Abstract
Background
Obesity, which is defined as the excess accumulation of body fat, poses metabolic diseases that result in significant health risks. Since conventional anti-obesity medications are known to have significant side effects, we tried a pharmacological approach with a natural product. Ginseng (Panax ginseng) is a traditional Asian medicine that possesses antioxidant, anti-inflammatory, and anti-obesogenic properties. However, the mechanism of the anti-obesity effects of ginseng leaf extract (GLE) is not yet understood.
Objective
We investigated the mechanism by which GLE inhibits the differentiation of 3T3-L1 preadipocytes.
Results
GLE treatment was administered throughout the 8 days differentiation period or at three stages of adipocyte differentiation (early: days 0–2; intermediate: days 2–4; or late: after day 4). During adipocyte differentiation, GLE treatment significantly inhibited 3T3-L1 preadipocyte differentiation at the early stage, leading to a notable reduction in lipid accumulation and a decrease in the expression of crucial adipogenic transcription factors that regulate adipocyte differentiation. GLE also increased the expression of HO-1 and Wnt/β-catenin signaling in a dose-dependent manner during adipocyte differentiation. To evaluate the role of HO-1 induced by GLE, we used HO-1 inhibitor SnPP and HO-1 siRNA. Attenuation of HO-1 function and expression inhibited the decrease in lipid accumulation and adipogenic transcription factor expression caused by GLE; furthermore, inhibition of HO-1 suppressed Wnt/β-catenin signaling.
Conclusions
Overall, our results suggest that GLE inhibits the differentiation of 3T3-L1 preadipocytes by regulating HO-1 expression and Wnt/β-catenin signaling. Therefore, GLE could have preventive uses as a natural product for the treatment of obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is defined as the abnormal or excessive fat accumulation in the body, which poses a health risk (Bluher 2020). Obesity is associated to several health complications such as lipid metabolism disorder, insulin resistance, type 2 diabetes, and ischemic heart disease (Harms and Seale 2013). Because of this risk of obesity, some reports predict that the life expectancy of modern adolescents may actually be shorter than that of their parents (Olshansky et al. 2005). Despite its significant health impact, current pharmacological treatments for obesity often have limited benefits and undesirable side effects (Cao 2010).
Differentiation of preadipocytes into mature adipocytes is a key process in the development of obesity. CCAAT/enhancer-binding protein α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ), which regulate the expression of fatty acid-binding protein 4 (FABP4), play an important role in the differentiation of preadipocytes into mature adipocytes (Farmer 2006). In addition, Wnt/β-catenin signaling plays a critical role in regulating adipocyte differentiation by inhibiting the expression of PPARγ and C/EBPα (Laudes 2011) and, therefore, adipocyte differentiation (Lee et al. 2011; Rahman et al. 2016; Tian et al. 2017).
Heme oxygenase-1 (HO-1) functions as an antioxidant by decomposing heme, a strong pro-oxidant, and producing antioxidant molecules such as CO and bilirubin; as such, it can attenuate the oxidative stress associated with diabetes and obesity (Tsai et al. 2017; Vanella et al. 2013). Since lipid accumulation in 3T3-L1 cells is regulated through the production of reactive oxygen species, HO-1 may exert anti-obesogenic effects via antioxidant mechanisms (Lee et al. 2012a, b). In addition, several studies have found that HO-1 can impact adipocyte differentiation and adipocyte expansion through its association with Wnt/β-catenin signaling (Kim et al. 2018; Tsai et al. 2020; Tsai et al. 2017; Vanella et al. 2013). Therefore, understanding the molecular mechanisms involved in adipocyte differentiation and the regulation HO-1 may represent a potential therapeutic target for the prevention and treatment of obesity-related diseases.
Ginseng (Panax ginseng) is a widely used, traditional medicine in Asia, including the Korean Peninsula. Various studies show that ginsenoside, the main pharmacologically active ingredient of ginseng, has antioxidant, anti-inflammatory, and anti-cancer effects (Lu et al. 2009). Furthermore, ginsenosides exert anti-obesogenic effects by suppressing appetite, food absorption, and lipogenesis, and promoting fat oxidation, energy consumption, and browning (Zhang et al. 2017). In addition, various ginsenosides, such as Rg1, Rg2, F2, regulate adipogenesis in 3T3-L1 preadipocytes and in a high-fat diet mouse model (Liu et al. 2019; Liu 2018; Zhou et al. 2021); in particular, ginsenosides F2 and Rc suppress adipocyte differentiation by downregulating PPARγ in 3T3-L1 preadipocytes (Siraj et al. 2015; Yang and Kim 2015). Ginsenoside Rg1 also upregulates the nuclear factor (erythroid-derived 2)-like 2 (Nrf2)/HO-1 pathway in type 1 diabetic mice (Gao et al. 2020) and ginsenosides Rb2, Rd, and Rg1 exhibit antioxidant effects by activating the Nrf2/HO-1 signaling pathway in various disease models (Li and Zhang 2022; Zeng et al. 2015; Zhang et al. 2022).
In addition to the pharmacological activity of ginseng root-derived ginsenosides, biological activity has been observed in the berries and leaves of ginseng (Shi et al. 2007). In fact, ginseng berry extract attenuates obesity in high-fat diet-induced mice and inhibits lipid accumulation in 3T3-L1 preadipocytes (Shin et al. 2022; Yang et al. 2014). In addition, Panax ginseng leaf extracts (GLEs) are anti-obesogenic in high-fat diet-induced obesity rat models (Lee et al. 2017, b); and red ginseng extract prevents obesity and enhance lipid metabolism in castrated mice fed a high-fat died (Shin and Yoon 2018). However, the mechanism underlying the anti-obesogenic effects of ginseng leaves is not fully understood.
Although various ginseng extracts and ginsenosides have been reported to inhibit adipocyte differentiation, the effect of ginseng leaf extract (GLE) on differentiation of 3T3-L1 preadipocytes has not yet been elucidated. Thus, we specifically investigated the effects of GLE on the antioxidant HO-1 in hormone cocktail-induced differentiation of 3T3-L1 preadipocytes, and explored its potential as a natural pharmacological product.
Results
GLE inhibits lipid accumulation by inhibiting the early phase of differentiation in 3T3-L1 preadipocytes
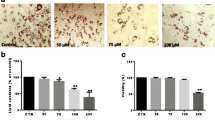
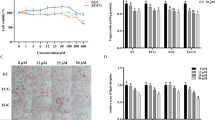
To determine whether GLE treatment affected the viability of 3T3-L1 preadipocytes, an MTT assay was performed; GLE had no significant cytotoxic effects at the tested concentrations (Fig. 1A). To investigate the effect of GLE during differentiation of 3T3-L1 preadipocytes to mature adipocytes, 1 mg/mL GLE was added at three stages (early: days 0–2; intermediate: days 2–4; and late: after day 4), or throughout the 8 days differentiation period (Fig. 1B). After 8 days, lipid accumulation was measured using Oil red O staining (Fig. 1C). Cells treated with GLE during the early stage of differentiation appeared to have significantly less lipid droplet accumulation than the control cells or those treated with GLE in the intermediate or late stages of differentiation. To make more precise assessment of the degree of lipid accumulation, Oil red O from stained cells was eluted with isopropanol and absorbance was measured at 500 nm with a spectrophotometer (Fig. 1D). Lipid accumulation was significantly reduced only when GLE was treated in the early stages of differentiation. However, cells treated with GLE during the intermediate and late stages exhibited no significant changes in lipid accumulation (Fig. 1D). Since GLE only had a significant effect during the early stages of differentiation, subsequent experiments were conducted during the early stage.
The effect of GLE on lipid accumulation during 3T3-L1 preadipocyte differentiation. A 3T3-L1 preadipocytes were treated with 0, 0.05, 0.1, 0.25, 0.5, or 1 mg/mL GLE for 24 h. Cytotoxicity was measured by MTT assay. B After 3T3-L1 preadipocytes reached confluence, differentiation was triggered by adding a hormone cocktail (MDI: IBMX, dexamethasone, insulin) in a medium containing DMEM and FBS (day 0). At days 2, 4, and 6 only insulin was added. 3T3-L1 adipocytes were treated with GLE for the indicated period. C After 8 days of differentiation, cells were stained with Oil red O. D Stained Oil red O was eluted and absorbance was measured at 500 nm. The results are expressed as mean ± SE (n = 4); statistical significance was based on one-way ANOVAs followed by Tukey’s post-hoc tests; **p < 0.01 compared with the control group
GLE inhibits adipocyte differentiation and downregulates expression of adipogenic markers
To investigate whether decreased lipid accumulation in GLE-treated 3T3-L1 preadipocytes was mediated by inhibiting preadipocyte differentiation, GLE treatment was performed in dose-dependent manner. Oil red O staining showed there was a significant, dose-dependent reduction in the number of lipid droplets and lipid accumulation in 3T3-L1 preadipocyte differentiation treated with 0.25, 0.5, and 1 mg/mL GLE; in cells treated with 1 mg/mL GLE, lipid accumulation was 40% lower than in control cells (Fig. 2A,B). Next, to further investigate the inhibitory effect of GLE on preadipocyte differentiation, the expression of adipogenic transcription factors was measured at day 5 of differentiation. The expression of PPARγ, C/EBPα, and FABP4 decreased in a dose-dependent manner with increasing GLE treatment (Fig. 2C–E). These results suggest that GLE may regulate adipocyte differentiation by inhibiting lipid accumulation through the modulation of adipogenic transcription factors.
The effect of GLE on adipogenesis and expression of adipogenic markers. Cells were treated with 0, 0.05, 0.1, 0.25, 0.5, or 1 mg/mL GLE during the early stage (days 0–2) of 3T3-L1 preadipocyte differentiation. After 8 days of differentiation, A cells were stained with Oil red O and B lipid accumulation was evaluated by elution of Oil red O and absorbance measurement at 500 nm. Protein expression levels of C PPARγ, D C/EBPα, and E FABP4 were evaluated by western blot on day 5 of differentiation. The results are expressed as mean ± SE (n = 4); statistical significance was based on one-way ANOVAs followed by Tukey’s post-hoc tests; **p < 0.01 compared with the untreated group
GLE increases the expression of HO-1 and Wnt/β-catenin signaling
To confirm the inhibitory effect of GLE on adipocyte differentiation, expression of Wnt/β-catenin and HO-1 were measured in response to GLE treatment during adipocyte differentiation. The expression of HO-1 increased in GLE-treated cells in a dose-dependent manner (Fig. 3A). In line with the upregulation of HO-1, expression of its transcription factor, Nrf2, also increased in dose-dependent manner in the nuclear fraction (Fig. 3B). In parallel with this finding, the levels of pGSK3β and β-catenin in GLE-treated cells also exhibited a dose-dependent increase (Fig. 3C,D). These results indicate that GLE upregulates HO-1 expression and Wnt/β-catenin signaling during 3T3-L1 preadipocyte differentiation.
The effect of GLE on HO-1 expression and Wnt/β-catenin signaling. Cells were treated with 0, 0.05, 0.1, 0.25, 0.5, or 1 mg/mL GLE for 24 h during the early stage (days 0–2) of 3T3-L1 preadipocyte differentiation. Protein expression levels of A HO-1, C β-catenin, and D p-GSK3β were evaluated by western blot on day 1 of differentiation. B Protein expression levels of Nrf2 were evaluated by western blot of the nuclear fraction of cells on day 1 of differentiation
The effects of GLE on 3T3-L1 preadipocyte differentiation are inhibited by the HO-1 inhibitor SnPP
Suppression of C/EBPα and PPARγ expression by Wnt/β-catenin signaling in adipocyte differentiation is well-known (Prestwich and Macdougald 2007). To examine whether HO-1 inhibits 3T3-L1 preadipocyte differentiation through the Wnt/β-catenin pathway, the HO-1 inhibitor SnPP was used. When GLE (1 mg/mL) and SnPP (20 μM) were administered together, suppression of lipid accumulation by GLE was negated, with the number of lipid droplets and lipid accumulation similar to that in control cells (Fig. 4A,B). Therefore, GLE-induced inhibition of adipogenesis appeared to be completely inhibited by SnPP. To further confirm the inhibition of adipocyte differentiation by HO-1, expression of differentiation-related proteins were measured. The addition of both GLE and SnPP resulted in higher expression of the adipogenic markers C/EBPα, PPARγ, and FABP4 than GLE treatment alone (Fig. 4C–E). Treatment with SnPP and GLE also attenuated the expression of pGSK3β and β-catenin compared with GLE treatment alone (Fig. 4F,G). These results suggest that GLE-induced HO-1 expression regulates adipocyte differentiation through Wnt/β-catenin signaling.
The effect of the HO-1 inhibitor SnPP on 3T3-L1 preadipocyte differentiation with GLE. After 8 days of differentiation, A cells were stained with Oil red O and B lipid accumulation was evaluated by elution of Oil red O and absorbance measurement at 500 nm. Protein expression levels of C PPARγ, D C/EBPα, and E FABP4 were evaluated by western blot on day 5 of differentiation. Protein expression levels of F β-catenin and G p-GSK3β were evaluated by western blot on day 1 of differentiation. The results are expressed as mean ± SE (n = 4); statistical significance was based on one-way ANOVAs followed by Tukey’s post-hoc tests; **p < 0.01 compared with the untreated group; ##p < 0.01 compared with the GLE-treated group
The effects of GLE on 3T3-L1 preadipocyte differentiation are inhibited by HO-1 knockdown
To further confirm that HO-1 regulates adipocyte differentiation, endogenous HO-1 was knocked down by siRNA in 3T3-L1 preadipocytes. First, the HO-1 siRNA efficiency was confirmed at the protein level: endogenous HO-1 expression was lower in cells treated with HO-1 siRNA than untreated cells and those treated with control siRNA (Supplementary Fig. 1A). In HO-1-knockdown cells, suppression of lipid accumulation by GLE (1 mg/mL) was inhibited (Fig. 5A). In line with this, lipid accumulation was significantly higher in HO-1 knockdown cells than cells treated with control siRNA in response to GLE treatment (Fig. 5B). Consistent with these results, HO-1-knockdown cells had higher expression of adipogenic transcription factors than control siRNA-treated cells, both with and without the addition of GLE (Fig. 5C–E). Finally, the increase in pGSK3β and β-catenin expression induced by GLE was attenuated by HO-1 knockdown (Fig. 5F,G). These results indicate that HO-1 inhibits adipocyte differentiation through the Wnt/β-catenin pathway.
The effect of HO-1 knockdown and GLE on 3T3-L1 preadipocyte differentiation. After 8 days of differentiation, A cells were stained with Oil red O and B lipid accumulation was evaluated by elution of Oil red O and absorbance measurement at 500 nm. Protein expression levels of C PPARγ, D C/EBPα, and E FABP4 were evaluated by western blot on day 5 of differentiation. Protein expression levels of F β-catenin and G p-GSK3β were evaluated by western blot on day 1 of differentiation. The results are expressed as mean ± SE (n = 4); statistical significance was based on one-way ANOVAs followed by Tukey’s post-hoc tests; **p < 0.01 compared with the control siRNA group; ##p < 0.01 compared with the GLE-treated group
Discussion
In this study, we aimed to elucidate the effects of GLE on differentiation in a 3T3-L1 adipocyte model. To the best of our knowledge, this is the first report to demonstrate that GLE inhibits the differentiation of 3T3-L1 preadipocytes into mature adipocytes via HO-1-mediated activation of Wnt/β-catenin signaling. Our findings show that GLE downregulates expression of the transcription factors PPARγ and C/EBPα, which are involved in 3T3-L1 preadipocyte differentiation, leading to the inhibition of lipid accumulation. Our results suggest that GLE increases expression of HO-1 and nuclear translocation of Nrf2, as well as activating Wnt/β-catenin signaling. Furthermore, we show that GLE-mediated inhibition of adipogenic transcription factors was suppressed by the HO-1 inhibitor SnPP and HO-1 knockdown. Overall, these findings suggest that GLE regulates 3T3-L1 preadipocyte differentiation by activating the Wnt/β-catenin pathway via HO-1. Therefore, GLE may serve as a natural product for obesity through inhibition of adipocyte differentiation.
Our previous study confirmed the presence of ginsenosides in GLE using LC–MS/MS analysis: specifically, ginsenosides Rb1, Rb2, Rc, Rd, Re, and Rg1 were identified (Hur et al. 2021). These findings were consistent with a previous report identifying ginsenosides Rb1, Rb2, and Rg1 in Korean ginseng leaves (Lee et al. 2012a, b). In addition, several other studies have identified the presence of bioactive ginsenosides, including Rb1, Rb2, and Rd, in the roots, leaves, stems, and berries of ginseng (Lee et al. 2017; Wang et al. 2006). In line with the present findings, a number of ginsenosides identified in GLE, such as ginsenoside Rb1 and Rb2, inhibit adipocyte differentiation in 3T3-L1 preadipocytes (Dai et al. 2018; Oh et al. 2012). Ginsenosides Rb1, Rb2, and Rg1 also exhibit an anti-obesity effect in high-fat diet-induced obese mouse models (Li et al. 2018; Liu et al. 2018; Yu et al. 2015). In particular, ginsenoside Rg1 attenuates adipocyte differentiation through suppressing the early stages of adipocyte development in 3T3-L1 preadipocyte differentiation (Koh et al. 2017). Furthermore, the anti-obesity effect on 3T3-L1 preadipocytes and obese mice was observed not only in the presence of pure ginsenosides, but also ginseng extracts (Zheng 2020). The results of our study support the potential of ginsenoside-containing GLE as an inhibitor of 3T3-L1 preadipocyte differentiation.
PPARγ and C/EBPα are key transcription factors involved in the early stages of adipocyte differentiation (Gregoire et al. 1998). C/EBPα regulates the expression of downstream target genes, while PPARγ is necessary for adipogenesis and the maintenance of the differentiated state. Both factors contribute to the expression of lipogenic genes such as FABP4 (Chang and Kim 2019). Several studies report that various ginsenosides (Oh et al. 2012; Yang and Kim 2015), as well as ginseng and red ginseng extracts (Shin and Yoon 2018; Zheng et al. 2020), downregulate PPARγ and C/EBPα during the differentiation of 3T3-L1 preadipocytes. Consistent with the effects of ginsenosides and ginseng extract, we found that GLE decreased expression of PPARγ and C/EBPα in the early stage of 3T3-L1 preadipocyte differentiation; this led to a decrease in the expression of the adipogenic enzyme FABP4, which suppressed lipid accumulation in 3T3-L1 adipocytes. Overall, our results indicate that GLE inhibits adipogenesis in 3T3-L1 preadipocytes by downregulating PPARγ and C/EBPα expression.
In the early stages of adipocyte differentiation, hormonal stimuli promote expression of C/EBPβ and PPARγ, leading to degradation of β-catenin, which suppresses Wnt/β-catenin signaling (Farmer 2006). By contrast, HO-1 expression activates Wnt/β-catenin signaling (Prestwich and Macdougald 2007; Vanella et al. 2013). Numerous studies report that activation of Wnt/β-catenin signaling inhibits differentiation of 3T3-L1 preadipocytes by stabilizing β-catenin and downregulating C/EBPβ and PPARγ signals (Christodoulides et al. 2009; Winter and Nusse 2021; Ross et al. 2000). We found that Wnt/β-catenin signaling was upregulated by GLE, thereby inhibiting adipogenic transcription factors and suppressing differentiation of 3T3-L1 preadipocytes. Previous reports demonstrate that the bioactive components of ginseng are capable of activating the Nrf2/HO-1 pathway (Li et al. 2021; Shan et al. 2022; Yan et al. 2022), and HO-1 can induce activation of Wnt/β-catenin signaling, which in turn regulates the inhibition of adipocyte differentiation (Khitan et al. 2014; Tsai et al. 2017; Vanella et al. 2013). In the present study, we confirmed that GLE promotes nuclear translocation of Nrf2 and induces HO-1 expression during adipocyte differentiation. Furthermore, the GLE-induced activation of Wnt/β-catenin signaling and downregulation of adipogenic transcription factor expression was inhibited by blocking the expression and function of HO-1 using siRNA and SnPP, respectively. These results demonstrate that the inhibitory effect of GLE on the differentiation of 3T3-L1 preadipocytes is mediated by HO-1-induced Wnt/β-catenin signaling.
Conclusion
Our findings demonstrate that GLE inhibits the differentiation of 3T3-L1 preadipocytes by HO-1-induced activation of Wnt/β-catenin signaling. Thus, this study suggests that natural products such as GLE have the potential to prevent the risk of obesity. However, further studies are needed at pre-clinical and clinical levels to ascertain its potential as an agent for anti-obesity products.
Materials and methods
Preparation of ginseng leaf aqueous extract (GLE)
The GLE extraction method was described in detail previously (Hur et al. 2021). Briefly, dried ginseng leaf powder was extracted twice by mixing in a rotator for 30 min with distilled water heated to 95 °C (1:10 w/v). The aqueous component was filtered with 3 M paper, frozen at – 80 °C and lyophilized. Lyophilized GLE was reconstituted in distilled water to a concentration of 50 mg/mL. An authenticated voucher specimen (KULBM-2103) was deposited in the Herbarium at the Department of Food Science and Biotechnology of Animal Products, Konkuk University (Seoul, Republic of Korea).
Cell culture and differentiation of 3T3-L1 preadipocyte
3T3-L1 preadipocytes were maintained in DMEM containing 10% newborn calf serum and 1% penicillin/streptomycin (Gibco) at 5% CO2 and 37 °C under a humidified atmosphere. Cells (1 × 105 cells) were plated into 60 mm cell culture dishes and differentiated. The differentiation was induced with differentiation medium supplemented with 10% FBS, 1% penicillin/streptomycin, and an adipogenic hormone cocktail (0.5 mM IBMX, 1 μM dexamethasone, and 1 μg/mL insulin). After incubation for 2 days, the medium was replaced every 2 days with DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, and 5 μg/mL insulin. During incubation for 8 days, the cells were treated with various concentrations of GLE for the indicated periods.
Oil red O staining and lipid accumulation
To confirm intracellular lipid accumulation of differentiated 3T3-L1 cells, Oil red O staining was performed. After washing twice with PBS, cells were fixed with 4% paraformaldehyde at room temperature for 20 min. Cells were treated with 60% isopropyl alcohol and dried completely. Cells were then stained with a 0.35% Oil red O solution for 10 min at room temperature. After removing the residual staining solution, cells were washed with distilled water. Images of stained intracellular lipid droplets were observed under an Eclipse Ti2 fluorescence microscope (Nikon, Minato, Tokyo, Japan). To elute the Oil red O dye, 100% isopropyl alcohol was added for 10 min, and absorbance was measured at 500 nm.
MTT assay
3T3-L1 preadipocytes were seeded into 24-well plates at 2.0 × 104 cells per well and cultured in growth medium for 48 h. GLE was treated with various concentration for 24 h. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (0.5 mg/mL) was added to the culture medium and incubated for 2 h. After removing the supernatant, the crystal formazan formed by the MTT solution in living cells was dissolved with 1 mL of acidified isopropanol; absorbance was measured at 570 nm.
Western blot analysis
3T3-L1 preadipocytes underwent differentiation and GLE treatment; protein expression was measured at day 1 (HO-1, β-catenin, and p-GSK3β) or day 5 (C/EBPα, PPARγ, and FABP4). Whole-cell lysates were prepared using a PRO-PREP Protein Extraction Solution (iNtRON Biotechnology, Seongnam, Korea). After centrifugation of lysates at 13,000 rpm at 4 °C for 15 min, the supernatant was collected and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis before transfer to Immobilon-P polyvinylidene difluoride membranes (Merck, Darmstadt, Germany). Membranes were incubated overnight at 4 ℃ with primary antibodies and probed with a peroxidase-conjugated secondary antibody for 1 h at room temperature. After washing the membrane, protein bands were detected using WesternBright ECL solution (Advansta Inc., Menlo Park, CA, USA).
Fractionation of cytoplasmic and nuclear proteins
3T3-L1 preadipocytes (1 × 105 cells) were plated into 60 mm cell culture dishes and treated for 12 h with the indicated concentration of GLE. The cells were washed with PBS and resuspended in hypotonic buffers (20 mM Tris–Cl, 10 mM NaCl, 3 mM MgCl2, pH 7.4), and allowed to swell on ice for 15 min. Lysates were vortexed for 10 s after the addition of 0.1% NP-40 detergent (Sigma-Aldrich). The supernatant, a cytosolic fraction, was obtained by centrifugation at 3000 rpm at 4 °C for 10 min. The pellet was washed several times with hypotonic buffer before resuspension in PRO-PREP Protein Extraction Solution. Nuclear fractions were sonicated for 3 cycles of 5 s (power 55 W, frequency 20 kHz). After incubation on ice for 1 h, the supernatant was collected by centrifugation at 13,000 rpm at 4 °C for 15 min. Supernatants from the cytoplasmic and nuclear fractions were then subjected to western blotting, as described above.
Gene silencing
3T3-L1 preadipocytes were transfected with 100 nM of a non-specific, control siRNA (Ambion, Austin, TX, USA) or HO-1 siRNA (Ambion) using PolyFect (Qiagen, Valencia, CA, USA) in serum-containing medium. Following incubation for 8 h, medium was replaced with fresh, complete medium and cultured for an additional 40 h. Cells were then differentiated in the presence or absence of GLE, as described above. The efficiency of gene silencing was verified by western blotting.
Statistical analysis
SigmaPlot 2001 software was used to visualize the data, which was expressed as means standard error (SE). Statistical significance was determined using one-way ANOVA followed by Tukey’s post-hoc test.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bluher M (2020) Metabolically healthy obesity. Endocr Rev. https://doi.org/10.1210/endrev/bnaa004
Cao Y (2010) Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov 9(2):107–115
Chang E, Kim CY (2019) Natural products and obesity: a focus on the regulation of mitotic clonal expansion during adipogenesis. Molecules. https://doi.org/10.3390/molecules24061157
Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A (2009) Adipogenesis and WNT signalling. Trends Endocrinol Metab 20(1):16–24
Dai S et al (2018) Ginsenoside Rb2 promotes glucose metabolism and attenuates fat accumulation via AKT-dependent mechanisms. Biomed Pharmacother 100:93–100
de Winter TJJ, Nusse R (2021) Running against the Wnt: how Wnt/beta-catenin suppresses adipogenesis. Front Cell Dev Biol 9:627429
Farmer SR (2006) Transcriptional control of adipocyte formation. Cell Metab 4(4):263–273
Gao Y et al (2020) Ginsenoside Rg1 protects mice against streptozotocin-induced type 1 diabetic by modulating the NLRP3 and Keap1/Nrf2/HO-1 pathways. Eur J Pharmacol 866:172801
Gregoire FM, Smas CM, Sul HS (1998) Understanding adipocyte differentiation. Physiol Rev 78(3):783–809
Harms M, Seale P (2013) Brown and beige fat: development, function and therapeutic potential. Nat Med 19(10):1252–1263
Hur J et al (2021) Ginseng leaf extract ameliorates the survival of endotoxemic mice by inhibiting the release of high mobility group box 1. J Food Biochem 45(7):e13805
Khitan Z, Harsh M, Sodhi K, Shapiro JI, Abraham NG (2014) HO-1 upregulation attenuates adipocyte dysfunction, obesity, and isoprostane levels in mice fed high fructose diets. J Nutr Metab 2014:980547
Kim CY, Kang B, Suh HJ, Choi HS (2018) Red ginseng-derived saponin fraction suppresses the obesity-induced inflammatory responses via Nrf2-HO-1 pathway in adipocyte-macrophage co-culture system. Biomed Pharmacother 108:1507–1516
Koh EJ et al (2017) Ginsenoside Rg1 suppresses early stage of adipocyte development via activation of C/EBP homologous protein-10 in 3T3-L1 and attenuates fat accumulation in high fat diet-induced obese zebrafish. J Ginseng Res 41(1):23–30
Laudes M (2011) Role of WNT signalling in the determination of human mesenchymal stem cells into preadipocytes. J Mol Endocrinol 46(2):R65-72
Lee SH et al (2011) Persicaria hydropiper (L.) spach and its flavonoid components, isoquercitrin and isorhamnetin, activate the Wnt/beta-catenin pathway and inhibit adipocyte differentiation of 3T3-L1 cells. Phytother Res 25(11):1629–1635
Lee HJ, Lee HS, Cho HJ, Kim SY, Suh HJ (2012a) Utilization of hydrolytic enzymes for the extraction of ginsenosides from Korean ginseng leaves. Process Biochem 47(3):538–543
Lee OH, Seo MJ, Choi HS, Lee BY (2012b) Pycnogenol(R) inhibits lipid accumulation in 3T3-L1 adipocytes with the modulation of reactive oxygen species (ROS) production associated with antioxidant enzyme responses. Phytother Res 26(3):403–411
Lee SG, Lee YJ, Jang MH, Kwon TR, Nam JO (2017) Panax ginseng leaf extracts exert anti-obesity effects in high-fat diet-induced obese rats. Nutrients. https://doi.org/10.3390/nu9090999
Lee JW et al (2017) Comprehensive profiling and quantification of ginsenosides in the root, stem, leaf, and berry of Panax ginseng by UPLC-QTOF/MS. Molecules. https://doi.org/10.3390/molecules22122147
Li Y, Zhang W (2022) Effect of ginsenoside Rb2 on a myocardial cell model of coronary heart disease through Nrf2/HO-1 signaling pathway. Biol Pharm Bull 45(1):71–76
Li JB, Zhang R, Han X, Piao CL (2018) Ginsenoside Rg1 inhibits dietary-induced obesity and improves obesity-related glucose metabolic disorders. Braz J Med Biol Res 51(4):e7139
Li J et al (2021) Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway. J Transl Med 19(1):96
Liu H et al (2018) Antiobesity effects of ginsenoside Rg1 on 3T3-L1 preadipocytes and high fat diet-induced obese mice mediated by AMPK. Nutrients. https://doi.org/10.3390/nu10070830
Liu H et al (2019) Ginsenoside Rg2 inhibits adipogenesis in 3T3-L1 preadipocytes and suppresses obesity in high-fat-diet-induced obese mice through the AMPK pathway. Food Funct 10(6):3603–3614
Lu JM, Yao Q, Chen C (2009) Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol 7(3):293–302
Oh J et al (2012) Ginseng and its active components ginsenosides inhibit adipogenesis in 3T3-L1 cells by regulating MMP-2 and MMP-9. Evid Based Complement Alternat Med 2012:265023
Olshansky SJ et al (2005) A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 352(11):1138–1145
Prestwich TC, Macdougald OA (2007) Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol 19(6):612–617
Rahman N, Jeon M, Kim YS (2016) Delphinidin, a major anthocyanin, inhibits 3T3-L1 pre-adipocyte differentiation through activation of Wnt/beta-catenin signaling. BioFactors 42(1):49–59
Ross SE et al (2000) Inhibition of adipogenesis by Wnt signaling. Science 289(5481):950–953
Shan Y et al (2022) Ginsenoside Rg3 ameliorates acute pancreatitis by activating the NRF2/HO-1-mediated ferroptosis pathway. Int J Mol Med. https://doi.org/10.3892/ijmm.2022.5144
Shi W, Wang YT, Li J, Zhang HQ, Ding L (2007) Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chem 102(3):664–668
Shin SS, Yoon M (2018) Korean red ginseng (Panax ginseng) inhibits obesity and improves lipid metabolism in high fat diet-fed castrated mice. J Ethnopharmacol 210:80–87
Shin JE, Jeon SH, Lee SJ, Choung SY (2022) The administration of Panax ginseng berry extract attenuates high-fat-diet-induced sarcopenic obesity in C57BL/6 mice. Nutrients. https://doi.org/10.3390/nu14091747
Siraj FM, SathishKumar N, Kim YJ, Kim SY, Yang DC (2015) Ginsenoside F2 possesses anti-obesity activity via binding with PPARgamma and inhibiting adipocyte differentiation in the 3T3-L1 cell line. J Enzyme Inhib Med Chem 30(1):9–14
Tian L et al (2017) Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death Dis 8(1):e2559
Tsai YC et al (2017) Heme oxygenase-1 mediates anti-adipogenesis effect of raspberry ketone in 3T3-L1 cells. Phytomedicine 31:11–17
Tsai YC et al (2020) Involvement of the p62/Nrf2/HO-1 pathway in the browning effect of irisin in 3T3-L1 adipocytes. Mol Cell Endocrinol 514:110915
Vanella L et al (2013) Increased heme-oxygenase 1 expression in mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation via upregulation of the canonical Wnt signaling cascade. Stem Cell Res Ther 4(2):28
Wang CZ, Wu JA, McEntee E, Yuan CS (2006) Saponins composition in American ginseng leaf and berry assayed by high-performance liquid chromatography. J Agric Food Chem 54(6):2261–2266
Yan X et al (2022) Ginseng oligosaccharides protect neurons from glutamate-induced oxidative damage through the Nrf2/HO-1 signaling pathway. Food Funct 13(16):8605–8615
Yang JW, Kim SS (2015) Ginsenoside Rc promotes anti-adipogenic activity on 3T3-L1 adipocytes by down-regulating C/EBPalpha and PPARgamma. Molecules 20(1):1293–1303
Yang SO et al (2014) Classification of ginseng berry (Panax ginseng C.A. MEYER) extract using 1H NMR spectroscopy and its inhibition of lipid accumulation in 3 T3–L1 cells. BMC Complement Altern Med. https://doi.org/10.1186/1472-6882-14-455
Yu X et al (2015) Ginsenoside Rb1 ameliorates liver fat accumulation by upregulating perilipin expression in adipose tissue of db/db obese mice. J Ginseng Res 39(3):199–205
Zeng X, Li J, Li Z (2015) Ginsenoside Rd mitigates myocardial ischemia-reperfusion injury via Nrf2/HO-1 signaling pathway. Int J Clin Exp Med 8(8):14497–14504
Zhang L, Virgous C, Si H (2017) Ginseng and obesity: observations and understanding in cultured cells, animals and humans. J Nutr Biochem 44:1–10
Zhang Z et al (2022) Ginsenoside Rg1 inhibits oxidative stress and inflammation in rats with spinal cord injury via Nrf2/HO-1 signaling pathway. NeuroReport 33(2):81–89
Zheng Y et al (2020) Preclinical research on a mixture of red ginseng and licorice extracts in the treatment and prevention of obesity. Nutrients. https://doi.org/10.3390/nu12092744
Zhou J et al (2021) Ginsenoside F2 suppresses adipogenesis in 3T3-L1 cells and obesity in mice via the AMPK pathway. J Agric Food Chem 69(32):9299–9312
Acknowledgements
We would like to appreciate bioedit [www.bioedit.kr] for English language editing.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2021R1A2C1007526).
Author information
Authors and Affiliations
Contributions
HGL, JH and HGS contributed to conceptualization and design. HGL and JH conducted methodology and investigation. JH and JPW validated the results. HGL visualized all data and drafted the original manuscript. HGS managed and supervised the entire study and handled manuscript review and editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Hyuk Gyoon Lee declares that he has no conflict of interest. Jinwoo Hur declares that he has no conflict of interest. Jun Pil Won declares that he has no conflict of interest. Han Geuk Seo declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, H.G., Hur, J., Won, J.P. et al. Heme oxygenase-1 mediates the inhibitory effect of ginseng (Panax ginseng) leaf extract on differentiation in 3T3-L1 adipocytes. Mol. Cell. Toxicol. 20, 699–708 (2024). https://doi.org/10.1007/s13273-023-00408-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-023-00408-4