Abstract
To prevent losses before consumption due to the rapid ripening of bananas, turning unripe bananas into flour and using it in bakery products can both enhance the functional properties of the product and transform bananas into a high-value product. In this study, it is aimed to enhance the functional properties of banana flour through fermentation, thereby investigating its potential use in the production of healthy snack biscuits which are widely consumed, especially by children and busy people. Different proportions (0%, 15%, and 30%) of unripe banana flour (UBF) and fermented unripe banana flour (FUBF) were added to biscuits, evaluating their impact on physical (color, diameter, thickness, spread ratio), textural (hardness), and functional properties (total phenolic content, antioxidant activity, dietary fiber, glycemic index). The effect of FUBF on biscuit spread ratio compared to UBF was positive (p < 0.05). The addition of UBF or FUBF significantly increased total phenolic content (TPC) and antioxidant activity (p < 0.05), with the highest TPC (1167.88 mg GAE/kg) observed in biscuits containing 30% FUBF (p < 0.05). Fermentation showed no significant effect on antioxidant activity of samples (p > 0.05). The glycemic index (GI) values were notably high across all samples, with the control at 78.59 and the 30% FUBF sample at 72.74 (p < 0.05), indicating all samples fell into the high GI food category. Biscuit hardness decreased significantly with UBF or FUBF addition (p < 0.05), while fermentation had no significant impact on hardness (p > 0.05). This study underscores the potential of UBF or FUBF to contribute to healthier snack options with improved functional characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biscuits, known for their convenience, affordability, and widespread popularity, rank as the most favoured bakery products globally. With an extended shelf life, they have become a widely embraced snack suitable for all age groups. Despite their popularity, many commercially available biscuits tend to be high in calories, sugar, and fats, while lacking sufficient fiber, essential vitamins, and minerals. This holds true especially for traditional biscuits consumed as snacks or treats, rather than specifically formulated or marketed as nutritious options. Therefore, while exceptions exist, it remains important for consumers to be mindful of these factors when choosing biscuits as part of their daily diet. There is a concerted effort to enhance the functionality of biscuits through fortification and nutritional improvements [1].
Due to their starchy nature and high sugar content, biscuits are not recommended for individuals suffering with diabetes and obesity. This has led to an emerging trend of substituting wheat flour with alternative flours high in dietary fiber, and the widespread adoption of composite flours in biscuit production. These changes are geared towards repositioning biscuits as a healthier snacking choice. Previous studies have revealed that unripe banana flour is suitable for use to meet consumer demand by adding functional properties to biscuits [2,3,4].
Bananas (Musa spp) rank among the globally most produced and consumed fruits and stand as the fourth largest export commodity, following rice, wheat, and corn [5]. Banana production, which totaled 67 million tons in 2002, surged to 114 million tons during the period of 2017–2019 and is anticipated to further rise to 128 million tons in 2022. Approximately 20% of banana fruits go unmarketed due to defects, leading to their disposal as waste. The production of flour from unripe bananas presents an opportunity to extend their shelf life, mitigating wastage resulting from inadequate post-harvest handling practices [6].
Bananas serve as a significant source of lignin, starch, cellulose, and hemicellulose when harvested in the green stage of immaturity. Processing the fruit into flour during this phase enhances its functional properties [7]. Banana flour, rich in bioactive food components and resistant starch (RS), presents an opportunity for developing novel functional products with considerable commercial potential. The incorporation of unripe banana flour can enhance various products, including pasta, bread, cookies, and biscuits [6].
Unripe banana flour encompasses bioactive compounds, including phenolics, flavonoids, carotenoids, and phytosterols [6]. Nutritionally, unripe banana flour stands out as a notable source of resistant starch (RS) and dietary fiber. Reported values indicate that the content of resistant starch falls within the range of 40.9–58.5%, while dietary fiber content ranges from 6.0 to 15.5% [5]. RS and the bioactive compounds present in UBF have the potential to modulate metabolic activities [6]. Moreover, it has been demonstrated that RS offers physiological benefits, including the modulation of glycemic response and the production of short-chain fatty acids [8].
Despite its high nutritive value, the utilization of UBF in bakery products can lead to the development of undesirable smells and tastes. This adverse effect can be mitigated through fermentation, a process known to enhance the formation of a substantial level of resistant starch (RS) [9] while simultaneously improving the solubility of bioactive compounds. Furthermore, fermentation induces the synthesis of new phenolic compounds, and the gluten content decreases due to the heightened activity of endogenous enzymes. The constituents generated during fermentation play a crucial role in conferring functional properties to the product, enhancing textural characteristics, and reducing the glycemic index [10].
This study aimed to enrich biscuit samples with dietary fiber and phenolic compounds through the addition of immature banana flour. Since immature banana flour contains a high amount of resistant starch, the goal was also to reduce the glycemic index values of the biscuits. It was intended to enhance the functional properties of immature banana flour through fermentation, as this process increases the solubility of bioactive components. The effects of both immature banana flour and fermented immature banana flour on the physical, functional and textural properties of the biscuit samples was determined. It is believed that this study will contribute to the production of healthier snacks and provide a new purpose for banana production, which experiences trading losses.
Materials and Methods
Materials
The unripe banana bunch (Musa cavendishii Lam.) was procured from a local farmer in Alanya, Turkey. Wheat flour, milk powder, shortening, baking powder, and salt were obtained from commercial suppliers in Konya, Turkey. Enzymes and agents utilized in starch hydrolysis were sourced from Megazyme (K-GLUC and K-TDFR, Megazyme, Ireland), while chemicals were acquired from Merck (Darmstadt, Germany).
Unripe Banana Flour (UBF) Production
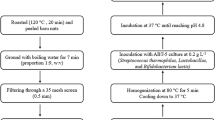
The unripe bananas, post-peeling, were sliced into 1 cm thick pieces and immersed in a 0.3% citric acid solution. Subsequently, they underwent drying at 50 °C in an oven until the moisture content dropped below 10%. The dried slices were then finely ground into powder and, finally, sieved through a 250-micron sieve to yield UBF.
Fermented Unripe Banana Flour (FUBF) Production
Fermentation was conducted using a starter culture comprising Lactobacillus fermentum and Lactococcus lactis, which were isolated from sourdough samples obtained in our previous studies. Lactic acid bacteria were inoculated into MRS broth and incubated at 30 °C for 24 h. Following incubation, the tubes underwent centrifugation, the medium was decanted, and the resulting pellet was washed twice with sterile ¼ Ringer’s solution.
UBF and water were were combined at a ratio of 1:1.5 (w/v). The mixture was then inoculated with a minimum of 106 cfu/ml of each starter culture and allowed to ferment at 30 °C for 24 h. Subsequent to fermentation, the mixture underwent drying at 50 °C in an oven until the moisture content dropped below 10%, after which it was finely ground into a powder. After fermentation, the total LAB count was determined to be 8.32 log cfu/g, and the pH and titration acidity were measured as 4.72 and 0.68%, respectively.
Biscuit Production
Biscuit production adhered to the formulation outlined in Table 1. Fermented or non-fermented UBF was substituted for wheat flour in ratios of 0:100% (control), 15:85%, and 30:70%, and combined with other ingredients. Then, all mixture was kneaded for 8 min. Subsequently, the dough was rolled out to a thickness of 5 mm using a dough sheeter (KitchenAid, 5KSM45, USA) and cut into round shapes with a cookie cutter. Finally, the biscuits were baked in an oven at 170 °C for 17 min and allowed to cool to room temperature before analysis. The samples containing 15% UBF, 30% UBF, 15% FUBF, and 30% FUBF were coded as 15B, 30B, 15FB, and 30FB, respectively.
Results and Discussion
The Diameter, Thickness and Spread Ratio Measurements of Biscuit Samples
The measured diameter, thickness and spread ratio values of biscuits are given in Table 2. The lowest diameter was measured in the control group as 44.46 mm, and the UBF or FUBF utilisation increased diameter. Contrary to the diameter values, the highest thickness was detected in the control as 5.18 mm. The thickness decreased as the increment of UBF or FUBF addition rate (p < 0.05). The thickness value was lower in 30B than in 30FB. The spread ratio values were compatible with the thickness values of the biscuits. The lowest spread ratios were obtained in the control sample as 8.58 and UBF or FUBF utilization lead to an increase in the spread ratio. The highest spread ratio was measured in 30B as 15.78, and it was followed by 30FB with 13.11. Since the spread ratio is calculated by dividing the diameter by thickness, the rise in diameter and the decline in thickness increases the spread ratio.
After baking biscuits, the higher spread ratio is generally preferred and influenced by a variety of factors [22]. The addition of UBF or FUBF which are not containing gluten decreased the rising ability of samples. The reduction in gluten concentration means weaker rheological properties and a greater spread ratio [22]. In addition, the decrease in the interaction between gluten and starch due to the high dietary fibre content of UBF, which is delayed gluten development, may have led to the weakening of dough hardness and increased spread during baking. It is worth noting that biscuits with a high spread ratio are generally considered desirable because of their crispiness [23]. Therefore, it was evaluated that the high utilisation ratio of UBF and FUBF improved the physical quality of biscuits, however, fermentation did not ensure any advance.
Textural Properties of Biscuits
The hardness of biscuits decreased with UBF or FUBF addition (p < 0.05) (Table 2). The highest hardness value was obtained in the control sample to be 1683.17 g. The differences between hardness values of UBF and FUBF-containing samples were statistically insignificant (p > 0.05), and lower than the control.
Hardness can be described as the peak force required to break the biscuit [24]. This parameter is varied due to some factors such as baking conditions, ingredient type and quantity, protein content and fibre content. The UBF addition to wheat flour is related to the reduction in carbohydrates, and rise in fibres, liquid oils and proteins. The gluten concentration is diluted by the substitution of banana flour for wheat flour, while protein content increases. The developing of biscuits structure is attributed to starch gelatinization, recrystallization and protein denaturation. It is normally expected that the hardness of biscuits increases with banana flour which is a source of high dietary fibre and protein [24]. RS has low molecular weight and an amorphous structure. Therefore, it makes dough sticky and runny due to the tendency of free hydroxyl groups to form strong bonds with water molecules [25]. However, it was not possible to add the same level of the water as the control biscuits, as it caused a denser and harder dough due to other components in the banana flour. More water was added to the samples with banana flour to obtain a workable dough of a similar consistency to the control. The less water in the dough leads to less expansion in the biscuit structure which resulted in biscuits having a harder texture. Moreover, there is a synergistic effect between RS and native starch. Gelatinized starch can retrograde, and increases the hardness of the biscuits due to the formation of a strong film during storage. Due to the higher hygroscopicity of resistant starch, the amount of water remaining for the gelatinization of wheat starch decreases during baking which reduces the biscuit hardness [25].
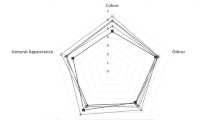
Colour Features of Biscuit Samples
The colour parameters of biscuits are shown in Fig. 1. The addition of UBF or FUBF decreased the L* and increased the L* values of samples (p < 0.05). The highest L* (78.56 ± 1.56) belonged to the control group, and the lowest L* (56.93 ± 0.51) belonged to 30FB. The effect of UBF or FUBF addition rate on a* value was found to be insignificant (p > 0.05). The lowest a* (1.77 ± 0.06) belonged to the control group. For the b* value, the value of 30FB was significantly different and lower than the control (p < 0.05). The b* values of the other samples were found to be similar to the control group (p > 0.05). The highest (25.16 ± 1.00) and the lowest (20.14 ± 0.19) b* values belonged to the control sample and 30FB, respectively. It can be said that the effect of fermentation on colour values, in general, was insignificant (p > 0.05).
Melanoidins occurring during the Maillard reaction between reducing sugar and amino acids at high temperature is important in colour formation [26]. The colour of the product darkens as the amount of sugar and amino acids used as substrates in the Maillard reaction increases. Although the amount of soluble sugar is lower in UBF than in ripe ones [27], it still contains more soluble sugar than wheat flour. The addition of banana flour containing glucose, fructose and protein to the sample increases the amount of substrate [24]. For this reason, it is expected result that the colour became darker with the addition of banana flour. Since the degradation of starch to glucose during fermentation increased the amount of substrate, the addition of FUBF also caused a more intense colour darkening. The increase in redness with the colour darkening caused an increase in the a* value and a decrease in b* value meaning yellowness.
Total Phenolic Content of Biscuits
The total phenolic contents of biscuits are presented in Table 3. The addition of UBF or FUBF increased the total phenolic content of samples (p < 0.05). However, it was found that the effect of fermentation on TPC was insignificant (p > 0.05). The lowest (468.64 mg GAE/kg) and the highest (1167.88 mg GAE/kg) TPC were determined in the control and 30FB samples, respectively.
Banana flour is a rich source of phenolic compounds and contains large amounts of catechins and flavonoids. It was reported that the TPC of peel and pulp were 907 and 232 mg/100 g of dry based (db) in Musa cavendish, respectively [28]. Fatemeh et al. [29] reported that the total phenolic and total flavonoid contents of unripe Cavendish banana were 373.88 and 281.18 mg CE/100 g db, respectively, and decreased to 230.21 and 196.45 mg CE/100 g db respectively, with ripening. Khoza et al. [30] reported that the total phenolic and total flavonoid contents of unripe banana flour ranged from 298.73 to 524.87 mg GAE/100 g dry matter and from 287.40 to 407.08 mg QE/100 g dry matter, respectively. The increase in the phenolic content of the samples due to the addition of banana flour to biscuits can be explained by reason that the total phenolics are more abundant in banana flour than in wheat flour [31].
Total Antioxidant Activity (TAA) of Biscuits
DPPH and FRAP antioxidant activity of biscuits are presented in Table 3. The addition of UBF or FUBF increased TAA of biscuits for both methods. In general, fermentation had insignificant effect on TAA of samples except 15FB. The fermentation had positive effect on DPPH result for 15% addition rate, and the DPPH scavenging activity of 15FB (1022.19 µM TE/g) was higher than 15B (889.53 µM TE/g) (p < 0.05). The lowest results which were 292.50 µM TE/g and 72.67 mg/kg for DPPH and FRAP, respectively, were detected in control group. In addition, the highest results were obtained in 30B and 30FB samples for both assays.
Khoza et al. [30] reported that the antioxidant activity of banana flour varies depending on the cultivar, and different structures in the same plant. They noted that there is a relationship between total phenolic and flavonoid contents of plant materials and DPPH inhibition. They reported that the DPPH and FRAP antioxidant activity of unripe banana flour ranged from 301.34 to 437.22 mg TE/100 g db and 324.27 to 474.23 mg TE/100 g db, respectively. Fatemeh et al. [29] reported the DPPH inhibition as 35.21% in unripe Cavendish banana and 29.38% in ripe Cavendish banana. Although the effect of fermentation on antioxidant activity was generally insignificant, the increase in solubility of bioactive compounds and the synthesis of new phenolic compounds during fermentation also affect antioxidant activity [10]. Consistent with the above results, the antioxidant activity of biscuits increased with a rise in the phenolic compound concentration in our study.
Total Dietary Fibre Content (TDF) of Biscuits
Total dietary fiber of biscuits is given in Table 3. The addition of UBF or FUBF at the rate of 15% had no significant effect on the dietary fibre contents of samples that were similar to the control group for 15B and 15FB (p > 0.05). However, TDF contents of 30B and 30FB were higher than other samples (p < 0.05). The effect of fermentation was statistically insignificant (p > 0.05).
UBF is a very good source of starch, resistant starch (RS) and dietary fibre. RS having similar physiological effects to dietary fibre is not digested in the small intestine and is used as a substrate for microflora in the colon. After the fermentation of RS in the colon, short-chain fatty acids such as butyrate, propionate, and acetate may occur. Each type of short-chain fatty acid, especially butyrate, is well-known for health enhancement and plays an important role in the human gut [30]. Therefore, unripe banana flour use as a functional ingredient increases the RS and TDF of samples. Menezes et al. [27] reported that unripe banana flour contained 56.24 g/100 g db of TDF, of which 48.99 g//100 g dry matter is resistant starch. Substituting flour containing such a high amount of dietary fibre is expected to increase the TDF of the samples. However, the preference of 30% or more of UBF or FUBF in production would be appropriate for a significant rise in the amount of dietary fibre content of biscuits.
Estimated Glycemic Index (GI) Value of Biscuits
The GI values of samples are presented in Table 3. The addition of UBF or FUBF at the rate of 30% decreased the GI of samples (p < 0.05), while GI values of 15B and 15FB were determined as similar to the control group (p > 0.05). The effects of UBF or FUBF addition rate and fermentation were statistically insignificant (p > 0.05). The highest and lowest GI values were detected in control (78.59) and 30FB (72.74), respectively. These results may be attributed to the high RS and TDF content of UBF and FUBF. It can be also concluded from these results that UBF or FUBF utilisation can be an effective way to control the glycemic response of foods.
The release of blood glucose can be controlled as a result of the reduction in leptin and post-prandial glucose reactions by consumption of foods with low soluble sugar content and high dietary fibre or RS content as long as there is no obstructive disease [27, 30]. RS is considered to improve glucose and lipid metabolism, and also have effects on colonic health. However, it must constitute at least 14% of the TDF to provide any benefits for glycemic or insulinemic responses [32]. Since the ratio of RS to TDF is high in UBF, it is expected that UBF and FUBF decreased the GI values of samples. However, the preference of more than 30% of UBF or FUBF in production would be appropriate for a significant reduction in the GI values of biscuits.
Foods can be classified according to their glycemic index values as high (GI ≥ 70), medium (GI 56–69), and low glycemic index foods (GI ≤ 55) [33]. In this study, although the glycemic index values of the samples decreased with the addition of 30% UBF or the same amount of FUBF, this value did not fall below 70. In a study by Juarez-Garcia et al. (2006), bread made with 100% unripe banana flour had a GI value of 65.08, whereas bread made with 100% wheat flour had a GI value of 81.88. Therefore, it can be inferred that bread with unripe banana flour may have a lower glycemic index. However, unlike our study where unripe banana flour was used at 100%, in our study, its maximum usage rate was 30%, replacing wheat flour. Hence, the GI value may not have dropped below 70. To produce biscuits with a lower glycemic index, it may be necessary to use higher proportions of unripe banana flour in the formulation [34].
Conclusion
This study highlights the potential of using FUBF to enhance the functional properties of bakery products. UBF is a rich source of dietary fiber and phenolic components, significantly increasing the functionality of biscuits. Our results indicate that biscuits enriched with UBF have superior phenolic content, antioxidant activity, and dietary fiber.
The study also found that UBF can be used without fermentation, and its inclusion decreased the hardness of the biscuits. This reduction in hardness is particularly beneficial for populations with weak chewing force, such as young children and the elderly.
Future research should focus on long-term health impacts of consuming biscuits enriched with UBF and explore the potential applications of UBF in other food products. Additionally, optimizing the fermentation process to maximize health benefits while maintaining product quality would be valuable.
In summary, biscuits containing UBF, especially at a 30% inclusion rate, are highly functional, making them a preferable option for many consumers who prefer foods enriched with dietary fiber and phenolic compounds, and with enhanced functional properties. Additionally, biscuits made with UBF can be a good alternative for those who need a quick meal or a snack. Since the beneficial effects of fermented products on health are well-known, the use of FUBF might have additional health benefits not determined in this study. It is recommended that future research should investigate the health effects of using FUBF in bakery products using advanced methods. Research can also be conducted on the use of FUBF in the formulation of special dietary foods that require specific nutrition.
Data Availability
The data that support the findings of this study are available upon reasonable request from the corresponding author.
References
Kārkliņa D, Gedrovica I, Reca M, Kronberga M (2012) Production of biscuits with higher nutritional value. in proceedings of the latvian academy of sciences. Proc Latv Acad Sci B: Nat Exact Appl Sci 66(3):113–116. https://doi.org/10.2478/v10046-012-0005-0
Mabogo F, Mashau M, Ramashia S (2021) Effect of partial replacement of wheat flour with unripe banana flour on the functional, thermal, and physicochemical characteristics of flour and biscuits. Int Food Res J 28:138–147. https://doi.org/10.47836/ifrj.28.1.14
Mashau ME, Rambau FD, Kgatla TE (2022) Influence of unripe banana flour incorporation on the physical, antioxidant properties and consumer acceptability of biscuits. J Microbiol Biotechnol Food Sci 12:e2632. https://doi.org/10.55251/jmbfs.2632
Sotiles AR, Daltoé MLM, de Lima VA, Porcu OM, da Cunha MAA (2015) Technological use of green banana and birdseed flour in preparing cookies. Acta Sci Technol 37:423–429. https://doi.org/10.4025/actascitechnol.v37i4.27200
Thakaeng P, Boonloom T, Rawdkuen S (2021) Physicochemical properties of bread partially substituted with unripe green banana (Cavendish spp.) flour. Molecules 26:2070. https://doi.org/10.3390/molecules26072070
Dibakoane SR, Du Plessis B, Da Silva LS, Anyasi TA, Emmambux MN, Mlambo V, Wokadala OC (2023) Nutraceutical properties of unripe banana flour resistant starch: a review. Starch-Stärke 75:2200041. https://doi.org/10.1002/star.202200041
Anyasi TA, Jideani AI, Mchau GR (2017) Effects of organic acid pretreatment on microstructure, functional and thermal properties of unripe banana flour. Food Meas Charact 11:99–110. https://doi.org/10.1007/s11694-016-9376-2
Bertolini AC, Bello-Pérez LA, Méndez‐Montealvo G, Almeida CA, Lajolo F (2010) Rheological and functional properties of flours from banana pulp and peel. Starch‐Stärke 62:277–284. https://doi.org/10.1002/star.200900216
Wolter A, Hager A-S, Zannini E, Arendt EK (2014) Influence of sourdough on in vitro starch digestibility and predicted glycemic indices of gluten-free breads. Food Funct 5:564–572. https://doi.org/10.1039/c3fo60505a
Çetin Babaoğlu H, Arslan Tontul S, Akin N (2021) Fiber enrichment of sourdough bread by inulin rich Jerusalem artichoke powder. J Food Process Preserv 45:15928. https://doi.org/10.1111/jfpp.15928
AACC (2010) Approved methods of the American Association of Cereal Chemists. St. Paul, MN, USA
AOAC (2012) Official Methods of Analysis. Gaithersburg, MD., USA
Aktaş K, Demirci T, Akin N (2015) Chemical composition and microbiological properties of tarhana enriched with immature wheat grain. J Food Process Preserv 39:3014–3021. https://doi.org/10.1111/jfpp.12554
AACC (2000) Approved methods of the American Association of Cereal Chemists. St. Paul, MN, USA
Candal C, Erbas M (2019) The effects of different processes on enzyme resistant starch content and glycemic index value of wheat flour and using this flour in biscuit production. J Food Sci Technol 56:4110–4120. https://doi.org/10.1007/s13197-019-03880-w
Škerget M, Kotnik P, Hadolin M, Hraš AR, Simonič M, Knez Ž (2005) Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem 89:191–198. https://doi.org/10.1016/j.foodchem.2004.02.025
Fernández-León M, Fernández-León A, Lozano M, Ayuso M, Amodio ML, Colelli G, González-Gómez D (2013) Retention of quality and functional values of broccoli ‘Parthenon’stored in modified atmosphere packaging. Food Control 31:302–313. https://doi.org/10.1016/j.foodcont.2012.10.012
Aktas RN, Tontul I (2021) Usability of soapwort and horse chestnut saponin extracts as foaming agents in foam mat drying of pomegranate juice. J Sci Food Agric 101:786–793. https://doi.org/10.1002/jsfa.10770
McCleary BV (2023) Measurement of Dietary Fiber: which AOAC Official Method of Analysis SM to Use. J AOAC Int 106:917–930. https://doi.org/10.1093/jaoacint/qsad051
Goñi I, Garcia-Alonso A, Saura-Calixto F (1997) A starch hydrolysis procedure to estimate glycemic index. Nutr Res 17:427–437. https://doi.org/10.1016/S0271-5317(97)00010-9
Englyst KN, Vinoy S, Englyst HN, Lang V (2003) Glycaemic index of cereal products explained by their content of rapidly and slowly available glucose. Br J Nutr 89:329–339. https://doi.org/10.1079/BJN2002786
Alıoğlu T, Özülkü G (2020) Evaluation of whole wheat flour sourdough as a promising ingredient in short dough biscuits. Food Sci Technol (Campinas) 41:1009–1016. https://doi.org/10.1590/fst.28820
Suriya M, Rajput R, Reddy CK, Haripriya S, Bashir M (2017) Functional and physicochemical characteristics of cookies prepared from Amorphophallus paeoniifolius flour. J Food Sci Technol 54:2156–2165. https://doi.org/10.1007/s13197-017-2656-y
Amarasinghe NK, Wickramasinghe I, Wijesekara I, Thilakarathna G, Deyalage ST (2021) Functional, physicochemical, and antioxidant properties of flour and cookies from two different banana varieties (Musa acuminata Cv. Pisang awak and Musa acuminata Cv. Red Dacca). Int J Food Sci 6681687. https://doi.org/10.1155/2021/6681687
Yu S, Dong K, Pora BL, Hasjim J (2022) The roles of a native starch and a resistant dextrin in texture improvement and low Glycemic Index of biscuits. Processes 10:2404. https://doi.org/10.3390/pr10112404
Hadiyanto AA, Van Straten G, Boom R, Esveld D, Van Boxtel A (2007) Quality prediction of bakery products in the initial phase of process design. Innov Food Sci Emerg Technol 8:285–298. https://doi.org/10.1016/j.ifset.2007.01.006
Menezes EW, Tadini CC, Tribess TB, Zuleta A, Binaghi J, Pak N, Vera G, Dan MCT, Bertolini AC, Cordenunsi BR (2011) Chemical composition and nutritional value of unripe banana flour (Musa acuminata, var. Nanicão). Plant Foods Hum Nutr 66:231–237. https://doi.org/10.1007/s11130-011-0238-0
Someya S, Yoshiki Y, Okubo K (2002) Antioxidant compounds from bananas (Musa Cavendish). Food Chem 79:351–354. https://doi.org/10.1016/S0308-8146(02)00186-3
Fatemeh S, Saifullah R, Abbas F, Azhar M (2012) Total phenolics, flavonoids and antioxidant activity of banana pulp and peel flours: influence of variety and stage of ripeness. Int Food Res J 19:1041–1046
Khoza M, Kayitesi E, Dlamini BC (2021) Physicochemical characteristics, microstructure and health promoting properties of green banana flour. Foods 10:2894. https://doi.org/10.3390/foods10122894
Mohapatra D, Mishra S, Sutar N (2010) Banana and its by-product utilisation: an overview. J Sci Ind Res India 69:323–329
Scazzina F, Siebenhandl-Ehn S, Pellegrini N (2013) The effect of dietary fibre on reducing the glycaemic index of bread. Br J Nutr 109:1163–1174. https://doi.org/10.1017/S0007114513000032
Atkinson FS, Brand-Miller JC, Foster-Powell K, Buyken AE, Goletzke J (2021) International tables of glycemic index and glycemic load values 2021: a systematic review. AJCN 114(5):1625–1632. https://doi.org/10.1093/ajcn/nqab233
Juarez-Garcia E, Agama-Acevedo E, Sáyago-Ayerdi SG, Rodríguez-Ambriz SL, Bello-Pérez LA (2006) Composition, digestibility and application in breadmaking of banana flour. Plant Foods Hum Nutr 61(3):131–137. https://doi.org/10.1007/s11130-006-0020-x
Funding
This study was financially supported by The Scientific and Technological Research Council of Türkiye (TUBITAK)(Project No: 1919B012100796).
Author information
Authors and Affiliations
Contributions
H. Ç.-B.: Data curation, Methodology, Investigation, Writing-original draft, Writing-review & editing. A.C: Data curation, Investigation. S.T: Data curation, Investigation. S. A.-T.: Data curation, Methodology, Investigation, Writing-original draft, Writing-review & editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Çetin-Babaoğlu, H., Coşkun, A., Taşçı, S. et al. Fermented Unripe Banana Flour Utilization as a Functional Ingredient in Biscuits. Plant Foods Hum Nutr (2024). https://doi.org/10.1007/s11130-024-01224-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s11130-024-01224-4