Abstract
The effect of SiO2/Al2O3 ratio on high silica content nano-sized HZSM-5 stability and selectivity in conversion of methanol to propylene (MTP) has been investigated in a continuous flow isotherm fixed-bed reactor. Highly crystalline HZSM-5 zeolite samples with SiO2/Al2O3 ratios ranging 150–200 have been prepared and characterized using XRD, ICP-AES analytical techniques, NH3-TPD, BET surface area and SEM imaging for studying their physical and chemical behavior. The reaction was conducted at 480 °C and 1bar with WHSV = 1 h−1 and a solution with 1:1 wt% of methanol and water as feed. Over thoroughly monitoring the catalyst synthesis conditions, the nanocrystal ZSM-5 zeolite samples were fabricated with fairly close microstructural properties and crystal sizes in order to be able to study the effect of SiO2/Al2O3 ratio on the ZSM-5’s performance in MTP. Results show that although the crystal size does not considerably affect the elementary structure of the zeolite active sites, it seems to be an influential factor in bond strength of Si–O versus Al–O. The results have also showed that increase of SiO2/Al2O3 ratio leads to higher selectivity and stability of catalyst.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zeolites, as aluminosilicate molecular sieves with molecular dimensional pores, has achieved widespread use due to their catalytic role in petroleum refining and petrochemical processes of methanol to gasoline (MTG), methanol to olefin (MTO) and as well ethanol to propylene reactions. Excellent physical and chemical properties of zeolite such as crystalline structure, high internal surface area, uniform pores, good thermal stability and crystal/particle size can influence the efficiency of the zeolite as catalyst [1–7].
Since the discovery of methanol to gasoline conversion reaction by Mobil Company, methanol to hydrocarbon (MTHC) technology, especially methanol to gasoline (MTG) and methanol to olefin (MTO) reactions over a zeolite based catalyst have been regarded as a new route for demanding light olefins production from natural gas [8–10]. It has been generally accepted that methanol in the MTO process is first dehydrated to dimethyl ether (DME) and water, and subsequently DME converts to light olefins. In tow are light olefins reactions to form paraffines, aromatics, naphthenes and higher olefins via hydrogen transfer, alkylation and polycondensation [6, 11–13].
Crystal size of zeolites has a great effect on the catalytic activity and selectivity. Recent reports indicate that when the crystal size of HZSM-5 zeolite is reduced to nanoscale, super catalytic properties like higher activity, lower coke content and better stability can be obtained in various reactions, conversion of methanol to hydrocarbons for instance [14, 15]. Mesoporous ZSM-5 in the form of nanosized crystals would provide significantly improved characteristics such as decreased diffusion path length, increased external surface area, higher catalytic performance, easier access to active sites on the external surface and enhanced resistance to coke formation [16–20]. Thus, the SiO2/Al2O3 ratio of ZSM-5 zeolite directly contribute to determining its activity and lifetime. Though high aluminum content ZSM-5 zeolite (i.e. low SiO2/Al2O3 ratio) has high quantity of acid sites and therefore high activity, it will also engender high coke formation rate which results in fast catalyst deactivation [21].
The zeolite has three-dimensional microporous channels in ZSM-5 with accessible diameter of 0.55 nm of 10-membered ring openings which cause diffusion restrictions and make the catalyst prone to coking [22, 23]. The formation of coke subsequently deactivates the catalyst by blocking pore entrances and covering active sites inside the channels. This has become of interest in industrial applications [24, 25].
Present study investigates the role of molar ratio on the activity and selectivity of the ZSM-5 toward the methanol to olefin reaction. Nano crystals of HZSM-5 zeolite samples with different SiO2/Al2O3 molar ratio in nanoparticles were synthesized and tested using analytical techniques and methods. Results were then studied to find methanol conversion and selectivity of the catalysts in their performance.
Experimental
Materials and sample preparation
Chemicals used in this work included Tetraethyl orthosilicate (TEOS, 98 wt%, Merck), Tetrapropyleammonium hydroxide (TPAOH,20 wt%, Merck), aluminum nitrate (Al(NO3)3.9H2O, Merck). To fabricate nanosized ZSM-5 zeolites, different molar compositions of Al2O3:150SiO2:53.5TPAOH:1625H2O and Al2O3:200SiO2:71.3TPAOH:2166.5H2O by Van Grieken and coworkers method [26] have been prepared which the aluminum nitrate was added to aqueous solution of TPAOH at 0 °C under stirring until a clear solution was obtained. The TEOS was added drop wise to the mixture and left to become clear. In order to hydrolyze TEOS, the above solution was stirred at room temperature for 48 h and then passed into a Teflon-lined stainless steel autoclave for crystallization under autogenous pressure and static conditions at 170 °C for 72 h. Subsequently, the acquired powder was separated from the solution by centrifugation and finally was washed by distilled water, dried overnight at 110 °C and calcined in air at 550 °C for 7 h.
Characterization techniques
ICP–AES apparatus was applied for analysis of trace elements in the solid particles. So as to dissolve the solid samples, 0.1 g of ZSM-5 was added to 30 ml distilled water (5 ml) in a HDPE bottle. The suspended ZSM-5 solution was then mixed with 1 ml of 4.8 wt% HF solution. For oxidizing silicon, addition of 1 ml of 70 wt% HNO3 was done and freshly distilled water was added to achieve a dilute 30 ml solution, and finally the solution was analyzed by ICP–AES.
Specific surface area was measured by N2 adsorption method (Quantachrome BET pore size analyzer, USA model Nova 2000). Before performing the adsorption–desorption measurements, obtained zeolite samples were degassed at 300 °C in N2 flow for 2 h. With applying water as dispersant particle size distribution (PSD) was studied with Malvern particle size analyzer MasterSizer 2000 model. X-ray diffraction pattern was recorded with PHILIPS XPert diffractometer with CuKα radiation in range 2θ 2–70.
The acidity of the samples was also assessed by temperature-programmed desorption of ammonia (NH3–TPD, BelCat) with TCD detector. SEM analysis were performed on the TESCAN model VEGA-II in low vacuum.
Catalytic performance
A fixed-bed micro-reactor of stainless steel tube with 10 mm inner diameter has been used for methanol to propylene (MTP) reaction. The crushed samples were sieved with mesh size of 18–25 or 750–1000 µm. 4g zeolite sample was loaded to the reactor for each test. Subsequently, it was heat-treated in situ at a heating rate of 5 °C/min under N2 flow (30 ml/min) at atmospheric conditions. Sample was maintained at 550 °C for 5 h.
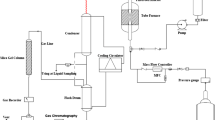
To prepare feed materials for the tests, a peristaltic pump sent a mixture of 50% methanol in water with a flow rate of 0.14 ml/min. The weight hourly space velocity (WHSV) was adjusted to 1 h−1. Performance tests were done at atmospheric pressure in 480 °C. To evade probable condensation of heavier hydrocarbons, the temperature of the waste line was always kept at above 150 °C. By cooling the outlet stream to 15 °C, the gas and liquid products were separated. A gas chromatography (GC) was used to analyze a small fraction of the outlet gas online. The GC (Varian CP, model 4900) equipped with TCD had four capillary columns for separating C1–C4 alkane, C2–C4 olefin, CO, CO2 and H2 compounds. Aqueous and organic phases in the liquid product of the reactor were separated by a decanter. A small portion of the aqueous phase was sent to another GC (Varian CP, model 3800), that three of its four capillary columns of this instrument were used for separating water, methanol and oxygenates. A small portion of the organic phase was also analyzed with the gas chromatograph, which was equipped with Flame Ionization Detector (FID) and had one capillary column for separating heavier hydrocarbons.
Result and discussion
Catalyst characterization
The XRD patterns of the fabricated ZSM-5 samples are represented in Fig. 1. The X-ray diffraction patterns of both samples are rather similar and fairly match with that of ZSM-5 with MFI structure [23]. Degree of crystallinity was determined from the intensity of the peak around 2θ = 23° based on the highly crystalline micro ZSM-5 sample as the reference. Both samples with different molar composition show relatively the same crystallinity (100%).
The chemical compositions of the both samples, as measured by ICP-AES, are reported in Table 1. They show the silicon and aluminum content of zeolites and the difference in molar ratio of SiO2/Al2O3.
The BET surface areas of the samples reported in Table 1 shows partial increasing in surface area with increasing the SiO2/Al2O3 ratio. These data can be predicted as regarding particle size analysis, with increasing the SiO2/Al2O3 molar ratio, the crystal sizes decrease and consequently, boost in surface area occurred. The relatively large BET results indicate presence of mesopores in the sample in addition to small particle size [27]. The practical shares outlined in Table 1, are actually the theoretical ratios for synthesis, while the actual shares of SiO2 and Al2O3 in nanostructure of obtained crystals is 100 and 150. This could be found more or less in all synthesis. In nano-ZSM-5 synthesis, this difference could be substantial due to the application of reagents in the form of solution and existence of some inactive fraction of SiO2 and Al2O3 which may not have participated in ZSM-5 crystal formation.
The particle size distribution was also measured and the results are illustrated in Fig. 2. It shows a shift to smaller size particles with increasing the SiO2/Al2O3 ratio. For sample with SiO2/Al2O = 150, the particle sizes are in the range of 25–110 nm, while in sample with SiO2/Al2O3 = 200, the particle sizes in range of 20–100 nm are observed. Although the SiO2/Al2O3 ratio is not a key factor in determination of the particle size for the samples, there is a minor difference between results for the samples. This could be attributed to different bond strengths of Si–O compared to Al–O. The most important parameters in this determination are reagent chemicals used for synthesis, temperature, stirring rate and generally the controlling factors of nucleation rate over nuclei growth. As could be observed, despite the similarity of these parameters in two samples, there is a minor difference between their particle sizes. This slight variance, however, could be attributed to the shorter bond length of Si–O in comparison with Al–O which induces stronger bond in Si–O. Altogether, increasing alumina in primary gel causes more base consumption which leads to reduce in basicity of the system. Thus, the number of nuclei declines and an increase in the particle size could be expected [28].
The corresponding SEM-image of a dried sample is shown in Fig. 3. Particles have spherical like morphologies, however, they were agglomerated at the calcinations temperature. According to the estimation based on SEM picture, the average particle sizes of the samples are quite the same as one that has been acquire from the particle size distribution results.
NH3–TPD measurements were carried out to determine the acid strength and the amounts of the acidic sites on the catalyst surface. Desorption peaks with maxima in the range 180–250 °C, 260–330 °C, and 340–500 °C are normally ascribed to the NH3 chemisorbed on weak, medium and strong acidic sites, respectively [29, 30]. The temperatures of the desorption peaks (Tdi) and the acid content of the catalysts are summarized in Table 2.
According to Table 2, the number of acidic sites decreases with increasing SiO2/Al2O3 ratio which agrees well with those reported in the literature [31]. This is primarily related to the increase in extra-framework aluminum content as well as in basic framework. Shifting the desorption temperature of ammonia from the strong acid sites to higher temperature as the SiO2/Al2O3 ratio decreased, also strongly suggests the existence of aluminum in extra-framework positions. The results revealed that the molar ratio of SiO2/Al2O3 of the initial gel mixture has a significant effect on chemical and physical properties of the final products.
Catalyst performance
Methanol conversion and hydrocarbon products selectivity, as the catalyst performance test were calculated according to below equations:
Here, n i and n o represent the number of moles of the component at the inlet and outlet streams, respectively.
The conversion of methanol by catalysts is shown in Fig. 4. The results recorded for 30 h of reaction show that the sample with higher SiO2/Al2O3 ratio had had more conversion of methanol to products. It has been assumed that catalyst deactivation in the MTP reaction is generally attributed to the coke deposition on the catalyst surface, which is caused by the side reactions that take place on the acid sites. It has been proved that the total acid sites of ZSM-5 decreased with increasing the SiO2/Al2O3 molar ratio [32]. Concerning the NH3-TPD results, the decrease of the acid sites would suppress these side reactions, leading to enhanced stabilities. Similarly both samples show superior conversions of methanol to propylene against same micro sizes catalysts, which is for diffusion limitation that rises with increasing in particle size [33].
The results of the selectivity of the samples to propylene are shown in Fig. 5. They show that sample with higher SiO2/Al2O3 ratio is more selective to propylene against the lower one. The main reason for the change in product distribution of the catalysts in MTO reaction could be their difference in Brǿnsted acidity. A decline in Brǿnsted acidity of the catalysts with the improvement of SiO2/Al2O3 ratio favors the formation of propylene in the reaction and results in an increase in propylene to ethylene (P/E) ratio. In NH3 TPD analysis performed in the present study, it was not possible to separate the exact contribution for each type of Brǿnsted or Lewis acid sites. Other methods such as NH3 stepwise temperature-programmed desorption (STPD) coupled with FT–IR [34] or DRIFTS (diffuse reflectance Fourier transform infrared spectra, using pyridine as probe molecule) [35] need separate measurement of the Brǿnsted and Lewis acid sites. Other studies have also reported that decreasing Brǿnsted acidity of HZSM-5 zeolite could improve its selectivity to propylene in MTO reaction [1].
Both samples show superior selectivity against same micro sizes samples since small crystals have shorter channels and the reactants contact with fewer number of acidic sites. Having said that, it could be observed that these excellent results were not stable over period of time as a consequence of high coke formation over the surface of catalysts. Therefore, higher quantities of light olefins are formed in the catalyst with smaller crystal size [36]. It should be noted that the selectivity of the catalyst samples to other compounds such as C1–C4 alkane, C4, C5 +, CO, CO2 and H2 was negligible compared to propylene.
Conclusion
Two samples of nanosized ZSM-5 catalysts with different SiO2/Al2O3 ratios of 150 and 200 but of similar structural properties were synthesized and characterized and their performances on MTO reaction were investigated. This enabled the study of the basic effect of SiO2/Al2O3 ratio on catalytic performance of ZSM-5 zeolite in a fixed-bed reactor. The increase of SiO2/Al2O3 led to decrease in total acidic sites, and increase of surface area in catalysts. No difference has been observed in crystallinity of samples. Though the desirable lifetime is yet to be assessed for industrial applications, the samples with higher SiO2/Al2O3 ratio showed higher selectivity and stability in MTP reaction.
References
Chang CD, Chu CTW, Socha RF (1984) J Catal 86:289–296
Chang CD, Lang WH, Smith RL (1979) J Catal 56:169–173
Chang CD, Silvestri AJ (1977) J Catal 259:249–259
Wu MM, Kaeding WW (1984) J Catal 88:478–489
Dehertog WJH, Froment GF (1991) Appl Catal 71:153–165
Dahl IM, Kolboe S (1993) Catal Lett 20:329–336
Song Z, Liu W, Chen C et al (2013) Reac Kinet Mech Cat 109:221–231
Stöcker M (1999) Microporous Mesoporous Mater 29:3–48
Chen JQ, Bozzano A, Glover B et al (2005) Catal Today 106:103–107
Hack M, Koss U, Rothaemel M, Holtmann HD (2006) US007015369B2
Dahl IM, Kolboe S (1994) J Catal 149:458–464
Mobinikhaledi A, Khajeh-Amiri A (2014) Reac Kinet Mech Cat 112:131–145
Mobinikhaledi A, Foroughifar N, Khajeh-Amiri A (2016) Reac Kinet Mech Cat 117:59–75
Sugimoto M, Katsuno H, Takatsu K, Kawata N (1987) Zeolites 7:503–507
Frey K, Lubango LM, Scurrell MS, Guczi L (2011) Reac Kinet Mech Cat 104:303–309
van Donk S, Janssen AH, Bitter JH, de Jong KP (2003) Catal Rev 45:297–319
Rezaei F, Webley P (2010) Sep Purif Technol 70:243–256
Egeblad K, Christensen CH, Kustova M, Christensen CH (2008) Chem Mater 20:946–960
Groen JC, Peffer LAA, Moulijn JA, Pérez-Ramírez J (2004) Coll Surf A 241:53–58
Tao Y, Kanoh H, Kaneko K (2003) J Am Chem Soc 125:6044–6045
Yamamura Masami, Chaki Kazutoshi, Toshiya Wakatsuki HO (1994) HO 14:643–649
Chester EGD AW (2009) Zeolite Characterization and Catalysis. Springer, Berlin
Olson DH, Kokotailo GT, Lawton SL, Meier WM (1981) J Phys Chem 85:2238–2243
Wang X, Lau KC, Turner CH, Dunlap BI (2010) J Power Sources 195:4177–4184
Wan Z, Wu W, Li (Kevin) G et al (2016) Appl Catal A Gen 523:312–320
Van Grieken R, Sotelo JL, Menéndez JM, Melero JA (2000) Microporous Mesoporous Mater 39:135–147
Zhou M, Wang F, Xiao W et al (2016) Reac Kinet Mech Cat 119:699–713
Hu Y, Liu C, Zhang Y et al (2009) Microporous Mesoporous Mater 119:306–314
Campelo JM, Garcia A, Herencia JF, Luma D, Marinas JM, Romero AA (1995) J Catal 151:307–314
Arena F, Dario R, Parmaliana A (1998) Appl Catal A Gen 170:127–137
Costa C, Dzikh IP, Lopes JM et al (2000) J Mol Catal A: Chem 154:193–201
Shirazi L, Jamshidi E, Ghasemi MR (2008) Cryst Res Technol 43:1300–1306
Chen D, Moljord K, Fuglerud T, Holmen A (1999) Microporous Mesoporous Mater 29:191–203
Zhang W, Burckle EC, Smirniotis PG (1999) Microporous Mesoporous Mater 33:173–185
Reddy CR, Bhat YS, Nagendrappa G, Jai Prakash BS (2009) Catal Today 141:157–160
Firoozi M, Baghalha M, Asadi M (2009) Catal Commun 10:1582–1585
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jabbari, A., Abbasi, A., Zargarnezhad, H. et al. A study on the effect of SiO2/Al2O3 ratio on the structure and performance of nano-sized ZSM-5 in methanol to propylene conversion. Reac Kinet Mech Cat 121, 763–772 (2017). https://doi.org/10.1007/s11144-017-1162-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-017-1162-6