
J.G. Topliss, J. Med. Chem. 15, 1006 (1972)
P. Ertl, J. Chem. Inf. Comput. Sci. 43, 374 (2003)
T.I. Oprea, A.M. Davis, S.J. Teague, P.D. Leeson, J. Chem. Inf. Comput. Sci. 41, 1308 (2001)
Acknowledgments
This work was supported by the CUD (Commission Universitaire pour le Développement, Belgium) within the framework of the P3 program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malek, F., Draoui, N., Feron, O. et al. Tridentate bipyrazole compounds with a side-arm as a new class of antitumor agents. Res Chem Intermed 40, 681–687 (2014). https://doi.org/10.1007/s11164-012-0993-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0993-z