Abstract
A hydrothermal procedure was utilized to synthesize high silica H-ZSM-5 catalysts for application in methanol to olefin (MTO) reaction by partial and total substitution of tetrapropyl ammonium hydroxide (TPAOH) with equivalent amount of the less expensive tetrapropyl ammonium bromide (TPABr) template. Five H-ZSM-5 zeolites with a similar Si/Al ratio (200) were synthesized using various proportions of TPAOH and TPABr. The TPA+ concentration (0.04 M) and initial gel pH (10.5) were constant. The catalysts were characterized by TG–DTA, XRD, ICP, FE-SEM, BET, FT-IR and NH3-TPD techniques. The samples showed cubic morphology, but their particle sizes and shapes were different. The highest crystallinity were obtained by single template synthesis for MFI structure whereas using mixed template synthesis led to lower crystallinity and larger crystallite sizes. Increasing molar ratio of TPAOH in initial gel from 0.33 to 3 decreased the purity of H-ZSM-5, and also affected its crystallinity and particle size. The NH3-TPD results indicated that both TPAOH and TPABr templates exhibited almost the same effect on the acidity of the as-synthesized catalysts, whereas mixing the two templates increased the total acidity and decreased the content of strong acid site. The catalytic performance of all samples was evaluated in MTO reaction in a fixed-bed reactor at 480 °C and atmospheric pressure using feed containing a mixture of 50 wt% methanol in water with methanol WHSV of 0.9 h−1. All the catalysts exhibited approximately similar methanol conversion though their propylene selectivities and products distributions were greatly dependent on the template composition. This work verified that despite providing the same TPA+ and OH− concentrations in the initial gel, the H-ZSM5 samples with different properties could form using diverse template combinations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Light olefins such as ethylene, propylene, and butylenes are widely used as raw materials in petrochemical industry and polyolefin production. Nowadays, light olefins are mostly produced as a by-products of steam cracking and fluid catalytic cracking (FCC) processes using naphtha, light diesel and other petroleum cuts as feedstock. From the perspective of increasing oil prices and environmental protection, the development of other routes for light olefins production, especially methanol to olefins (MTO) process as a proper subset of methanol-to-hydrocarbons (MTH) technology, has recently attracted considerable attention. Simple processing and non-petroleum sources as feed were the main reasons for many endeavors to develop the MTO process that is an acid catalyzed reaction [1,2,3,4]. It has been suggested that methanol is first dehydrated to dimethyl ether (DME) which is subsequently converted to ethylene, propylene, higher olefins and other hydrocarbon by-products [5, 6]. The formation of DME from methanol is directly related to weak acid sites. In other words, weak acid sites are not active in the conversion of methanol to light olefins, while strong acidic sites favor secondary reactions as well as the MTO reaction. The main products are CH4, olefinic/paraffinic C2-C4 hydrocarbons, LPG, gasoline, CO and H2 [7, 8]. Consequently, selecting an appropriate catalyst can play a significant role in the success of the process. Catalysts used in the MTO reaction are typically microporous solid acids including zeolites. Many zeolites have been evaluated in the MTO process, among which SAPO-34 with CHA type framework and H-ZSM-5 with MFI type framework exhibited the best catalytic performance in the MTO and MTP reactions, respectively [9,10,11]. H-ZSM-5 has more favorable properties compared to other zeolites owing to it give high propylene yield and selectivity with limited coke deposition [12,13,14].
The physicochemical properties and catalytic performance of H-ZSM-5 catalyst are dependent on nature, strength and amount of acidic sites, porosity, and crystallite size [15, 16]. Among them, acidity is the main effective parameter which can be controlled by different synthesis variables such as synthesis gel composition, type of template, pH of gel, aging time and temperature of the gel and/or hydrothermal treatment conditions [17,18,19,20]. Since it was first reported in 1972, the synthesis of ZSM-5 for either exploring an effective method by adding different templates or enhancing its properties and morphology through in-situ/ex-situ ways, never seem to stop. H-ZSM-5 catalyst can be synthesized with diverse templates including tetraalkyl ammonium ions, amines and alcohols [21,22,23,24]. The template plays important roles in the synthesis of zeolites so that it can act as a structure-directing agent (SDA), space-filling and charge-compensating species, being necessary for the successful synthesis of such materials [25].
Schwarz et al. [23] investigated different templates such as tetraalkyl ammonium ions (alkyl=methyl, ethyl, propyl, butyl), amines and alcohols in the synthesis ZSM-type zeolites. They found that the best template for the synthesis of ZSM-5 zeolite under the conditions examined was tetrapropyl ammonium ion. Among these templates, tetrapropyl ammonium hydroxide (TPAOH) has been most commonly used for the synthesis of ZSM-5. Using TPAOH as the template, small particle size of ZSM-5 could be achieved. However, TPAOH is an expensive material, and its high price limits its application in industrial scale. The cost of tetrapropyl ammonium bromide (TPABr) is much lower than that of TPAOH and it was successfully applied as a cheap template in the synthesis of H-ZSM-5. Nevertheless, it results in large particle size which affects the catalytic performance in the MTO reaction due to higher diffusion limitation [26,27,28]. One of the strategies proposed is using mixed template synthesis and in recent years this method has repeatedly been applied in the production of SAPO-34 [29,30,31].
TPAOH had a dual role in zeolite synthesis. It serves as a structure-directing agent in the assembly of the zeolite framework and also maintains the alkalinity of the synthesis solution due to it is a very strong base [32]. TPABr similarly produces TPA+ cation, although it is a weaker base and less hydrophobic compared to TPAOH.
Although TPAOH, is one the best known SDAs in the nanosized ZSM-5 synthesis, as mentioned in literatures, it is not reasonable economically. Therefore, it would be worthwhile to find a suitable substitute for this SDA. One of the methods that are recently applied is the combination of different SDAs and compensate the changed pH by addition of a suitable base. In this way, SDAs can modify each other’s structure to result in a highly crystalline nanosized ZSM-5. Of course, in this method, the final result is strongly depending on the ratio of the SDAs to each other [32, 33].
In recent years, few studies have been published on the combination of two SDAs for the synthesis of H-ZSM-5 catalyst, in a number of open literatures [20, 33,34,35,36]. Chauhan et al. prepared H-ZSM-5 catalysts by changing the molar ratios of two templates {tri-ethyl butyl ammonium bromide (TEBA) and ethylene diamine (EDA)}. In that research, ZSM-5 catalysts were hydrothermally synthesized by commercial sodium silicate (28 wt% SiO2, 7 wt% Na2O), aluminum sulfate (Al2(SO4)3.16H2O) as the silica and alumina sources. It was reported that the crystallite morphology and size of the ZSM-5 synthesized samples varied with proportions of templating agents. They reported that the crystallite size in the range of 100–400 nm was observed with equimolar templates [32]. Soltanali et al. reported the preparation of ZSM-5 zeolite using different combinations of two SDAs, TPAOH and TPABr. They also studied the effects of different TPAOH/TPABr molar ratios on the size, crystallinity, and surface area of obtained ZSM-5 zeolites. Their results showed that in 2:1 molar ratio of TPAOH to TPABr, the smallest size with the biggest surface area and high crystallinity was achieved [34]. Abbasian and Taghizadeh, synthesized a series of nano-sized H-ZSM-5 using mixed template method and investigated the effect of different TPABr/TPAOH molar ratios on the catalytic performance of methanol dehydration to dimethyl ether (DME) in a fixed bed reactor. They found that the crystal sizes of the zeolites were enlarged with increasing the concentration of TPABr in the synthesis reaction mixture. However, the addition of up to 10 mol% TPABr to TPAOH in the synthesis reaction mixture did not significantly affect the properties and performance of prepared catalysts; an economic saving in preparation cost could be achieved [35]. Alipour et al. investigated the effect of different SDAs such as TPAOH, TPABr, EDA and ethanol solely or as dual, triple or quad mixture on the synthesis and characteristics of nano-ZSM-5 zeolite such as crystallinity and crystal size, and also its catalytic properties in methanol to gasoline (MTG) process. They found that application of a quad mixture of different SDAs not only reduced the synthesis cost compares to samples synthesized with one template but also ZSM-5 with small particle size, high crystallinity and improved catalytic properties for MTG reaction was obtained in a short crystallization period [36].
In this work, the H-ZSM-5 catalysts were synthesized hydrothermally using a single template as well as mixed templates of TPAOH and TPABr and characterized by TG–DTA, XRD, ICP, FE-SEM, BET, FT-IR and NH3-TPD analyses. The effects of the molar ratio of TPAOH/TPABr on the crystallite size, acidity, morphology and catalytic performance of the catalysts in the MTO reaction were investigated in detail. To, the best of our knowledge, there is no open report on the use of combination of the two above-mentioned compounds as the templates in H-ZSM-5 synthesis for the MTO reaction. This work is part of an extensive effort to prepare a suitable H-ZSM-5 catalyst for the methanol conversion to light olefins especially with high propylene selectivity in commercial scale by Petrochemical Research and Technology Company.
Experimental details
Materials
The starting materials were sodium aluminate (NaAlO2, Al2O3 wt% = 55), silicic acid (SiO2.xH2O, ≥ 99 wt%), ammonium nitrate (NH4NO3, 99 wt%), sodium hydroxide (NaOH, 99.6 wt%) and sulfuric acid (H2SO4, 98 wt%). Tetrapropyl ammonium hydroxide (C12H29NO, 40 wt%) and tetrapropyl ammonium bromide (C12H28BrN) were used as the templates. All the materials were extra pure from Merck Company and were used as received without any further treatments.
Catalyst preparation
High silica H-ZSM-5 catalysts were synthesized by the hydrothermal method using silicic acid and sodium aluminate as Si and Al sources, respectively. The molar ratio of the starting gel was 20SiO2:0.05Al2O3:xTPAOH:yTPABr:1.5Na2O:200H2O, where x + y = 1.0. The synthesis procedure for gel preparation with x = 1.0 is summarized as follow: solution A was prepared as a mixture of NaOH (1.36 g), sodium aluminate (0.227 g), template and distilled water (14.95 g). Solution B was composed of silicic acid (22.91 g) and distilled water (59.81 g). Templates were used according to Table 1. Solution A was added drop wise to solution B during 3 h under vigorous stirring and then concentrated H2SO4 was added to the mixture to adjust gel pH. The crystallization was carried out in stainless-steel Teflon-lined autoclaves at 180 °C for 48 h without stirring under autogenous pressure. Afterwards, the solid product was recovered by filtration, washed several times with deionized water until pH neutral, and then dried overnight at 105 °C. Finally, the catalyst samples were calcined in a muffle furnace under a flow of air at 530 °C for 12 h to remove the organic templates completely. Ion exchange of as-synthesized Na-ZSM-5 samples were carried out four times with 1 M NH4NO3 solution for 10 h at 90 °C under continuous agitation. The samples were then washed and dried at 105 °C for 12 h, followed by calcination at 530 °C for 12 h (at heating rate of 3 °C/min) to obtain the H-form of the zeolites. The resulting powders were formed by tableting and then 16–25 mesh catalysts were made by crushing and sieving for catalytic evaluation in the reactor. The molar composition of the reaction mixtures and the synthesis conditions for the different ZSM-5 materials obtained are given in Table 1.
Catalyst characterization
Thermo-gravimetric analysis (TGA) and differential thermal analysis (DTA) were applied to detect the thermal decomposition of templates in as synthesized un-calcined samples using TG Instrument (Pyris Diamond TG/DTA, PerkinElmer) in the temperature range of 30–700 °C with heating rate of 10 °C/min in N2 atmosphere. TGA/DTA profiles showed that various decomposition reactions occur in as dried precursors as a function of temperature and we could decide the calcination temperature of the as-dried precursors.
The powder X-ray diffraction (XRD) pattern was used to identify the type of crystalline phase and crystallinity of the powder in calcined samples. XRD studies were carried out by a Bruker d8 advance diffractometer using Cu Kα radiation (λ = 1.5406 Å) in the 2θ range from 5° to 80° with the rate of 2°/min. The crystallinity was calculated as ratio of the large peak intensity found at 2θ = 23° to that of ZSM-5/TPABr. The average crystallite sizes (DXRD) of the samples were estimated using the Scherer's equation [37].
The chemical compositions of H-ZSM-5 catalysts (Al, Si) were determined by an inductively coupled plasma-optical emission spectrometer (ICP-OES, Thermo) and using Ar as the plasmogene.
The morphology and particle size of catalyst samples were carried out using a field emission-scanning electron microscopy (FE-SEM KYKY-EM3200, China) operating at 26 kV.
The surface functional groups were qualitatively measured using Fourier transform infrared (FT-IR) spectra of the calcined catalysts were measured in the range of 400–4000 cm−1 using a Bruker Vertex80 spectrometer for addressing the surface functional groups.
The textural properties of all catalysts were measured using N2 adsorption–desorption technique at 77 K on NOVA 2000 instrument (Quanta-chrome USA) in the relative pressure range from 0.05 to 0.99. Before measurements, all samples were evacuated at 300 °C under N2 flow for 3 h. The BET surface area was calculated from linear part of adsorption plot according to IUPAC recommendations [38]. The total pore volume was estimated based on the amount of nitrogen adsorbed at a p/po = 0.99. The micropore area and micropore volume of the ZSM-5 samples were calculated from the t-plot curve at thickness range between 3.5 and 5.4 Å. The mesopore volume is difference of the calculated total volume and the corresponding micropore data. The pore size distribution was calculated using the Barrett-Joyner-Halenda (BJH) method on the adsorption branch of the isotherm.
The surface acidity of the prepared catalysts was measured by temperature-programmed desorption of ammonia (NH3-TPD) with an online thermal conductivity detector (TCD) using a conventional flow apparatus (BELCAT-A, BEL Japan, Inc.) In a typical analysis, 35 mg of each sample was initially degassed with helium at 300 °C for 2 h at a heating rate of 10 °C/min, and then cooling down to 60 °C. Afterwards, the sample was saturated with NH3 for 1 h. After saturation, the sample was purged with helium for 30 min to remove the physically and weakly adsorbed ammonia on the surface of the catalyst. Subsequently, the sample was heated from 35 to 850 °C with a heating rate of 5 °C/min. The amount of ammonia desorbed from the catalyst surface was quantified by comparing the area under TPD curve with the peak areas of standard sample using a thermal conductivity detector (TCD).
Catalytic performance tests
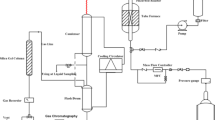
The performance of all prepared catalyst for methanol to olefins (MTO) reaction was investigated in a fixed-bed continuous-flow reactor (450 mm length, 11 mm I.D., S.S. 316) under atmospheric pressure at 480 °C. Fig. S1 provides a schematic outline of the experimental set-up constituted of a liquid/gas feeding part, a fixed-bed reactor, and an analysis part. A temperature-controlled three-zone furnace supplied the constant temperature to the entire reactor. A K-type thermocouple probe was placed in the center of catalyst bed in order to monitor the reaction temperature. In each run, 4 g of catalyst (mesh size: 16–25) was loaded in the middle section of the reactor tube and a mixture of 50 wt% methanol in water was pumped into the catalyst bed by a WellChrom HPLC K-120 pump to provide a methanol WHSV of 0.9 h−1. Prior to the MTO reaction, the sample was activated in-situ, a heating rate of 3 °C/min under highly purified N2 flow at 300 °C for 2 h. Subsequently, the nitrogen gas flow was stopped and the mixture of methanol and water was injected from the feed tank to pre-heater. To prevent any product condensation, external heating was performed on the transfer line from the reactor outlet to the separator vessel inlet, and the temperature was kept constant at 120 °C. On-line testing of gas phase was carried out by the micro-GC (Varian CP-4900) equipped with a TCD detector, where four channels were utilized to separate the products [(CH4, C2-C5 olefin/paraffin, DME, C6-cut) and (H2, CO, and CO2)]. The bottom stream in two aqueous and organic phase was separated. Separated aqueous phase of the liquid product was analyzed by an off-line gas chromatograph of Varian CP-3800 equipped with FID and TCD detectors, where a CP-Wax52CB column was utilized to separate the components (methanol and water). The organic phase was also analyzed with the off-line gas chromatograph of Varian CP-3800 equipped with a FID detector, in which a PIONA column was utilized to separate the components (C6-C16 hydrocarbons). Then the reaction performance results, namely methanol conversion and hydrocarbon products selectivity were carried out. As the equilibrium reaction of methanol to DME occurred very rapidly, for the conversion and hydrocarbon products selectivity calculations, those two species could be combined into one lumped reactant species (oxygenates). Hence, the calculation related to the conversion of methanol in the MTO reaction was carried out as follows (Eq. 1):
The product selectivity was denoted as the mole ratio of each of the products (on the basis of CH2), thereinafter signified as the moles of converted methanol (Eq. 2):
where superscript i denotes the components at the inlet of system and superscript o refers to the components at the system outlet, and subscript x denotes the number of carbon atoms.
Results and discussion
Catalyst characterization
Thermo-gravimetric analysis
Thermo-gravimetric/differential thermal analysis (TGA/DTA) were performed aiming to examine the complete elimination of organic template molecules from the structure of as-synthesized after calcination. Fig. S2 demonstrates TGA/DTA profiles of synthesized samples. The TGA/DTA results for samples S-1, S-2, S-3, S-4 and S-5 exhibited nearly similar weight loss patterns in the temperature ranges of 25–150 °C and 150–500 °C. All the synthesized samples indicated low weight losses at temperatures below 150 °C. This first weight loss (I), accompanied with endothermic peak appearing in DTA curve, can be ascribed to desorption of physically adsorbed water associated with H-ZSM-5 samples. The second weight loss (II), between 150 and 500 °C, along with strong exothermic DTA peaks, is corresponded to the thermal decomposition of the organic template. The thermographs suggest a stepwise decomposition of the organic template, tetrapropyl ammonium ion (TPA+). TGA profiles (Fig. S2a) revealed that the main weight losses for S-1, S-3, S-4 and S-5 samples were centered at around 410–480 °C, while in case of S-2 sample, there is a sharp peak in the temperature range 200–320 °C. The DTA diagram in Fig. S2b exhibits that decomposition of the template in sample S-2 takes place in a different way. Unlike other samples which showed a single sharp peak at 430 °C, the S-2 decomposition occurred over a wide temperature range, so that it has a board peak in the DTA profile. The fact that the template decomposition began at a lower temperature in this sample could possibly be attributed to its smaller crystal size and therefore less diffusion limitations. However, different decomposition mechanisms of TPABr and TPAOH could not be excluded as an additional cause.
The main weight loss data are presented in Table 2. The weight losses associated with template eliminations from TGA (step II) showed fair agreements with the results of the organic content obtained in calcination step (Table 2), confirming the weight losses results. The second weight loss increased with increase of TPAOH content in the gel from sample S-3 to S-5, which is related to the loss of template trapped at ion-exchange site within zeolite cages. The highest weight loss and the strongest exothermic DTA peak were observed for the samples S-1 and S-4 synthesized exclusively with TPAOH and equimolar molar mixture of templates. The TGA/DTA study indicated that complete decompositions of organic templates occur at temperatures greater than 530 °C, because as-dried precursors do not demonstrate any weight losses at temperatures higher than 530 °C. Consequently, the calcinations were carried at this temperature during the present investigation. Our experimental results are in excellent agreement with literature [39].
Crystal structure and morphological analysis
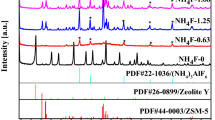
Fig. 1 displays the X-ray diffraction patterns of the H-ZSM-5 catalysts. All samples showed XRD patterns that are typical of the MFI-structure for H-ZSM-5. It must be noted that almost all the samples had some impurity. Also, all patterns exhibited a very low background signal and sharp reflections indicating high crystallinity of all the samples. The main peaks appearing at 2θ = 7.8, 8.7, 22.9, 23.8 and 24.2° were assigned to the formation of ZSM-5 orthorhombic structure (JCPDS 42-23) [40, 41] which implied ZSM-5 was successfully crystallized not only with the single SDAs, but also with their mixtures. Meanwhile, additional peaks can be observed in the diffraction patterns (2θ = 25.5–27°) which could be attributed to amorphous SiO2 formation along with ZSM-5, because the intensities of latter peaks were different for various samples. This impurity phase caused a critical decrease in the intensity of the characteristic peak of ZSM-5 around 2θ = 7.8–9.0°. This may be explained by different alkalinity and perhaps hydrophobicity of the templates. According to the literature, zeolite synthesis is usually performed under basic or strongly basic conditions. For our specific system, the alkalinity is defined as the OH−/Si ratio. The alkalinity in the synthesis batch is one of the most important parameters for controlling the crystallization of zeolites. An enhancement in alkalinity increases the crystallization rate through increasing crystal growth rate and/or nucleation leading in an enrichment in the concentration of reactive species in the liquid phase of the crystallizing system [42,43,44]. Herein, the alkalinity was partially supplied by the templates so that the alkalinity was increased with TPAOH portion of the templates in the solution (Table 1). Increasing alkalinity of the synthesis mixture results in much more dissolving and remaining silica in the liquid phase. Hence, less silica becomes incorporated into the crystalline solid phase and this favors crystallization of a mordenite phase which is more stable than ZSM-5. The mordenite phase then gradually transforms into the most stable amorphous SiO2. In case of both S-1 and S-5, the characteristic peaks of amorphous SiO2 were intense. From the XRD patterns presented in Fig. 1, it can be observed that the intensities of the amorphous SiO2 characteristic peaks decrease with increasing TPABr content in the liquid phase. Thus, it can be concluded that the pure ZSM-5 phase can be synthesized only when the molar ratio of TPAOH/ TPABr is equal to 1.0, because in case of sample S-4, no diffraction peaks other than those of ZSM-5 could be observed indicating the high purity of the product. These results are in good consistence with the literature [22, 45]. Also, both XRD pattern and SEM images of S-2 sample indicated the presence of lamellar magadiite (Na2Si14O29.9H2O [46].
The relative crystallinity was measured from the intensity of the (051) peak in the XRD pattern, which is characteristic of the ZSM-5 structure. Relative crystallinity data, based on the S-2 catalyst, and the position of the (051) peak (at 2θ ≈ 23°) of the samples are given in Table 3. The results showed that changing the TPAOH/TPABr ratio in S-3, S-4 and S-5 samples affect the crystallinity of ZSM-5. According to the XRD results, relative crystallinity was increased by increasing the amount of TPAOH template in the preparation gel. It was found that both the prepared samples with a single template retain a relative crystallinity higher than 80%, whereas the relative crystallinity of catalysts prepared using mixtures of different combinations of templates decreased to 78% or lower values. Both XRD and FT-IR results proved that the lowest and highest crystallinity belonged to the S-2 and S-3 samples prepared with TPABr as the only template and the TPAOH/TPABr ratio of 0.25:0.75.
In order to evaluating the effects of alkalinity on zeolite growth, the synthesis solutions containing a mixture of TPAOH and TPABr were used. In the solution, the quantity of tetrapropyl ammonium (TPA+) ion was kept constant at 0.04 M, while the concentration of OH− was systematically changed. Fig. S3 shows the plot of crystallinity as a function of hydroxide concentration. The best crystallinity was gained for the synthesis solution containing 0.0003 M hydroxide ion. Higher alkalinity represented poorer crystallinity. The results suggest that alkalinity has a stronger influence on crystallinity than [TPA+] in the range of concentrations applied in these experiments. Our experimental results show very good agreement with the literature [47].
One of the most effective synthesis parameters on the particle/crystal size and morphology was found to be the template source. Different template sources have various reactivity, alkalinity and solubility. It is noteworthy that increasing the alkalinity causes more rapid decrease in the solubility of the amorphous silicate precursor as well as increase in the solubility of crystallized zeolite [30]. A high TPAOH/TPABr molar ratio in the mixed template could result in high hydrophobicity and reduced solubility in the aqueous synthesis medium thereby decreasing directing function to form zeolites. As can be seen in Table 3, TPABr as the template caused the smallest mean crystallite size of the catalysts. However, crystallite sizes of ZSM-5 catalysts were increased by increasing the TPAOH content of the mixed templates. Furthermore, the position of the (051) peak is slightly affected by different combinations of TPAOH and TPABr templates. Table 3 also shows the effects of the different combinations of two templates on structural parameters of the prepared catalysts compared with the standard ZSM-5 sample. The results demonstrate that the dimensions (a, b, c) and the volume of the unit cell do not change significantly and the structural parameters of as-synthesized ZSM-5 samples are comparable to those of the standard sample.
Fig. 2 displays some FE-SEM micrographs of the H-ZSM-5 samples synthesized with a single template and different combinations of two various templates. It is seen in the images that the templates influence the morphology and the particle size of H-ZSM-5 samples. Both samples S-1 and S-2 exhibited almost similar morphologies but different sizes in which the cubic particles are clearly obtained. In the case of sample S-1, synthesized with TPAOH, although the predominant morphology is a typical cubic like rectangle morphology, the FE-SEM images indicated strong agglomeration of particles in which the morphology is not quite distinctive. On the other hand, sample S-2 synthesized with TPABr presents two quite different particle shapes and morphologies including cubic crystals and sheet particles simultaneously. In comparison (based on the FE-SEM images) particles with smaller sizes were obtained by applying TPABr (sample S-2). These small particles reveal a high nucleation density and slow crystal growth after nucleation in the synthesis gel. The existence of large grains in sample S-1 may be the reason for its sharp XRD peaks in Fig. 1. Also, it is found that although the cubic particles are clearly observed, mixed templates affected the crystal morphologies and the particle sizes of the H-ZSM-5 materials. Sample S-3 exhibits two quite different particle shapes and morphologies, that is, cubic crystals and sheet shapes simultaneously, which is obtained when the TPAOH/TPABr ratio of 0.25:0.75 is used as a template agent. In this sample, the cubic, smaller particle, and sheet aggregates be assigned to ZSM-5, quartz, and other unknown phases (maybe Kenynaite). However, the as-synthesized sample S-4 (with TPAOH and TPABr in equal amounts) presents a quite different crystal shape and size. In fact, sample S-4 shows spherical aggregates of the uniform cube type H-ZSM-5 crystals and these aggregates demonstrate the average crystal size of approximately 11 µm with homogeneous size distribution. The sample S-5 indicated agglomeration of particles, where the irregular surface of spherical shaped particle was aggregated with H-ZSM-5 cubic crystals. Thus, the FE-SEM study confirms that morphology of the final H-ZSM-5 product is dependent on the nature and composition of a mixture of templates used during synthesis. Since the differences in size, alkalinity, hydrophobicity and geometric shape of template affect the filling and directing manners, various crystal orientations and morphologies were achieved. According to the FE-SEM images, comparing the single template and mixed templates synthesized samples reveals that when the TPAOH/TPABr = 1.0, cubic H-ZSM-5 crystals with good dispersion and homogeneous size distribution could be obtained, while when the TPAOH/TPABr < 1.0 or > 1.0, the synthesized H-ZSM-5 samples exhibit irregular or aggregated crystallites.
Table 4 presents the particle sizes of the samples prepared with diverse combinations of the templates. It can be seen that particle sizes for the five samples varied modestly by changing the proportion of the templating agents. For S-1 and S-2 samples, with 100% TPAOH and only TPABr, average particle sizes equal to 14 and 11 μm were achieved. The sample S-2 had the smallest average particle size. This can be due to its enhanced particle growth and aggregation in the presence of TPAOH. However, for the sample S-4, with a 50:50 mixture of TPAOH and TPABr the particle size reached to 11.4 μm. Increasing TPAOH proportion in template mixture to 75% (sample S-5) led to increased particle size again (≈ 12.8 μm), though this is lower than that of sample S-1. This indicates that the particle size has increased by an increase in the TPAOH content from 0.33 to 3.0 in the gel mixture. Interestingly, the particle size of samples S-3 and S-4, prepared with mixed templates, remained almost the same in contrast to that of sample S-5.
It may also be noted that in order to obtain the desired pure ZSM-5 phase the use of mixed templates necessitated longer hydrothermal treatment than that for the control test with TPABr alone. It can be seen that by employing different template combinations, noticeable differences in particle sizes are observed. The main reason for the various morphologies and particle sizes in zeolites synthesized is the alkalinity of the synthesis solution. At a low alkalinity, only a small amount of TPA-aluminosilicate species is formed due to the limited solubility of silica at low pH and scarcity of TPA+ ion. Under this condition, the zeolite growth is slow and the shape of the crystal grains is elongated. Increasing the alkalinity (or [OH]−) can increase the induction period, crystallization (crystal growth) and nucleation rate. It is known that the high [OH]− concentration can cause zeolite dissolution. In addition, the high alkalinity promotes secondary nucleation that can lead to the formation of smaller crystals on top of the primary growth, thereby average particle size increases [42,43,44,45,46,47].
Textural properties
Nitrogen adsorption–desorption isotherm measurements were carried out at 77 K in order to investigate the effect of template composition on the textural properties of ZSM-5. Fig. 3 illustrates the N2 adsorption–desorption isotherms of different H-ZSM-5 catalysts. It is seen that all the samples exhibited a combination of types I and IV isotherm with rectangular type H4 hysteresis loops at high relative pressures (P/Po = 0.5–0.95), implying the micropores are the major component of all samples. As can be observed in Fig. 3, the differences of the isotherms between samples are negligible. There are distinctions in the hysteresis loops at high relative pressures as are seen by the shapes of the isotherms related to the mesopore feature. The textural properties listed in Table 4 confirm that the formation of mesoporous structure is relatively low. The hysteresis of sample S-1 is higher than that of other catalysts which is due to the increase in capillary condensation in inter-crystalline voids owing to the aggregation of nanoparticles. Furthermore, it was found that the isotherms (the hysteresis loops) did not change significantly by the different combinations of the two templates [6, 48, 49]. Fig. S4 shows the corresponding BJH pore size distributions of the samples prepared with different combinations of the templates. The narrow pore size distribution curves indicate that all samples have uniform mesopores centered at around 3.6 nm. These mesopores attributed to tensile strength effect. Also, the sample S-1 showed a sharper peak in the range 2.9–4.2 nm, which may be due to its narrower crystal size distribution.
Data of BET surface area, micropore volume, mesopore volume and external surface area of ZSM-5 catalysts, calculated from the N2 adsorption/desorption isotherms, are summarized in Table 4. It was established that these characters are weakly affected by template type and combination. The sample synthesized solely by TPABr template had a higher BET surface than the sample prepared using TPAOH. The lower surface area of the sample S-1 could be due to the presence of amorphous SiO2. This situation was modified by addition of TPABr to the TPAOH system. The surface area decreased from 289 to 227 m2/g when TPAOH content in the mixed template was increased from x = 0 to x = 1 during gel synthesis. Gradual decrease was observed in the surface area by changing TPAOH content from x = 0.75 to x = 1.0. This observation strongly suggests that the sample partially lost its crystallinity and the crystal and particle size of the catalysts became larger with increasing TPAOH. This result is in consistent with the XRD and FE-SEM discussions already presented. The variations of the other data listed in Table 4 are similar to those for the BET surface areas. The sample with larger crystal size (sample S-1) possesses nearly similar pore volume around 0.13 cm3/g, small value of external surface area (58 m2/g) and very low mesoporous pore volume (0.058 cm3/g). Only sample S-2, synthesized using TPABr with small crystal size represents a higher value of pore volume (0.168 cm3/g) and external surface area (93 m2/g). These differences can be explained based on the smaller crystal size and the inter-crystalline porosity [50].
The Si/Al molar ratios of the different prepared catalysts have been exhibited in Table 2. In all cases, the real Si/Al mole ratio was very similar to the nominal one.
High specific surface area is one of the significant factors which can influence the catalyst activity. Using TPABr seems to be responsible for the obtained relative high specific surface area. This observation is in excellent agreement with FE-SEM and XRD analyses which was previously presented, confirming smaller crystals/particles of the catalyst would result in a higher specific surface area. Higher specific surface area and appropriate crystal/particle size of H-ZSM-5 would be balanced together in the MTP reaction.
FT-IR analysis
The FT-IR spectra of the H-ZSM-5 catalysts with different combinations of the two templates were recorded in the range of 400–4000 cm−1. Using the FT-IR bands, useful information can be achieved on the framework vibrations of zeolites. The FT-IR spectra of these samples in the range of 400–1400 cm−1 have been shown in Fig. S5. It is seen that all the samples present the main characteristic vibrations of TO4 units (SiO4 and AlO4 internal tetrahedron units) (798 and 791 cm−1) as well as the asymmetric and symmetric vibrations of T-O binding/double rings (455, 550 and 626 cm−1) belonging to H-ZSM-5. The intensities of the bands related to the T-O binding/double rings vibrations (455, 550 and 626 cm−1) are slightly decreased by increasing the TPAOH content in the mixed templates. Furthermore, FT-IR crystallinity has been defined as intensity ratio of the 550 and 450 cm−1 bands [6, 51, 52]. The results show that the ratio, and therefore the relative crystallinity increased in the following order: S-2 > S-4 ≈ S-5 > S-1 > S-3 that is in close agreement with that calculated from the XRD (Table 3). Weak adsorption intensities at 550 cm−1 for samples S-1 and S-5 reveal that the H-ZSM-5 with TPAOH template should have more micropore structure, which was confirmed by the BET analysis (Table 4). The absorptions at ~ 965 and ~ 1100 cm−1 are assigned to the symmetric stretching vibrations of the external and internal linkages, respectively. The positions of these bands for samples including the mixed templates slightly shifted toward the low wave numbers with respect to those for S-1 and S-2 samples which prepared using only TPAOH and TPABr templates, respectively. The existence of structures containing four chains of 5-rings caused an external asymmetric stretching vibration at 1225 cm−1 with its intensity for all samples is almost the same. A big band at ~ 3666 cm−1 attributed to Lewis acid site is questionable [53,54,55].
Brønsted acid sites can be investigated with and without probe molecules using the FT-IR spectroscopy while Lewis acid sites can only be studied with the help of probe molecules. Many probe molecules such as ammonia, pyridine and acetonitrile have been reported for zeolites acidity measurements in the literature [56]. Fig. S6 shows the FT-IR spectra of the different H-ZSM-5 samples in the range of 1400–4000 cm−1. In the OH-stretching vibrations region, the spectrum of H-ZSM-5 materials exhibited the two major characteristic bands at ~ 3610 and ~ 3745 cm−1. These are attributed to acidic bridging hydroxyl group of Si(OH)Al (Brønsted acid sites) and ν(OH) stretching mode of free or very weakly perturbed silanol groups on internal and external surfaces. A big band appearing at ~ 3666 cm−1 usually is attributed to Lewis acid sites that could be extra-framework aluminum connected to the zeolite structure. The quantitative investigation of FT-IR analysis showed that intensities of the bands at ~ 3610 and 3745 cm−1 were slightly increased by increasing the TPAOH content of the mixed templates. It was found that the samples S-2 and S-5 synthesized using only TPABr and a 50:50 mixture of TPAOH and TPABr templates have higher Brønsted acid sites than other samples. Furthermore, in the region 2980–2880 cm−1, weak C–H stretching absorption bands are attributed to the presence of the template. The later bands were detected on the surfaces of the H-ZSM-5 samples synthesized by a single template (TPAOH or TPABr). However, by mixing the two templates, the band is almost completely disappeared [19, 57, 58].
Acidity
The NH3-TPD profiles of the H-ZSM-5 catalysts prepared with different combinations of the templates are shown in Fig. 4. The acidity of the samples determined from the TPD peak areas and the temperatures of desorption maxima are summarized in Table 5. Generally, the NH3-TPD profile of the H-ZSM-5 zeolite indicates two peaks including a low temperature peak (TP1, weak acid sites) at around 170 °C and a high temperature peak (TP2, strong acid sites) near 380 °C, which are in accordance with the reported literature data for the acidity of MFI materials [59,60,61]. The peak area gives the number of acid sites and peak temperature evaluates acid strength. Even if the NH3-TPD curves cannot discriminate between Brønsted and Lewis acidity, the low temperature peak is assigned to ammonia desorbed from weak acid Lewis sites and the high temperature peak is ascribed to ammonia desorption from the more strong Brønsted acid sites [5, 7, 62].
As it is seen in Fig. 4, all catalysts show two dispersed NH3 desorption peaks centered at around 190 and 390 °C, which are attributed to weak and strong acid sites, respectively. Comparing samples S-1 or S-2 synthesized in the presence of only one template (TPAOH or TPABr) with the S-3, S-4 and S-5 samples synthesized using different combinations of the two templates exhibits a significant difference between the two general peaks. As can be observed in Table 5, acidity of S-1 catalyst is almost identical to that of the S-2 catalyst, while mixing the two templates significantly increases the total acidity as well as the densities of weak acid sites on the S-3, S-4 and S-5 catalysts surfaces. Also, the number of acid sites corresponding to the high temperature desorption peak of NH3-TPD and is related to the framework Al atoms of fresh H-ZSM-5 zeolites, is decreased by the mixing the templates. However, the changes in the combinations of the two templates (TPAOH/TPABr ratios) in the as-synthesized samples do not significantly vary the acidities of the catalysts. The number and strength of total acid sites increase in the following order according to the mixed templates used.
Various parameters such as Si/Al ratio and hydrothermal treatment could affect the strength and amount of the acid sites in ZSM-5 [22, 23, 39, 63]. Consequently, it would be expected that the peak area should be similar because the samples were synthesized at the same synthesis condition. The differences between acidities may be caused by the diverse combinations of the two templates we used. As can be seen in Fig. 4, the amounts of strong acid sites on the S-3 and S-4 samples are lower than other samples. This phenomenon may be related to existence of extra framework Al atoms [22, 64]. These active sites had a low capability of adsorbing ammonia compared to the other sites; hence they had fewer strong acid sites. The concentrations of extra framework Al atoms are related to the band intensities at ~ 3643 cm−1 through FT-IR spectra (Fig. S6) which is in good agreement with the NH3-TPD results.
It is extensively believed that weak acid sites have little activity in methanol conversion to light olefins, whereas strong acid sites on the H-ZSM-5 catalyst surface are accessible to reactants. Therefore, they should be active for the MTO reaction and also be the main reason for catalyst deactivation in this reaction [5, 7, 29, 65]. From the NH3-TPD curves, it is obvious that high ratio of strong acid sites to weak acid sites for the two samples S-1 and S-2 is in good agreement with their high activities compared to those of other samples.
Catalytic performance
The catalytic performance of a zeolite catalyst in the MTO reaction depends both on the nature (acidity) of active sites and the structure (crystallite size) of the catalyst due to the reaction is severely diffusion limited [5, 48]. The catalytic activity of the samples was tested for the MTO reaction under the same operation conditions (T = 480 °C, P = 1 atm and WHSV = 0.9 h−1) over a time on stream (TOS) of 120–170 h. The variations of methanol conversion over different synthesized catalysts are shown in Table 6. All samples were effective in the MTO reaction and all, except for S-1, exhibited nearly complete methanol conversions. Over sample S-1, the transformation of methanol remained high up to 70 h on stream and then declined to 93.8% at TOS of 120 h. The catalyst decay could be attributed to coking on acidic sites, which was consistent with the FE-SEM and NH3-TPD data. The sample S-1 possessed the nearly highest concentration of strong acid sites (Table 5) and the largest crystallite and particle size among all the samples (Tables 3 and 4) both of which accelerated deactivation by coking. Too strong acid strength along with large diffusion path length can promote hydrogen transfer reaction to form aromatics and branched hydrocarbons, which could be trapped inside the cavities as coke and result in fast coke formation [2, 66]. The amount of heavy hydrocarbons formed on S-1 catalyst had the most value among those of other catalysts (Table 6). In contrast, samples S-2 to S-5 showed stable methanol conversions within 170 h runs. From the time-dependent conversion data, it was found that sample S-2 prepared with TPABr had a good stability in the MTO reaction at 480 °C and it was more stable than sample S-1. This superior performance of the catalyst prepared with TPABr can be attributed to the smaller crystallite size compared to sample S-1.
The effects of template type and combinations on the time-dependent products selectivity are plotted in Fig. 5 and Fig. S7. Also, the products distributions using as-synthesized H-ZSM-5 catalysts in the methanol to olefin are listed in Table 6. The highest selectivities to light olefins (C2=-C4=) were observed for samples synthesized with a single template compared to co-templated samples. These results could be well explained by the acidity of the different catalysts. From the NH3-TPD data (Table 5), the samples synthesized with a single template showed the highest strong acid sites. Although the samples synthesized with mixed templates demonstrated higher densities of acid sites, the number of strong acid sites on these catalysts was approximately low. As mentioned in acidity section, strong acid sites on the H-ZSM-5 catalyst are active for the MTO reaction and it is mainly the number of strong acid sites which is important not the strength. As a consequence, samples S-3 to S-5 revealed lower activities.
Fig. 5 illustrates the propylene selectivity and total light olefin (ethylene, propylene and butenes, C2=-C4=) compounds with time on stream (TOS) for the different catalysts prepared with both single template and mixed templates. It is seen that combinations of TPAOH and TPABr templates do not enhance the propylene and total light olefin selectivities compared to the single template. The reason confirmed by the NH3-TPD analyses could be the reduction of strong acid sites. The propylene selectivities by these catalysts were increased regularly (from 41.37 to 46.61%) with increasing the molar ratios of TPAOH/TPABr from 0.25:0.75 to 0.75:25. These results are principally because of increasing the TPAOH content in the mixed template leading to increasing the strong acid sites density. Among the two catalysts prepared with a single template, sample S-2 obtained by TPABr template presented enhanced products selectivity. The superior catalytic performance of sample S-2 can be attributed to its higher surface area (288.9 vs. 226.7 m2g−1), smaller crystallite size (37.9 vs. 40.0 nm) and higher number of strong acid sites (0.111 vs 0.109) in comparison with S-1 which has been associated with minimal side reactions.
The selectivity of ethylene, C1-C4 paraffinic hydrocarbons and liquid heavy hydrocarbons (C5-C16 alkane/alkene hydrocarbons) with time on stream over each catalyst are shown in Fig. S7a-Fig. S7c. In case of ethylene in Fig. S7a, all the catalysts illustrated a descending function with time. Comparison of Fig. 5a and Fig. S7a indicated that the production of propylene was increased by the reduction of ethylene produced that is owing to the fact that ethylene and butenes can oligomerize to bigger oligomers through metathesis, which crack to form propylene. That is why selectivity of propylene has maximum values at TOS, while the selectivity of ethylene is steadily decreased. The selectivity for ethylene using catalysts synthesized with a single template is lower than those of the samples prepared with mixed templates. The NH3-TPD results exhibited that combination of two templates in S-3 to S-5 catalysts led to decreased strong acid sites and subsequently minimized side reactions. However, catalyst S-1, synthesized only with TPAOH, demonstrates a severe slope in ethylene selectivity. This result may be explained by its crystal/particle size, specific surface area and strong acid sites density. The catalyst S-1 had the largest particle size (14.09 µm), the lowest surface area (226.7 m2g−1) and the highest density of too strong acid sites compared with other catalysts.
The selectivity for alkanes (C1-C4) and heavy hydrocarbons (C5-C16) were an ascending function with time for all catalysts, as shown in Fig. S7b and S7c. It is observed that the average selectivity of light alkanes (C1-C4) is almost the same on the all catalysts, whereas there were noticeable differences in heavy hydrocarbons selectivities on the various samples. Also, catalysts synthesized with single template illustrated the highest heavy hydrocarbons selectivities. The main reason confirmed by the NH3-TPD characterization could be the greater number of strong acid sites. As can be seen in Table 5 and Fig. 4, the number of strong acid sites on the catalysts S-1 and S-2 is higher than those of samples synthesized using the mixed templates. The strong acidity of S-1 and S-2 catalysts promoted hydrogen transfer reaction of olefins to saturated hydrocarbons and aromatics at the beginning reaction, which are precursors of coke formation and subsequently deactivation of the catalyst. Furthermore, the selectivity of heavy hydrocarbons was decreased with TOS, and this phenomenon is related to decreasing the strong acid sites by coke blocking [5, 48, 67, 68]. Among the recent two catalysts, the sample S-1 synthesized with only TPAOH showed the highest heavy hydrocarbons selectivities at the beginning of the reaction and with TOS. The NH3-TPD indicated that some of the acid sites on the surface of catalyst S-1 are too strong in comparison with those of the sample S-2. These are faster deactivated by coke formation and subsequently the possibility of hydrogen transfer reaction on the catalyst S-1 is increased.
Conclusion
In this research, five various H-ZSM-5 zeolites were synthesized by hydrothermal method under the same synthesis conditions using diverse combinations of TPAOH and TPABr as templates with the same TPA+ concentrations and pH in the precursor gel. The catalysts were different in average crystallite/particle size, morphology, surface area, acidity and catalytic performance. Some characters such as weight loss, crystallite and particle size were correlated with TPAOH concentration. The crystal/particle size was decreased by increasing the TPABr content in the mixed templates and the morphology of the crystal was changed to spherical due to aggregation of nanocrystals. The relative crystallinity and acid strengths were the highest for samples synthesized by single templates while utilization of mixed templates decreased the crystallinity and increased total acidity. The catalytic performance depending on several factors such as acidity and crystallite size showed a very complex dependence on template combination. The methanol conversion demonstrated a relation to acidity whereas the higher hydrocarbon selectivity was related to crystal size. The highest propylene, ethylene and total olefin (C2=-C4=) selectivity and methanol conversion and also the lowest by-products formation were found for the sample synthesized using TPABr as a template. Among the catalysts synthesized with mixed templates, the sample prepared with a 0.75:0.25 molar ratio of TPAOH/TPABr exhibited the best activity and the highest propylene selectivity. Overall, the catalyst prepared by single inexpensive template TPABr would be proposed for the MTO/MTP reaction.
References
Chang CD, Silvestri AJ (1977) The conversion of methanol and other O-compounds to hydrocarbons over zeolite catalysts. J Catal 47:249–259
Bleken FL, Chavan S, Olsbye U, Boltz M, Ocampo F, Louis B (2012) Conversion of methanol into light olefins over ZSM-5 zeolite: strategy to enhance propene selectivity. Appl Catal A Gen 447:178–185
Khanmohammadi M, Amani S, Garmarudi AB, Niaei A (2016) Methanol-to-propylene process: perspective of the most important catalysts and their behavior. Chin J Catal 37:325–339
Chen H, Wang Y, Meng F, Sun C, Li H, Wang Z, Gao F, Wang X, Wang S (2017) Aggregates of superfine ZSM-5 crystals: The effect of NaOH on the catalytic performance of methanol to propylene reaction. Microporous Mesoporous Mater 244:301–309
Yaripour F, Shariatinia Z, Sahebdelfar S, Irandoukht A (2015) Effect of boron incorporation on the structure, products selectivities and lifetime of H-ZSM-5 nanocatalyst designed for application in methanol-to-olefins (MTO) reaction. Microporous Mesoporous Mater 203:41–53
Rahmani M, Taghizadeh M (2017) Synthesis optimization of mesoporous ZSM-5 through desilication- reassembly in the methanol-to-propylene reaction. Reac Kinet Mech Cat 122:409–432
Ahmadpour J, Taghizadeh M (2015) Selective production of propylene from methanol over high-silica mesoporous ZSM-5 zeolites treated with NaOH and NaOH/tetrapropylammonium hydroxide. C R Chim 18:834–847
Rostamizadeh M, Taeb A (2015) Highly selective Me-ZSM-5 catalyst for methanol to propylene (MTP). J Ind Eng Chem 27:297–306
Ghalbi-Ahangari M, Ranjbar PR, Rashidi A, Teymuri M (2017) The high selectivity of Ce-hierarchical SAPO-34 nanocatalyst for the methanol to propylene conversion process. Reac Kinet Mech Cat 122:1265–1279
Javdani A, Ahmadpour J, Yaripour F (2019) Nano-sized ZSM-5 zeolite synthesized via seeding technique for methanol conversions: A review. Microporous Mesoporous Mater 284:443–458
Zhang S, Zhang B, Gao Z, Han Y (2010) Ca modified ZSM-5 for high propylene selectivity from methanol. Reac Kinet Mech Cat 99:447–453
Chang CD, Chu CT-W, Socha RF (1984) Methanol conversion to olefins over ZSM-5: I. Effect of temperature and zeolite SiO2Al2O3. J Catal 86:289–296
Rostamizadeh M, Yaripour F (2017) Dealumination of high silica H-ZSM-5 as long-lived nanocatalyst for methanol to olefin conversion. J Taiwan Inst Chem Eng 71:454–463
Liu J, Zhang C, Shen Z, Hua W, Tang Y, Shen W, Yue Y, Xu H (2009) Methanol to propylene: Effect of phosphorus on a high silica HZSM-5 catalyst. Catal Commun 10:1506–1509
Ahmadpour J, Taghizadeh M (2016) One-pot synthesis of hierarchically mesoporous ZSM-5 using different combinations of mesogenous templates. Synth React Inorg Met Org Chem 46:1133–1141
Jabbari A, Abbasi A, Zargarnezhad H, Riazifar M (2017) A study on the effect of SiO2/Al2O3 ratio on the structure and performance of nano-sized ZSM-5 in methanol to propylene conversion. Reac Kinet Mech Cat 121:763–772
Shao J, Fu T, Ma Q, Ma Z, Zhang C, Li Z (2019) Controllable synthesis of nano-ZSM-5 catalysts with large amount and high strength of acid sites for conversion of methanol to hydrocarbons. Microporous Mesoporous Mater 273:122–132
Kim S, Park G, Woo MH, Kwak G, Kim SK (2019) Control of hierarchical structure and framework-Al distribution of ZSM-5 via adjusting crystallization temperature and their effects on methanol conversion. ACS Catal 9:2880–2892
Abrishamkar M, Azizi SN, Kazemian H (2011) Using Taguchi robust design method to develop an optimized synthesis procedure for nanocrystals of ZSM-5 zeolite. Z Anorg Allg Chem 637:154–159
Petushkov A, Yoon S, Larsen SC (2011) Synthesis of hierarchical nanocrystalline ZSM-5 with controlled particle size and mesoporosity. Microporous Mesoporous Mater 137:92–100
Argauer RJ, Landolt GR (1972) Crystalline zeolite ZSM-5 and method of preparing the same. US Patent 3702886
Sang S, Chang F, Liu Z, He C, He Y, Xu L (2004) Difference of ZSM-5 zeolites synthesized with various templates. Catal Today 93:729–734
Schwarz S, Kojima M, O'Connor CT (1991) Effect of tetraalkylammonium, alcohol and amine templates on the synthesis and high pressure propene oligomerisation activity of ZSM-type zeolites. Appl Catal A Gen 73:313–330
Ma T, Zhang L, Song Y, Shang Y, Zhai Y, Gong Y (2018) A comparative synthesis of ZSM-5 with ethanol or TPABr template: distinction of Brønsted/Lewis acidity ratio and its impact on n-hexane cracking. Catal Sci Technol 8:1923–1935
Fouad OA, Mohamed RM, Hassan MS (2006) Ibrahim IA. Effect of template type and template/silica mole ratio on the crystallinity of synthesized nanosized ZSM-5. Catal Today 116:82–87
Aramburo LR, Karwacki L, Cubillas P, Asahina S, de Winter DM, Drury MR, Buurmans IL, Stavitski E, Mores D, Daturi M, Bazin P (2011) The porosity, acidity, and reactivity of dealuminated zeolite ZSM-5 at the single particle level: the influence of the zeolite architecture. Chem-Eur J 17:13773–13781
Losch P, Boltz M, Bernardon C, Louis B, Palčić A, Valtchev V (2016) Impact of external surface passivation of nano-ZSM-5 zeolites in the methanol-to-olefins reaction. Appl Catal A Gen 509:30–37
Khatamian M, Khandar AA, Haghighi M, Ghadiri M, Darbandi M (2010) Synthesis, characterization and acidic properties of nanopowder ZSM-5 type ferrisilicates in the Na+/K+ alkali system. Powder Technol 203:503–509
Ye L, Cao F, Ying W, Fang D, Sun Q (2011) Effect of different TEAOH/DEA combinations on SAPO-34’s synthesis and catalytic performance. J Porous Mater 18:225–232
Salmasi M, Fatemi S, Hashemi SJ (2012) MTO reaction over SAPO-34 catalysts synthesized by combination of TEAOH and morpholine templates and different silica sources. Sci Iran 19:1632–1637
Wang P, Lv A, Hu J, Xu JA, Lu G (2012) The synthesis of SAPO-34 with mixed template and its catalytic performance for methanol to olefins reaction. Microporous Mesoporous Mater 152:178–184
Chauhan NL, Das J, Jasra RV, Parikh PA, Murthy ZV (2012) Synthesis of small-sized ZSM-5 zeolites employing mixed structure directing agents. Mater Lett 74:115–117
Alipour SM, Halladj R, Askari S (2014) Effects of the different synthetic parameters on the crystallinity and crystal size of nanosized ZSM-5 zeolite. Rev Chem Eng 30:289–322
Soltanali S, Halladj R, Rashidi A, Bazmi M (2014) Mixed templates application in ZSM-5 nanoparticles synthesis: effect on the size, crystallinity, and surface area. Adv Powder Technol 25:1767–1771
Abbasian S, Taghizadeh M (2014) Preparation of H-ZSM-5 Nano-zeolite using mixed template method and its activity evaluation for ethanol to DME reaction. Int J Nanosci Nanotechnol 10:171–180
Alipour SM, Halladj R, Askari S, BagheriSereshki E (2016) Low cost rapid route for hydrothermal synthesis of nano ZSM-5 with mixture of two, three and four structure directing agents. J Porous Mater 23:145–155
Akbari B, Tavandashti MP, Zandrahimi M (2011) Particle size characterization of nanoparticles-a practicalapproach. Iran J Mater Sci Eng 8:48–56
Sing KS (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 57:603–619
Singh M, Kamble R, Viswanadham N (2008) Effect of crystal size on physico-chemical properties of ZSM-5. Catal Lett 120:288–293
Baerlocher C, McCusker LB, Olson DH (2007) Atlas of zeolite framework types. Elsevier, Amsterdam
Yaripour F, Shariatinia Z, Sahebdelfar S, Irandoukht A (2015) Conventional hydrothermal synthesis of nanostructured H-ZSM-5 catalysts using various templates for light olefins production from methanol. J Nat Gas Sci Eng 22:260–269
Persson AE, Schoeman BJ, Sterte J, Otterstedt JE (1995) Synthesis of stable suspensions of discrete colloidal zeolite (Na, TPA) ZSM-5 crystals. Zeolites 15:611–619
Grand J, Awala H, Mintova S (2016) Mechanism of zeolites crystal growth: new findings and open questions. Cryst Eng Comm 18:650–664
Hur YG, Kester PM, Nimlos CT, Cho Y, Miller JT, Gounder R (2019) Influence of tetrapropylammonium and ethylenediamine structure-directing agents on the framework Al distribution in B-Al-MFI zeolites. Ind Eng Chem Res 58:11849–11860
Qin Z, Lakiss L, Tosheva L, Gilson JP, Vicente A, Fernandez C, Valtchev V (2014) Comparative study of nano-ZSM-5 catalysts synthesized in OH− and F− media. Adv Func Mater 24:257–264
Wang YR, Wang SF, Chang LC (2006) Hydrothermal synthesis of magadiite. Appl Clay Sci 33:73–77
Wong WC, Au LT, Ariso CT, Yeung KL (2007) Effects of synthesis parameters on the zeolite membrane growth. J Membrane Sci 191:143–163
Ahmadpour J, Taghizadeh M (2015) Catalytic conversion of methanol to propylene over high-silica mesoporous ZSM-5 zeolites prepared by different combinations of mesogenous templates. J Nat Gas Sci Eng 23:184–194
Rostamizadeh M, Yaripour F (2016) Bifunctional and bimetallic Fe/ZSM-5 nanocatalysts for methanol to olefin reaction. Fuel 181:537–546
Uguina MA, de Lucas A, Ruiz F, Serrano DP (1995) Synthesis of ZSM-5 from ethanol-containing systems. Influence of the gel composition. Ind Eng Chem Res 34:451–456
Koekkoek AJJ, Xin H, Yang Q, Li C, Hensen EJM (2011) Hierarchically structured Fe/ZSM-5 as catalysts for the oxidation of benzene to phenol. Microporous Mesoporous Mater 145:172–181
Salmasi M, Fatemi S, Najafabadi AT (2011) Improvement of light olefins selectivity and catalyst lifetime in MTO reaction; using Ni and Mg-modified SAPO-34 synthesized by combination of two templates. J Ind Eng Chem 17:755–761
Jansen JC, Van der Gaag FJ, Van Bekkum H (1984) Identification of ZSM-type and other 5-ring containing zeolites by ir spectroscopy. Zeolites 4:369–372
Gorzin F, Darian JT, Yaripour F, Mousavi SM (2019) Synthesis of highly crystalline nanosized HZSM-5 catalyst employing combined hydrothermal and sonochemical method: Investigation of ultrasonic parameters on physico-chemical and catalytic performance in methanol to propylene reaction. J Solid State Chem 271:8–22
Rostamizadeh M, Yaripour F, Hazrati H (2018) High efficient mesoporous HZSM-5 nanocatalyst development through desilication with mixed alkaline solution for methanol to olefin reaction. J Porous Mater 25:1287–1299
Lercher JA, Gründling C, Eder-Mirth G (1996) Infrared studies of the surface acidity of oxides and zeolites using adsorbed probe molecules. Catal Today 27:353–376
Park JW, Seo G (2009) IR study on methanol-to-olefin reaction over zeolites with different pore structures and acidities. Appl Catal A: Gen 356:180–188
Sazama P, Wichterlova B, Dedecek J, Tvaruzkova Z, Musilova Z, Palumbo L, Sklenak S, Gonsiorova O (2011) FTIR and 27Al MAS NMR analysis of the effect of framework Al-and Si-defects in micro-and micro-mesoporous H-ZSM-5 on conversion of methanol to hydrocarbons. Microporous Mesoporous Mater 143:87–96
Martins GV, Berlier G, Bisio C, Coluccia S, Pastore HO, Marchese L (2008) Quantification of Brønsted acid sites in microporous catalysts by a combined FTIR and NH3-TPD study. J Phys Chem C 112:7193–7200
Zhou M, Wang F, Xiao W, Gao L, Xiao G (2016) The comparison of mesoporous HZSM-5 zeolite catalysts prepared by different mesoporous templates and their catalytic performance in the methanol to aromatics reaction. Reac Kinet Mech Cat 119:699–713
Gorzin F, Darian JT, Yaripour F, Mousavi SM (2019) Novel hierarchical HZSM-5 zeolites prepared by combining desilication and steaming modification for converting methanol to propylene process. J Porous Mater 26:1407–1425
Martin A, Berndt H (1994) Neutralization of HZSM-5 Brönsted acid sites by shaping with boehmite. React Kinet Mech Catal 52:405–411
Zhang J, Zhang H, Yang X, Huang Z, Cao W (2011) Study on the deactivation and regeneration of the ZSM-5 catalyst used in methanol to olefins. J Nat Gas Chem 20:266–270
Gorzin F, Darian JT, Yaripour F, Mousavi SM (2018) Preparation of hierarchical HZSM-5 zeolites with combined desilication with NaAlO2/tetrapropylammonium hydroxide and acid modification for converting methanol to propylene. RSC Adv 8:41131–41142
Li H, Wang Y, Fan C, Sun C, Wang X, Wang C, Zhang X, Wang S (2018) Facile synthesis of a superior MTP catalyst: hierarchical micro-meso-macroporous ZSM-5 zeolites. Appl Catal A Gen 551:34–48
Ivanova S, Lebrun C, Vanhaecke E, Pham-Huu C, Louis B (2009) Influence of the zeolite synthesis route on its catalytic properties in the methanol to olefin reaction. J Catal 265:1–7
Beheshti MS, Behzad M, Ahmadpour J, Arabi H (2020) Modification of H-[B]-ZSM-5 zeolite for methanol to propylene (MTP) conversion: Investigation of extrusion and steaming treatments on physicochemical characteristics and catalytic performance. Microporous Mesoporous Mater 291:109699. https://doi.org/10.1016/j.micromeso.2019.109699
Kharaji AG, Beheshti M, Repke JU, Tangestani-nejad S, Görke O, Godini HR (2019) Using response surface method to analyze the effect of hydrothermal post-treatment on the performance of extrudates HZSM-5 catalyst in the methanol to propylene reaction. Reac Kinet Mech Cat 127:375–390
Acknowledgements
The authors gratefully acknowledge the Petrochemical Research and Technology Company of the National Petrochemical Company for the financial support of the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Beheshti, M.S., Ahmadpour, J., Behzad, M. et al. Hydrothermal synthesis of H-ZSM-5 catalysts employing the mixed template method and their application in the conversion of methanol to light olefins. Reac Kinet Mech Cat 130, 493–518 (2020). https://doi.org/10.1007/s11144-020-01771-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01771-2