Abstract
Green tea possesses a range of beneficial effects, including anti-obesity, antioxidant, and anti-inflammatory properties, owing to its biologically active components, primarily catechins such as epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), and epigallocatechin gallate (EGCG). However, few studies have investigated the four catechin monomers simultaneously, and the molecular mechanisms of their anti-obesity effects have not been fully elucidated. In this study, we investigated the effects of four catechin monomers on the differentiation of 3T3-L1 preadipocytes of mice. Our findings demonstrated that four catechin monomers EC/ECG/EGC/EGCG (12, 25, 50 µM) dose-dependently inhibited the differentiation of 3T3-L1 preadipocytes and reduced triglyceride content. EGCG exhibited the most potent inhibitory effect with an optimal concentration of 50 µM. In addition, transcriptome sequencing and lipidomic analysis of EGCG-treated 3T3-L1 preadipocytes revealed that Ptgs2 and Pim1 were the most differentially expressed genes involved in regulating adipocyte differentiation. The results suggested that EGCG up-regulated the expression of the Pla2g2e gene and down-regulated the expression of the Pla2g4a and Pla2g2a genes via the glycerophospholipid metabolic pathway, which subsequently elevated lysophosphatidylcholine (LPC) levels, influencing the differentiation process of 3T3-L1 preadipocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a metabolic disorder that has become one of the biggest global health crises [1]. When the energy intake of the body is greater than the energy consumed, it leads to the transformation of excess energy in the body into triglycerides and accumulation in abdominal adipose tissue or subcutaneous adipocytes, resulting in metabolic derangement and triggering a series of diseases, such as cardiovascular disease, diabetes mellitus, non-alcoholic fatty liver disease, and tumors [2, 3]. The estimates for global overweight and obesity (BMI ≥ 25 kg/m²) from the World Obesity Federation suggest that more than 4 billion people could be affected by 2035, compared to more than 2.6 billion in 2020. This reflects an increase from 38% of the world’s population in 2020 to over 50% by 2035 [4]. Current weight loss strategies include lifestyle interventions, medication, and surgeries, but these treatment options have drawbacks such as difficulties in adherence, gastrointestinal damage, and risks of complications. Thus, natural products or new drugs with potential therapeutic effects for weight loss are significant.
Catechins have been widely recognized as the main functional components in tea including mainly epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), and epigallocatechin gallate (EGCG) [5]. Numerous studies have shown that catechins have anti-hyperlipidemia, antioxidant, anti-inflammatory, and cardiovascular disease prevention properties, among others [6,7,8]. The continuous ingestion of catechins, especially in high amounts, has been shown to lower body fat and cholesterol levels in humans, suggesting that the intake of a green tea extract rich in catechins may act as a preventive measure against obesity [9]. EGCG is considered to be the most abundant and important functional component of catechins, which may be related to its chemical structure, especially the number of hydroxyl groups [10]. Several in vivo experiments have shown that EGCG significantly reduces body weight gain, serum total cholesterol, and low-density lipoprotein in obese mice [11, 12].There are many reports on the molecular and cellular bases for antiobesity effects of green tea catechins [13, 14]. However, Most experiments are conducted based on mechanisms that operate through speculation, and few studies utilize the combined application of transcriptomics and lipidomics to reveal the complexity of biological systems at a more comprehensive level.
This study aims to integrate transcriptomics and lipidomics methodologies in order to obtain a more comprehensive array of biological information, thereby advancing scientific research in related fields. Four catechin monomers were selected (epicatechin, EC; epicatechin gallate, ECG; epigallocatechin, EGC; and epigallocatechin gallate, EGCG) to compare their inhibitory effects on the differentiation of 3T3-L1 cells. Furthermore, a comprehensive analysis of transcriptomics and lipidomics was conducted between the control group and the EGCG treatment group to delve deeper into the regulatory mechanisms by which EGCG inhibits adipocyte differentiation, potentially paving new avenues in the field of biomedicine.
Materials and Methods
The section on materials and methods is summarized in supplementary material.
Results and Discussion
Effects of Catechins on Cytotoxic and Differentiation of 3T3-L1 Preadipocytes
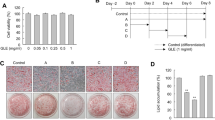
To assess the cytotoxicity of the Catechins in 3T3-L1 cells, the cells were treated with different concentrations of Catechins such as 1, 3, 6, 12, 25, 50, 100, 200, and 400 µM for 24 h. Thereafter, cytotoxicity was measured through the CCK-8 cell activity assay. Consequently, the significant reduction in cell viability caused by EGC and EGCG treatment with 400 µM represents a toxic effect on the cells (Fig. 1-A), indicating that a high concentration of EGC and EGCG inhibited the proliferation of 3T3-L1 preadipocytes and not be a safe concentration. For EC (1, 3, 6, 12, 25, 50, 100, 200, and 400 µM), ECG (1, 3, 6, 12, 25, 50, 100, 200, and 400 µM), EGC (1, 3, 6, 12, 25, 50, 100 and 200 µM) and EGCG (1, 3, 6, 12, 25, 50, 100 and 200 µM), no alterations in cell viability were observed.
Since the high concentrations of EGC and EGCG had a strong inhibitory effect on the cells, three concentrations of 12, 25, and 50 µM with cellular activity above 90% were chosen for subsequent experiments. In the present study, the determination of intracellular triglyceride content revealed that the four catechin monomers had a concentration-dependent tendency to reduce the triglyceride content (Fig. 1-B), with the treatment of EGCG at a concentration of 50 µM resulting in the lowest triglyceride content.
The results (Fig. 1-C) revealed that the three experimental groups of EGC, the 25 µM and 50 µM EGCG groups showed different degrees of reduction in the stained area by microscopic observation of different concentrations of the four catechins stained with oil red O dye in the treatment of 3T3-L1 preadipocytes, in which the areas stained by 25 µM and 50 µM EGC, and 50 µM EGCG were less. The quantitative results of oil red O staining are shown in Fig. 1-D. The above results suggested that EGC and EGCG could reduce the degree of differentiation of 3T3-L1 preadipocytes, thus reducing the accumulation of cellular lipid droplets.
The imbalance between energy intake and consumption of the organism leads to the occurrence of obesity, and its core physiological mechanism is that there are too many adipocytes in the body or their size is too large so that the mass of adipose tissue in the body increases [15,16,17]. By inhibiting the proliferation and differentiation of adipocytes, promoting fat decomposition in adipocytes, and reducing fat deposition, the purpose of obesity prevention can be achieved. In this study, induced differentiated 3T3-L1 preadipocytes were cultured in vitro, and high concentrations of EGC and EGCG were found to inhibit the proliferation of 3T3-L1 preadipocytes by CCK-8 assay significantly. Upon oil red O staining, it was found that 25 µM and 50 µM of EGC, and 50 µM of EGCG stained less area, suggesting that EGC and EGCG can reduce the degree of differentiation of 3T3-L1 preadipocytes, thus reducing the accumulation of cellular lipid droplets. Research has shown that the cytoplasmic triglyceride content increased significantly when 3T3-L1 preadipocytes were induced to differentiate into mature adipocytes by acylation-promoting protein [18]. It was demonstrated that successful differentiation of mature adipocytes leads to an increase in cytoplasmic triglyceride content. In this study, four catechin monomers were found to reduce intra-cellular triglyceride content in a concentration-dependent manner and inhibit 3T3-L1 preadipocyte differentiation.
Effects of catechins on the activity of 3T3-L1 preadipocytes (A); changes of triglyceride content after treatment with EC, ECG, EGC and EGCG (B); cells stained with oil red O after treatment with 0, 12, 25, 50 µM EC, ECG, EGC, EGCG for 24 h (C); quantitative results of oil red O (D). Findings are displayed as the mean ± SD from three replicates. The same small-letter means no significant difference, different small-letter means significant difference. * p < 0.05, ** p < 0.01 compared to the control group. Significant differences between different treatments are showed by different letters (p < 0.05)
Transcriptome Analysis of EGCG-treated 3T3-L1 Preadipocytes
To further investigate the mechanism of the inhibitory effect of catechins on 3T3-L1 preadipocytes, the treatment group with the most significant inhibitory effect of 50 µM EGCG intervening in adipocyte differentiation, and the control group was selected for transcriptome sequencing in this study. Using Fold Change ≥ 2 and FDR < 0.05 as the screening criteria, a total of 4299 differentially expressed genes were identified, of which 2628 were up-regulated and 1671 were down-regulated (Fig. 2A). Functional annotation of the differentially expressed genes showed (Fig. 2B) that the differentially expressed genes were mainly associated with molecular function regulators, transcriptional regulatory activity, catalytic activity, and binding in the Molecular function category. In the Biological process category, they are mainly associated with immune system processes, cellular component organization or biogenesis, localization, multicellular bioprocesses, developmental processes, response to stimuli, metabolic processes, bioregulation, and cellular processes. In the Cellular component category, they are primarily related to extracellular region parts, protein-containing complexes, membranes, membrane parts, organelle parts, organelles, and cellular parts. The top 20 Gene ontology (GO) entries were selected, which had the highest number of genes enriched to regulation of adipocyte differentiation and more genes enriched to positive regulation of adipocyte differentiation in GO entries (Fig. 2C). The results of GO enrichment were screened against significant differential genes to identify the top 10 genes with significant differential expression regarding the regulation of adipocyte differentiation and positive regulation of adipocyte differentiation. The results showed that the significant up-regulated differential genes for regulating adipocyte differentiation were Cmklr1, Trib3, Ccn4, Sort1, Dlk1, and Zbtb7c, and the significant down-regulated differential genes were Ptgs2, Pim1, Id2, and Klf5. Significantly up-regulated differential genes regarding positive regulation of adipocyte differentiation were Cmklr1, Zbtb7c, and significantly down-regulated differential genes were Ptgs2, Pim1, Id2, Klf5, Medag, Gm26885, Snai2, and Zbtb16. The genes with the largest multiplicity of differences in the two GO Terms were Ptgs2 and Pim1.
Transcriptomic analysis of 3T3-L1 preadipocytes treated with EGCG. The volcano plot of DEGs in the experimental group and the control group (A); GO classification statistics of differential genes between the experimental group and the control group (B); GO enrichment analysis bubble plot (top 20) (C); the statistical chart of KEGG classification of differential genes between the experimental group and the control group (up and down) (D); KEGG enrichment analysis bubble plot (top 20) (E). n = 3
The Kyoto Encyclopedia of Genes and Genomes (KEGG) annotation analysis showed 42 pathways involved in up-regulated differential genes and 43 pathways involved in down-regulated differential genes after EGCG intervention (Fig. 2D). Further enrichment analysis was performed to analyze the significance with Padjust < 0.05, and the result analysis showed that a total of 48 significant differential KEGG pathways were screened, and the top 20 signaling pathways were displayed in Fig. 2E. The figure shows that the top 20 significant signaling pathways are mainly related to inflammatory response, immune function, and cancer.
The most effective experimental group of 50 µM EGCG was selected for transcriptomic sequencing analysis, which revealed that the expression of Ptgs2 and Pim1 genes were significantly reduced. Pim1 is the gene encoding serine/threonine kinase, which has the role of regulating cell proliferation, apoptosis, and tumorigenesis. During precursor adipocyte differentiation, the transcription factors PPARγ, C/EBPα, and SREBP-1c facilitate the development of adipocytes, the synthesis of lipids, and the maturation of adipocytes via triglyceride accumulation [19, 20]. One study indicated that Pim1 knockout in senescent mice revealed that Pim1 reduced intramuscular fat content in senescent mice by inhibiting the expression of C/EBPδ [21]. AZD1208 functions as a Pim kinase inhibitor, leading to a significant reduction in triglyceride levels in 3T3-L1 adipocytes treated with AZD1208. Furthermore, this treatment resulted in diminished expression levels of C/EBPα, PPARγ, FAS, and ACC [22]. The Ptgs2 gene mainly encodes COX-2, which is involved in the regulation of cholesterol transport leading to hepatocyte lipid accumulation and inflammatory response [23]. Studies have shown that tea polyphenols reduce hepatic fat content in a high-fat dog model by decreasing the expression of COX-2 and inflammatory factors such as TNF-α and IL-6 [24]. The above studies are consistent with the results of the present experiment. KEGG pathway analysis enriched to MAPK signalling pathway and FoxO signalling pathway. MAPK signalling pathway plays a key role in the process of lipidogenic differentiation [25]. In terms of metabolic regulation, the activation of the FoxO signalling pathway can inhibit the expression of the mitochondrial respiratory chain complex and oxidative phosphorylation, resulting in reduced ATP content and metabolic level of cells, while simultaneously promoting the level of fatty acid oxidation and glucose glycolysis, thus mitigating the development the occurrence of metabolic diseases such as obesity and diabetes [26].
Lipidomic Analysis of EGCG-treated 3T3-L1 Preadipocytes
Based on the results of oil red O staining and triglyceride, the 50 µM EGCG dose group with the best effect was selected for the lipidomic study. Partial least squares discriminant analysis (PLS-DA) and Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) showed a significant separation of metabolite distribution between the experimental group and the control group (Fig. 3A), indicating that the two groups were classified significantly, and there were a high number of different metabolites between the groups. p < 0.05 and VIP value > 1 were used as the criteria to further screen the EGCG experimental and control groups for differential metabolites, and the results revealed that there was a total of 146 differential lipids in the experimental group compared with the control group, with 99 being up-regulated and 47 being down-regulated (Fig. 3B). Significantly different lipids were demonstrated using a clustered heatmap, with significant lipid differences in the experimental and control groups and more minor differences in lipids within the groups (Fig. 3C). KEGG pathway enrichment analysis showed the highest enrichment in the glycerophospholipid metabolism pathway (Fig. 3D), where there were 22 significantly different lipids, 16 of which were lysophosphatidylcholine: LPC (15:0), LPC (16:0), LPC (16:1), LPC (17:0), LPC (18:1), LPC (18:3), LPC (20:0), LPC (20:3), LPC (20:4), LPC (20:5), LPC (22:0), LPC (22:4), LPC (22:5), LPC (22:6), LPC (24:0), LPC (18:0); 2 phosphatidylcholines: PC (20:4/20:4), PC (22:0/20:4); 1 phosphatidylserine: PS (18:0/20:4); 1 lysophosphatidic acid: LPA (18:0) expression was up-regulated, and the expression of 2 phosphatidylcholines: PC (16:0/14:0), PC (18:1/18:1) were down-regulated. It is speculated that the glycerophospholipid metabolic pathway is a closely related metabolic pathway to the inhibition of 3T3-L1 preadipocyte differentiation by EGCG in this experiment. Some studies have shown a strong negative correlation between lysophosphatidylcholine and BMI, the expression of lipid LPC (20:4(8Z), 11Z, 14Z, 17Z) is significantly reduced in obese people. To further investigate the relationship between LPC and obesity, the experimenter tested the plasma of normal and obese adolescents and found that LPC (18:2), LPC (18:1), LPC (20:1), LPC (20:2), LPC (20:0) were significantly decreased in the obese group as compared to the normal group [27]. LPC can increase the expression of PPAR and GLUT4, thereby regulating blood glucose and cholesterol [28, 29]. Lipidomic analysis in this study showed that there were 16 LPCs in the glycerophospholipid metabolic pathway in the EGCG experimental group: LPC (15:0), LPC (16:0), LPC (16:1), LPC (17:0), LPC (18:1), LPC (18:3), LPC (20:0), LPC (20:3), LPC (20:4), LPC (20:5), LPC (22:0), LPC (22:4), LPC (22:5), LPC (22:6), LPC (24:0), and LPC (18:0) expression were elevated. In addition, four significantly different lipid PCs. LPA are fundamental precursors in the biosynthetic pathway of phospholipids, particularly during the early stages of eukaryotic cellular development. They play a crucial role in modulating numerous biological processes including cellular growth, development, proliferation, differentiation, and signal transduction [30].
Analysis of Integrated Lipidomics and Transcriptomics
Combined with transcriptome sequencing results, it was found that Pemt, Pld3, Mboat2, Plpp1, Pla2g2e, Plpp5, Dgki, Plpp2, and Plaat3 gene expression was significantly up-regulated in glycerophospholipid metabolism pathway, and Pla1a, Lpgat1, Lpin2, Pla2g4a, Etnk1, Dgke, Dgkh, Pla2g2a, Gm37988, and Abi3 gene expression was significantly down-regulated. These genes are involved in the expression of phospholipid-related enzymes, and it is speculated that the mechanism of EGCG inhibition of adipocyte differentiation may be related to its induction of the activity of LPC-related enzymes, such as phospholipase A2 (PLA2). PC can produce LPC in the presence of PLA2, and the Pla2g2e, Pla2g4a, and Pla2g2a genes all belong to the phospholipase A2 family, which is one of the key enzymes in biochemical reactions and cellular metabolic processes in vivo [31]. Among them, Pla2g2e encodes LPC acyl hydrolase, which is involved in the hydrolysis of PC to LPC, which indicates that Pla2g2e plays a regulatory role in LPC modulation [32]. Another Pla2g2e is associated with lipid metabolism, and Pla2g2e-knockout mice have severely elevated blood lipids and a considerable accumulation of cholesterol occurs in their macrophages, resulting in foam cells [33]. This aligns with our findings that EGCG enhances the expression of the Pla2g2e gene, thereby reducing lipid accumulation in adipocytes. Additionally, Pla2g2a participates in regulatory mechanisms associated with obesity and type II diabetes. An increase in the expression of Pla2g2a is observed in rats fed with a high-fat diet, suggesting a positive correlation between lipid biosynthesis and the expression of the Pla2g2a gene. [34]. Meanwhile, Pla2g4a is associated with non-alcoholic fatty liver disease, for which triglyceride content and epididymal fat weight were much lower, and adipocyte volume was smaller in Pla2g4a-knockout mice than in wild-type mice, regardless of whether they were fed normal or high-fat chow, indicating that Pla2g4a is likely positively correlated with triglyceride levels [35]. Therefore, as shown in Fig. 4, it was hypothesized that EGCG inhibited adipocyte differentiation by upregulating LPC expression through upregulation of the Pla2g2e gene and downregulation of the Pla2g4a and Pla2g2a genes. Our data analyzed by combined transcriptomic and lipidomic analyzes revealed that Ptgs2 and Pim1 were the most significantly altered genes between the 50 µM EGCG-treated group and the control group. Additionally, it appears that EGCG may have influenced the differentiation of 3T3-L1 preadipocytes differentiation through the glycerophospholipid metabolic pathway. However, some functional validations need to be further researched. For instance, a time-course experiment needs to be verified at what stage in the differentiation process the key genes are functioning, as well as overexpression or knockdown of Ptgs2, Pim1, Pla2g2e, Pla2g4a, and Pla2g2a genes need to be verified their effects on cell differentiation.
Conclusions
In this study, we compared the effects of four catechin monomers on the differentiation of 3T3-L1 preadipocytes, and the most significant inhibitory effect was observed at an EGCG concentration of 50 µM. By transcriptome and lipidome analyzes, it was found that EGCG may affect the differentiation process of 3T3-L1 preadipocytes by up-regulating the Pla2g2e gene and down-regulating the Pla2g4a and Pla2g2a genes to up-regulate the expression of LPC via the glycerophospholipid metabolic pathway. We will further construct animal models to determine the lipid-lowering effect and dosage of EGCG in vivo. In addition, catechins have a generally low bioavailability, and we will design in vitro simulated digestion experiments to explore the degree of digestion of EGCG. This study provides a basis for the application of EGCG to functional foods with weight loss and lipid-lowering properties.
Data Availability
No datasets were generated or analysed during the current study.
Abbreviations
- ACC:
-
Acetyl CoA carboxylase
- BMI:
-
Body mass index
- COX-2:
-
Cyclooxygenase 2
- C/EBP:
-
CCAAT enhancer binding protein
- DEGs:
-
Differentially expressed genes
- EC:
-
Epicatechin
- ECG:
-
Epicatechin gallate
- EGC:
-
Epigallocatechin
- EGCG:
-
Epigallocatechin gallate
- FAS:
-
Fatty acid synthase
- FDR:
-
False discovery rate
- FoxO:
-
Forkhead box O
- GLUT4:
-
Glucose transporter 4
- GO:
-
Gene ontology
- HSL:
-
Hormone-sensitive triglyceride lipase
- IL-6:
-
Interleukin-6
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- LPA:
-
Lysophosphatidic acids
- LPC:
-
Lysophosphatidylcholine
- MAPK:
-
Mitogen-activated protein kinase
- PC:
-
Phosphatidylcholine
- PLA2:
-
Phospholipase A2
- PPAR:
-
Peroxisome proliferators-activated receptors
- PS:
-
Phosphatidylserine
- SREBP-1c:
-
Sterol regulatory element-binding protein-1c
- TNF-α:
-
Tumor necrosis factor-α
References
Lahey R, Khan SS (2018) Trends in obesity and risk of cardiovascular disease. Curr Epidemiol Rep 5:243–251. https://doi.org/10.1007/s40471-018-0160-1
Padwal R, Li SK, Lau DCW (2003) Long-term pharmacotherapy for overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Int J Obes Relat Metab Disord 27:1437–1446. https://doi.org/10.1038/sj.ijo.0802475
Saunders KH, Umashanker D, Igel LI, Kumar RB, Aronne LJ (2018) Obesity pharmacotherapy. Med Clin North Am 102:135–148. https://doi.org/10.1016/j.mcna.2017.08.010
World Obesity Atlas (2023) https://data.worldobesity.org/publications/?cat=19((accessed on March 2023).
Cyboran S, Strugala P, Włoch A, Oszmiański J, Kleszczyńska Y (2015) Concentrated green tea supplement: biological activity and molecular mechanisms. Life Sci 126:1–9. https://doi.org/10.1016/j.lfs.2014.12.025
Bhardwaj P, Khanna D (2013) Green tea catechins: defensive role in cardiovascular disorders. Chin J Nat Med 11(4):345–353. https://doi.org/10.1016/S1875-5364(13)60051-5
Wang D, Gao Q, Wang T, Zhao G, Qian F, Huang J, Wang H, Zhang X, Wang Y (2017) Green tea infusion protects against alcoholic liver injury by attenuating inflammation and regulating the PI3K/Akt/eNOS pathway in C57BL/6 mice. Food Funct 8(9):3165–3177. https://doi.org/10.1039/c7fo00791d
Tian Y, Zou B, Yang L, Xu SF, Yang J, Yao P, Li CM (2011) High molecular weight persimmon tannin ameliorates cognition deficits and attenuates oxidative damage in senescent mice induced by D-galactose. Food Chem Toxicol 49(8):1728–1736. https://doi.org/10.1016/j.fct.2011.04.018
Nagao T, Hase T, Tokimitsu I (2007) A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obes (Silver Spring) 15(6):1473–1483. https://doi.org/10.1038/oby.2007.176
Peluso I, Serafini M (2017) Antioxidants from black and green tea: from dietary modulation of oxidative stress to pharmacological mechanisms. Br J Pharmacol 174(11):1195–1208. https://doi.org/10.1111/bph.13649
Klaus S, Pültz S, Thöne-Reineke C, Wolfram S (2005) Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes (Lond) 29:615–623. https://doi.org/10.1038/sj.ijo.0802926
Grove KA, Sae-tan S, Kennett MJ, Lambert JD (2012) (-)-Epigallocatechin-3-gallate inhibits pancreatic lipase and reduces body weight gain in high fat-fed obese mice. Obes (Silver Spring) 20:2311–2313. https://doi.org/10.1038/oby.2011.139
Kao YH, Chang HH, Lee MJ, Chen CL (2006) Tea, obesity, and diabetes. Mol Nutr Food Res 50(2):188–210. https://doi.org/10.1002/mnfr.200500109
Ku HC, Chang HH, Liu HC et al (2009) Green tea (-)-epigallocatechin gallate inhibits insulin stimulation of 3T3-L1 preadipocyte mitogenesis via the 67-kDa laminin receptor pathway. Am J Physiol Cell Physiol 297(1):C121–C132. https://doi.org/10.1152/ajpcell.00272.2008
Spiegelman BM, Flier JS (2001) Obesity and the regulation of energy balance. Cell 104:531–543. https://doi.org/10.1016/s0092-8674(01)00240-9
Wang X, Liu XH (2006) Proliferation, differentiation and regulation of proadipocytes. Chin J Huaihai Med 04:350–352. https://doi.org/10.3969/j.issn.1008-7044.2006.04.063
Dai B, Xiao ZZ, Liu L et al (2007) Effects of the original constituent of Liu-Wei Di-huang pill morroniside on the proliferation and differentiation of rat preadipocyte. Chin J Clin Pharmacol Ther 12:1245. https://doi.org/10.3969/j.issn.1009-2501.2007.11.010
Wang HW, Lu HL, Wen Y, Cianflone K (2005) Expression of DGAT, LPL and Adipsin mRNA during differentiation of 3T3-L1 Preadipocyte Induced by Acylation stimulating protein. Chin J Acta Medicinae Universitatis Sci Technologiae Huazhong 6:707–710. https://doi.org/10.3870/j.issn.1672-0741.2005.06.016
Gregoire FM, Smas CM, Sul HS (1998) Understanding adipocyte differentiation. Physiol Rev 78:783–809. https://doi.org/10.1152/physrev.1998.78.3.783
Lamichhane R, Kim SG, Kang S et al (2017) Exploration of underlying mechanism of anti-adipogenic activity of Sulfuretin. Biol Pharm Bull 40:1366–1373. https://doi.org/10.1248/bpb.b17-00049
Shang GK, Han L, Wang ZH et al (2021) Pim1 knockout alleviates Sarcopenia in aging mice via reducing adipogenic differentiation of PDGFRα(+) mesenchymal progenitors. J Cachexia Sarcopenia Muscle 12:1741–1756. https://doi.org/10.1002/jcsm.12770
Park YK, Obiang-Obounou BW, Lee KB et al (2018) AZD1208, a pan-pim kinase inhibitor, inhibits adipogenesis and induces lipolysis in 3T3-L1 adipocytes. J Cell Mol Med 22:2488–2497. https://doi.org/10.1111/jcmm.13559
Zhan DL (2020) Cyclooxygenase 2 regulates cholesterol transport of hepatocytes expressed hepatitis B virus X and induces inflammatory activation of macrophages. Dissertation, Xiamen University. https://doi.org/10.27424/d.cnki.gxmdu.2020.002593
Rahman SU, Huang Y, Zhu L et al (2020) Tea polyphenols attenuate liver inflammation by modulating obesity-related genes and down-regulating COX-2 and iNOS expression in high fat-fed dogs. BMC Vet Res 16:234. https://doi.org/10.1186/s12917-020-02448-7
Su J, Su H (2016) The regulation mechanism of MAPK signal pathway in adipocyte differentiation. Chem Life 36:252–256. https://doi.org/10.13488/j.smhx.20160219
Behl T, Kaur I, Sehgal A et al (2021) Exploring the genetic conception of obesity via the dual role of FoxO. Int J Mol Sci 22(6):3179. https://doi.org/10.3390/ijms22063179
Wang Y, Jiang CT, Song JY, Song QY, Ma J, Wang HJ (2019) Lipidomic profile revealed the association of plasma lysophosphatidylcholines with adolescent obesity. Biomed Res Int 2019:1382418. https://doi.org/10.1155/2019/1382418
Yea K, Kim J, Yoon JH et al (2009) Lysophosphatidylcholine activates adipocyte glucose uptake and lowers blood glucose levels in murine models of diabetes. J Biol Chem 284:33833–33840. https://doi.org/10.1074/jbc.M109.024869
Klingler C, Zhao X, Adhikary T et al (2016) Lysophosphatidylcholines activate PPARδ and protect human skeletal muscle cells from lipotoxicity. Biochim Biophys Acta 1861:1980–1992. https://doi.org/10.1016/j.bbalip.2016.09.020
Wu XN, Ma YY, Hao ZC, Wang H (2020) Research progress on the biological regulatory function of lysophosphatidic acid in bone tissue cells. West China J Stomatology 38:328–333. https://doi.org/10.7518/hxkq.2020.03.017
Kim M, Jung S, Kim SY, Lee SH, Lee JH (2014) Prehypertension-associated elevation in circulating lysophosphatidlycholines, Lp-PLA2 activity, and oxidative stress. PLoS ONE 9:e96735. https://doi.org/10.1371/journal.pone.0096735
Sato H, Kato R, Isogai Y et al (2008) Analyses of group III secreted phospholipase A2 transgenic mice reveal potential participation of this enzyme in plasma lipoprotein modification, macrophage foam cell formation, and atherosclerosis. J Biol Chem 283:33483–33497. https://doi.org/10.1074/jbc.M804628200
Qu LB (2015) ERRγ mediates insulin-dependent regulation of VLDL secretion through PLA2G12B and ablation of sPLA2G2E reduces cholesterol efflux from macrophages and promotes the development of atherosclerosis. Dissertation, University of Chinese Academy of Sciences
Iyer A, Lim J, Poudyal H et al (2012) An inhibitor of phospholipase A2 group IIA modulates adipocyte signaling and protects against diet-induced metabolic syndrome in rats. Diabetes 61:2320–2329. https://doi.org/10.2337/db11-1179
Ii H, Yokoyama N, Yoshida S et al (2009) Alleviation of high-fat diet-induced fatty liver damage in group IVA phospholipase A2-knockout mice. PLoS ONE 4:e8089. https://doi.org/10.1371/journal.pone.0008089
Funding
This work was supported in part by the National Key R&D Project (2022YFE0111200 & 2021YFD1601104), the Key R&D Project of Hunan Province (2021NK1020-3), and the Natural Science Foundation of Hunan Province Youth Fund (No. 2021JJ40251).
Author information
Authors and Affiliations
Contributions
Ranran He: Methodology, Software, Writing—original draft preparation, Writing—review and editing. Yu Shi: Conceptualization, Methodology, Data curation, Writing—original draft preparation. Xiaoshuang Lu: Writing—original draft preparation, Writing—review and editing. Yufei Zhou: Writing—review and editing, Writing—review and editing. Zhonghua Liu: Project administration. Sheng Zhang: Conceptualization, Resources, Funding acquisition. Ailing Liu: Conceptualization, Writing—review and editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, R., Shi, Y., Lu, X. et al. Inhibitory Effect and Mechanism of Epigallocatechin Gallate on the Differentiation of 3T3-L1 Preadipocytes. Plant Foods Hum Nutr (2024). https://doi.org/10.1007/s11130-024-01229-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s11130-024-01229-z