Abstract
Enzyme resistant starch (ERS) is a indigestible form of starch and it can behave like dietary fibre that are the indigestible food components essential for a healthy lifestyle. The aim of this study was to examine the changes of the ERS content of wheat flour with ultrasonication, at four different flour/water ratios (1/5, 1/10, 1/15 and 1/20), three different pH values (6.1, 5.5 and 4.5) and three different temperatures (80 °C, 100 °C and 121 °C). Furthermore, possibility of addition this flour in four different proportions (0%-control, 25%, 50% and 75%) for biscuit production as a dietary fibre source was also investigated. The applied processes increased the dark colour and water holding capacity, but decreased the moisture content of flour samples. The ERS content of wheat flour increased about 30-fold and the sample that had the highest ERS content (ERS-enriched flour) of 3.30% was produced at 121 °C (autoclaving), pH 6.1 and 1/15 flour/water ratio with ultrasonication. ERS-enriched flour had higher melting point and lower estimated glycemic index (eGI) value compared to the wheat flour. In biscuit production, the biscuits with medium eGI (61.82–67.31) was obtained and the increase of ERS-enriched flour proportion increased the ERS content of biscuits, decreased the eGI, hardness and fracturability. During storage, while the light colour and moisture content of biscuits increased; eGI, hardness and fracturability decreased. The biscuits were considered as sensory acceptable by panellists. As conclusion; the ERS-enriched flour can contribute to the production of the medium or low GI foods rich in carbohydrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Good nutrition is a top of the preventive action that must be taken for a healthy lifestyle. Nowadays health conscious consumers are demanding functional foods that have nutritive and health promoting properties for their normal diet (de Boer and Bast 2015). Therefore, the food industry is interested in more research about the development of innovative food products that are beneficial to health (de Boer and Bast 2015; Zhang and Jin 2011). The glycemic index (GI), that is a relative number referred to glucose or to white wheat bread and shows the effect of carbohydrate-rich foods on blood glucose level, has been one of the intensively studied research topics because of the connection to chronic disorders such as diabetes, cardiovascular diseases and obesity (Pinhero et al. 2016; Bordenave et al. 2015). In this context, many studies have been done on carbohydrates and carbohydrate foods regarding decreasing their glycemic effect, due to be the first of the three main energy sources and a major part of the human diet (Zhang and Jin 2011; Ek et al. 2012). Carbohydrate-rich foods can be modified to lower their GI, and in this manner they can assist in the control of chronic disorders (Zhang and Jin 2011). They can be transformed into functional food products that support human health by adding healthy ingredients, or modifying their ingredients by technological methods, to produce dietary fibre (Olmedilla-Alonso et al. 2013).
Dietary fibre cannot be digested in the stomach and small intestine, because they are not hydrolysed by digestive enzymes. For this reason, they do not increase the blood glucose level. However, they are broken down in the large intestine by bacteria, so they act as prebiotics (Liu et al. 2015). Such bioactive functionality suggests to categorize them as functional food components.
Starch can be transformed into a form resistant to digestive enzymes with some technical methods, and thus it can behave like dietary fibre, physically and nutritionally (Lertwanawatana et al. 2015). Such starches are called enzyme resistant starch (ERS) because they pass through the small intestine without being hydrolysed by enzymes, and broken down by microorganisms in the large intestine (Hu et al. 2015). According to its physical and chemical properties, ERS has been classified into four different groups: Type 1, Type 2, Type 3 and Type 4. Type 1 is the starch that is not physically accessible in unground or partially ground cereals, grains and tubers. Type 2 is the non-gelatinized starch which is hydrolysed slowly by the α-amylase enzyme due to its structural properties. Type 3 is retrograded starch, and it occurs as a consequence of food processing techniques. Type 4 is the type of chemically modified starch (Ashwar et al. 2016).
It has been found many studies in the literature on ERS production made by using hydrothermal processes, chemical modification, microwave process etc. However, diversely from these methods, there was limited study on ERS production with ultrasonication. It was considered that ultrasonication can ease breaking and reorganising the starch chains going out from granules, due to the produce strong sound waves. These changes occur in starch chains can increase the recrystallization of them and the ERS formation. Ultrasonication, used for modification of the gelatinised starch or starch suspension in water, induces the morphological and physicochemical changes in starch granules via disrupting granule integrity and depolymerizing starch chains. These changes are connected with the frequency and intensity of ultrasound, temperature and moisture content, and they can effects the starch digestibility (Flores-Silva et al. 2017). In addition it was evaluated that pH variation can effect these circumstance.
The aim of this study was to increase the Type 3 ERS content of wheat flour with a different flour/water ratio, pH value, temperature and ultrasonication, and to investigate possibilities of using this flour in biscuit production as a dietary fibre source and bran alternative.
Materials and methods
Materials
Resistant starch assay kit (Megazyme Int. Wicklow, Ireland) and other chemicals (Merck, Darmstadt, Germany and Sigma, Taufkirchen, Germany) used in this study were analytical and microbiological grade. The foodstuffs (flour, hydrogenated vegetable oil, salt, fat-free milk and baking powder) used in the production of the biscuits were obtained from well-known local markets in Antalya, Turkey, in 2016. Physical analyses of the biscuits were performed in the whole biscuits, and chemical analyses were conducted with milled biscuits by using a blender (HGB2WTS3, 400 W, Waring, USA).
Research design and statistical analysis
A 2 × 4 × 3 × 3 factorial research design was used in production of flour samples. A total of 72 different samples were produced with and without ultrasonication, at four different flour/water ratios (1/5, 1/10, 1/15 and 1/20), three different pH values (6.1-natural pH value of the flour, 5.5 and 4.5-adjusted with 1 M citric acid) and three different temperatures (80, 100 and 121 °C). In production of biscuit samples, the four different ERS-enriched flour proportions were used.
The study was carried out in two replicates, and all analyses were conducted in duplicate. All statistical calculations were performed using SAS Statistical software (SAS Institute Inc., Cary, NC, USA). The significance was evaluated by analysis of variance (ANOVA), followed by Duncan’s multiple range test (p < 0.05). Results were presented as mean ± standard error.
Preparing of flour samples
Production flow diagram that was formed for the preparation of flour samples is shown in Fig. 1.
Application of ultrasonication
For the production of flour samples, the flour mixtures were prepared per the factorial research design and their internal temperatures were increased to 75 °C in a hot water bath for 20 min to the starch gelatinization, after adjusting the pH value. Ultrasonication was applied to these mixtures by using a sonicator at a power of 96 W (Sonopuls HD 3200, Bandelin, Berlin, Germany) combined with a cylindrical probe (6 mm) at 100% power and amplitude of 245–249 µm, for 15 min with 10-s on and 5-s off pulsation.
Drying of mixtures and mill
Ultrasonicated mixtures were spread out at about 2 mm of thickness on a clear flat surface, and were retrograded and dried to 14% moisture content under continuous air circulation for 3 days in a room maintained at 25 °C. In this way, the mould growth was prevented and it was ensured that the ERS content of the samples was improved safely. Dried samples were subjected to coarse (Waring blender, USA) and fine (Bosch, Germany) grinding, in two stages, to produce a particulate size of about 150 µm.
Experimental biscuit production and storage
The biscuit method used by Gündoğdu Sertakan (2006) has been modified for production. The amount of materials and the baking time of the biscuits have been determined by preliminary tests.
For the biscuit production, the mixture containing flour (234.4 g), salt (2.4 g), skimmed milk powder (4.8 g) and baking powder (8 g), including sodium bicarbonate and sodium acid pyrophosphate, was added to hydrogenated vegetable oil (40 g). The mixture was mixed manually for 1 min. Finally, the water (114 mL) was added to the mixture, and it was kneaded manually for 5 min to form biscuit dough. Entire mixing and kneading process was conducted by the same person and it was paid attention using the approximately same force for these processes. The obtained dough was rolled-out about the thickness of 1.5 mm. The rolled-out dough was placed on baking paper and cut circularly (D = 52 mm). The cut dough and the baking paper were taken on a tray heated to initial baking temperature (100 °C), and the tray was placed in an electric oven at 100 °C (HC744540 Siemens, Munich, Germany). After the dough was baked for 5 min at this temperature, the oven temperature was gradually increased from 100 to 180 °C for 15 min. Biscuits were baked for a total of 20 min, removed from the oven and left to cool at room temperature.
ERS-enriched flour with contents of 0%, 25%, 50% and 75% replaced the wheat flour in the formula, and the biscuit production was made with these flour mixtures. The biscuit produced with 0% ERS-enriched flour (100% wheat flour) was evaluated as the control sample.
Biscuits produced by adding different ERS-enriched flour proportions were rested for 3 h, then placed into zip lock plastic bags and stored in a cupboard at room temperature for 60 days.
Analysis methods
Determination of colour values
The L*, a* and b* colour values of the flour and biscuit samples were measured according to the CIELAB system by using a CR-400 chromameter (Minolta CR 400, Konica Minolta, Japan). The L*, a* and b* values in the CIELAB system express blackness-whiteness, greenness-redness and blueness-yellowness, respectively (Erbas 2010). For the measurement, flour samples were placed in the measurement cup and their colour values were determined by measuring at two different points. The colour of the biscuit samples was determined by measuring at four different points.
Determination of moisture content
The moisture content of the flour and biscuit samples were determined by drying 1 g of the sample at 105 °C until they reached a constant weight.
Determination of water holding capacity
To determine the water holding capacity (WHC) of the flour samples, 0.1 g of the sample was weighed into the tared tubes and then 1 mL of distilled water was added. These tubes were mixed for 5 s with a vortex mixer. The samples were incubated at 25 °C for 20 min by mixing in 5 min intervals. After 20 min, the samples were centrifuged (Centrifuge 5430, Eppendorf AG, Hamburg, Germany) at 2000×g for 10 min and the liquid fraction was removed. The WHC of the samples was determined by measuring the weight of the remaining pellet, and was calculated using the following equation as g water/g dry matter (Karaoğlu and Aktaş 2006):
Determination of enzyme resistant starch
ERS analysis of flour and biscuit samples was performed using a resistant starch assay kit (Megazyme Int. Wicklow, Ireland), according to the standard method AACC 32-40 (AACC 2010). The ERS content of the samples was calculated by the following equation:
where ∆E, F and W represent the absorbance of the sample, conversion from absorbance to micrograms and the dry weight of the sample, respectively.
Determination of thermal properties
The thermal properties were determined for only the flour sample that had the highest ERS content (ERS-enriched flour), and wheat flour using differential scanning calorimetry (DSC) instrument (DSC 6000, Perkin-Elmer Inc., Wellesley, USA). For this purpose, 5 mg of sample was weighed into an aluminium capsule and 5 μL of water was added on it. The capsule was tightly closed and conditioned at room temperature for 20 h (Wasserman et al. 2007). The thermal properties were determined by heating it from 0 to 200 °C with a heating rate of 10 °C/min. An empty capsule was used as a reference (Kapelko-Żeberska et al. 2015).
In vitro starch digestibility and determination of estimated glycemic index
The rate of the starch hydrolysis during in vitro digestion at different times (30, 60, 90, 120 and 180 min), and the estimated glycemic index (eGI) value of the ERS-enriched flour, wheat flour and biscuit samples were determined according to the method of Goñi et al. (1997) by using Megazyme resistant starch assay kit.
Hydrolysis curves were created by drawing a graph of the hydrolysed starch content against time. Hydrolysis curves modelled as non-linear by Goñi et al. (1997) were evaluated using SigmaPlot Systat 12 (Erkrath, Germany) program according to following equation:
where C is the concentration of hydrolysed starch at t time, C∞ is the equilibrium concentration of starch hydrolysed after 180 min, k is the kinetic constant.
The hydrolysis index (HI) was defined as the ratio of the area under the starch hydrolysis curve of the sample to the reference sample’s. The eGI value of the samples was calculated according to the following equation by using the hydrolysis index:
where the numbers in this in vitro eGI equation express the constant values used for calculating the eGI value from the total starch hydrolysis rate obtained within 180 min, after the food is subjected to digestive enzymes.
Freshly baked white bread crumbs were taken as a reference for the calculation of eGI, but the results were given by converting to glucose reference according to the following equation (Schakel et al. 2008):
Determination of dimensions and weight of biscuits
The dimensions and weight of the biscuits were determined by measurements made before and after baking, by randomly selecting five biscuits. Their weight and dimensions were measured with a precision scale and a calliper, respectively.
Determination of hardness and fracturability of biscuits
The hardness and fracturability of the biscuits were determined by using a texture analyser device (TA-XT plus, Stable Microsystems, Surrey, UK) equipped with three-point bend rig (HDP/M3PB). The analysis was carried out with a 5 kg load cell at a probe test speed of 3 mm s−1 and a trigger force of 5 g (Yeo and Seib 2009).
Determination of sensorial properties of biscuits
Sensory analysis was performed on the biscuits produced by adding ERS-enriched flour in different proportions (0%, 25%, 50% and 75%). The selected parameters were colour, straightness of baking, texture, brittleness, odour, aroma and flavour and overall parameters. Sensory evaluation was performed by 8 trained postgraduate students, using a 5-point hedonic scale (1 extremely disliked; 5 extremely liked).
Results and discussion
Physical and chemical properties of wheat flour and the effects of different applications on flour samples
The L*, a*, b* values, moisture content and WHC value of wheat flour used in the ERS-enriched flour production were 91.55, − 5.25, 15.90, 13.51%, 1.07 g w/g dm, respectively. The ERS content and the eGI value of wheat flour were determined as 0.11% and 71.19, respectively. In the literature, it is stated that the ERS content of wheat flour is about 0.09% (Tharanathan and Tharanathan 2001), and the eGI of foods prepared with wheat flour are about 70 in glucose reference (Foster-Powell et al. 2002).
The colour values, moisture, WHC and ERS contents of flour samples obtained after the different applications are given in Table 1.
The colour values of flour samples
It was determined that the L* values of the samples were affected significantly (p < 0.01) by the pH and temperature, whereas the b* values were affected from ultrasonication (p < 0.05) and pH (p < 0.01). The a* values were not affected (p > 0.05) by any parameters.
While the increasing temperature was darkened the colour, decreasing pH value caused light colour. These changes may have took place because the Maillard reaction occurs faster with the higher pH and temperature (de Oliveira et al. 2016). The decrease of the b* value of flour samples is indicated decreasing of yellowness. It is may be sourced from the oxidation of flour carotenoids of ERS produced with ultrasonication in low pH conditions. Similarly, in a study producing ERS from rice starch by autoclaving, it was determined that the a* value was not significantly affected by the applied processes, but lower L* value and higher b* value were determined (Ashwar et al. 2016).
The moisture content of flour samples
The moisture content of flour samples was average 7.88%, and this value is considered suitable for safe storage. It was reported in the literature that the moisture content of stored samples is usually below 14% (Dundar and Gocmen 2013).
The water holding capacity of flour samples
The WHC value of flour samples was significantly (p < 0.05; p < 0.01) affected by all applications. These applications increased the WHC content of the samples to about 5 times that of the wheat flour (1.07 g w/g dm). In addition, it was evaluated that the increased ERS content after applications effected the WHC content, too. This trend was reasoned to be due to the disintegration of the starch granule structure and reorganisation of amylose and amylopectin polymers. Trapping more water among these reorganised polymers increased the water holding capacity.
WHC decreased with the ultrasonication, increasing temperature and decreasing pH values. The amylopectin is particularly responsible for the WHC of gelatinised starch (Iftikhar and Dutta 2019) and it contains a lot of branches according to the amylose. It was considered that when the hydrothermal process and ultrasonication were applied to the starch, the breaking down the branches caused the active ends free. The decrease in the WHC might be resulted from closing a lot the active ends of the starch chains overlap, as a result of the reorganisation of the hydrogen bonds. The reorganisation occurred because of making sonication in the flour/water mixture, and this effect was increased by temperature and pH. Mudgil and Barak (2013) reported that the polysaccharide components of dietary fibres are highly hydrophilic, and WHC varies according to the applied process. Wang et al. (2014) determined that the WHC of ERS produced by applying a debranching enzyme (1.98 g/g) was higher than heat-moisture treatment (0.96 g/g). In another study, the WHC of the dried wheat starch after gelatinisation was found as about 2.00–2.50 g/g (Karaoğlu and Aktaş 2006). Ashwar et al. (2016) found that the rice starch held about twice as much water after autoclaving.
The ERS contents of flour samples
The ERS content of flour samples was significantly (p < 0.05; p < 0.01) affected by all applications.
The samples produced with ultrasonication contained more ERS than the samples produced without ultrasonication. It was considered that this trend was sourced from the increasing crystallization due to the more amylose and amylopectin chains escaped from the starch granules by the ultrasonication.
It was determined that as the temperature increased and the samples were processed with the autoclave, the ERS content of the samples increased. It was considered that this change occurred due to the increase in occurring and breaking of the hydrogen bond with the temperature. It was reported by Dundar and Gocmen (2013) that the ERS contents of the starch increased with increasing process temperature.
It was determined that the ERS content of samples decreased with low pH values. This trend might be resulted from increasing the solubility of the recrystallized starch in acidic conditions. It was reported in the literature that organic acids at low pH values broke down the amylose and amylopectin and they decreased the crystallinity of starch films (Majzoobi and Beparva 2014). Therefore, it was evaluated that the increase in hydrolysing the amylose and amylopectin and the decrease in starch crystallinity degree decreased the ERS formation, in which the starch becomes insoluble form.
The ERS content of the flour samples increased with the increase of the water content until reaching 1/15 flour/water ratio. After this ratio, an increase or a decrease did not occur statistically in ERS content. It might have occurred because the increasing water content, and also with the effect of high temperature, caused to break loose more amylose and amylopectin chains from starch granule as is in ultrasonication process, due to the high hydrogen bond capacity of water. They recrystallized firmly with retrogradation during drying. With the retrogradation occurred after hydrothermal processes, straight chains closed the overlap and winded up in the form of double helices gradually (Ashwar et al. 2016; Raigond et al. 2015). Therefore, this rearrangement increase the recrystallization and consequently the ERS content. It is stated in the literature that the amount of water added to the starch increases the formation of the ERS (Li et al. 2011). Furthermore, Li et al. (2011) noted that an increase of water content more than 20% caused a decrease in the amount of mung bean ERS. Zeng et al. (2015) determined that when applying the combination of ultrasonication and autoclaving to the starch, much more ERS was produced than applying the autoclaving alone.
The ERS content, that was determined to be 0.11% in the wheat flour, increased nearly 30-fold times and it was found an average of 3.30% in the processed samples. The ERS-enriched flour sample that had the highest ERS content was produced at 121 °C (autoclaving), pH 6.1 and 1/15 flour/water ratio with ultrasonication.
Thermal properties of ERS-enriched flour sample
The DSC graphs of wheat flour and ERS-enriched flour are given in Fig. 2. The melting temperature (Tm) and the melting enthalpy (ΔH) of the wheat (control) and ERS-enriched flour were determined as 141.17 °C and 1.10 J/g and 152.94 °C and 1.09 J/g, respectively. It was considered that the high melting temperature of the ERS-enriched flour sample indicated the occurrence of a new and different crystal structure for the starch as compared to the wheat flour. Wasserman et al. (2007) found that the melting temperatures and the melting enthalpy of the ERS produced from different wheat starches ranged from 120.0 to 138.0 °C and 1.1–2.0 J/g, respectively. In ERS produced from rice flour, the melting temperature was determined to be 162.08 °C (Moongngarm et al. 2015).
The parameters of hydrolysis kinetics and eGI of ERS-enriched flour sample
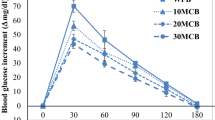
The total starch hydrolysis curve, parameters of hydrolysis kinetics and eGI of the wheat flour and ERS-enriched flour are given in Fig. 2 and Table 4. Significant (p < 0.01) differences were determined between wheat flour and ERS-enriched flour in terms of C∞, k, HI and eGI. The HI value, which is indicative of the availability of hydrolysis, was determined as 114.91 for wheat flour and 98.30 for ERS-enriched flour. Similarly, the eGI value of the ERS-enriched flour was found as 65.51 with respect to the glucose reference, and it was lower than the wheat flour (71.88) used as a control. The decrease in the HI and eGI showed the high ERS content of the sample.
The foods are divided into 3 groups as high (GI ≥ 70), medium (56 ≤ GI ≤ 69) and low (GI ≤ 55) GI foods in terms of the GI values. According to this classification, the control sample was in the food group with high GI, whereas the ERS-enriched flour sample was in the food group with medium GI. In a study that was used taro starch for ERS production, the eGI value was determined to decrease from 60.58 to 51.86 after starch retrogradation (Simsek 2011).
Biscuit production and storage
The changes that occurred after baking for the dimensions and weight of biscuits produced by adding different ERS-enriched flour proportions are given in Table 2. The effect of ERS-enriched flour proportion and the storage on the colour values, hardness, fracturability, moisture and ERS contents of biscuits are given in Table 3. The total starch hydrolysis curve and the effect of the ERS-enriched flour proportion and the storage on the hydrolysis kinetics parameters and eGI values for the biscuits are given in Fig. 2 and Table 4.
Dimensions and weight of biscuits
Increasing the ERS-enriched flour proportion in the biscuits significantly increased biscuits’ diameter (p < 0.01) and decreased their thickness (p < 0.05), but the weight loss remained stable. It was considered that the biscuit dough spread out during baking with increasing ERS-enriched flour proportion, because the proteins of this flour became denatured before. Since the parameters promoted the structure formation, such as elasticity and bulking of dough, takes place with the gluten, the structure of the biscuits was not provided due to the denaturation of this protein.
The colour values of biscuits
The L* values of the biscuits were significantly (p < 0.01) affected by the proportion of ERS-enriched flour and the storage, but a* and b* values were only affected (p < 0.01) by the storage. As expected, increasing the ERS-enriched flour proportion in biscuits darkened the biscuit colours. Because, when the wheat flour was heated for ERS production, the colour of flour darkened due to the occurred Maillard reaction. Using this dark flour in biscuit production was darkened the colour of biscuits. It was determined that the biscuit colour became lighter with storage.
The hardness and fracturability of biscuits
The hardness and fracturability of the biscuits were significantly (p < 0.05; p < 0.01) affected by both the ERS-enriched flour proportion and the storage. It was determined that the hardness of the biscuits decreased as the ERS-enriched flour proportion added to the biscuit increased. Similarly, Wang et al. (2014) found that the hardness values of the biscuits differed according to the added ERS source, and the hardness generally decreased with an increase of the added ERS amount.
The highest fracturability value was determined for the biscuit samples produced by the addition of 0% and 25% ERS-enriched flour. In products produced with wheat flour, the gluten protein ensures the robust formation of the structure and the improvement of the texture (Dizlek 2012). It was observed that the hardness and fracturability values of the biscuits decreased as the added proportion of ERS-enriched flour increased, due to the denaturation of gluten and the high WHC content of ERS-enriched flour. In addition, increasing the moisture content of biscuits, together with the increasing ERS-enriched flour proportion, may influence this behavior.
The moisture content of biscuits
Both the ERS-enriched flour addition and the storage significantly (p < 0.01) affected the moisture content of biscuits. With the increase in the added ERS proportion and the storage period, the moisture content of the biscuits increased. The moisture content of the biscuits may have increased with the increasing ERS-enriched flour proportion due to the high WHC (Table 1) of it. High WHC causes the more interaction between water vapour and starch. This trend might have increased the moisture content of biscuits. The increase in the moisture content from 4.39 to 6.18% during storage may be due to the absorption of moisture from the environment by the biscuits.
The ERS content of biscuits
As expected, the added proportion from 0 to 75% of ERS-enriched flour significantly (p < 0.01) affected the ERS content from 0.44 to 2.84% for the biscuits. Also, the ERS content of the control biscuits was found to be 0.44%, differently from the wheat flour (0.11%). It was likely due to gelatinisation during baking and then retrogradation.
The parameters of hydrolysis kinetics and eGI value of biscuits
The added proportion of ERS-enriched flour and the storage significantly (p < 0.05; p < 0.01) affected the C∞, HI and eGI values. These parameters decreased with increasing ERS-enriched flour proportion and storage time. However, the biscuit samples had medium GI value and the place of them in the GI scale was not changed. In a study, it was determined that the eGI value of the biscuits was 87.00 when the white bread was taken as reference (Goñi et al. 1997). When the glucose conversion was performed, this value was found to be 60.84. Therefore, these biscuits were evaluated as medium GI foods, too. Frei et al. (2003) determined that the eGI values were lowered by retrograding the different rice varieties after heat treatment.
Although the ERS content of the biscuits (Table 3) did not increase during the storage, the eGI values decreased. It might be sourced from the increase in the interactions between the starch, fat and protein during storage, and therefore α-amylase had difficulties in accessing the starch in the interacted structure during in vitro digestion. In some studies, it was also determined that the fat and protein amount decreased the eGI values of the foods, by forming the barrier on starch granules and prevent the enzyme access to starch with this way (Ren et al. 2016).
The sensorial properties of biscuits
The effect of the ERS-enriched flour proportion and the storage on the sensorial properties of the biscuits are given in Table 3. While the storage did not affect (p > 0.05) of any sensorial properties of biscuits, the added proportion of the ERS-enriched flour affected (p < 0.05; p < 0.01) all sensorial properties significantly, except for aroma and flavour. Considering all sensorial parameters, increasing the ERS-enriched flour proportion was found to reduce sensory qualities of the biscuits. However, biscuits were evaluated to be sensory acceptable because the assessment score for all sensorial parameters of the biscuits was over 3 according to the 5-point hedonic scale. For this reason, it was evaluated that ERS-enriched flour can be used in biscuit production up to the 75% proportion.
Conclusion
The ERS is generally produced from pure starch, and the studies that used wheat flour in production of ERS are limited. It was deduced that the applied processes increase the ERS content of the wheat flour nearly 30-fold times, and this content can be further increased by optimizing the processing time and conditions. However, the proteins of ERS-enriched flour became denatured as a result of the applied processes. Therefore, it was considered that this flour can be added to the formulation of flat bakery products, such as cookies and biscuits in different proportions. Thus, ERS-enriched flour will contribute to the production of the low GI foods, which are beneficial to health.
References
AACC (2010) Approved methods of the American Association of Cereal Chemists (Method 32-40). American Association of Cereal Chemists, St. Paul
Ashwar BA, Gani A, Wani IA, Shah A, Masoodi FA, Saxena DC (2016) Production of resistant starch from rice by dual autoclaving-retrogradation treatment: invitro digestibility, thermal and structural characterization. Food Hydrocoll 56:108–117. https://doi.org/10.1016/j.foodhyd.2015.12.004
Bordenave N, Kock LB, Abernathy M, Parcon JC, Gulvady AA, van Klinken BJ-W, Kasturi P (2015) Toward a more standardised and accurate evaluation of glycemic response to foods: recommendations for portion size calculation. Food Chem 167:229–235. https://doi.org/10.1016/j.foodchem.2014.06.124
de Boer A, Bast A (2015) International legislation on nutrition and health claims. Food Policy 55:61–70. https://doi.org/10.1016/j.foodpol.2015.06.002
de Oliveira FC, Coimbra JSdR, de Oliveira EB, Zuñiga ADG, Rojas EEG (2016) Food protein-polysaccharide conjugates obtained via the maillard reaction: a review. Crit Rev Food Sci Nutr 56(7):1108–1125. https://doi.org/10.1080/10408398.2012.755669
Dizlek H (2012) Wheat gluten proteins: Gliadin. Acad Food J/Akademik Gida 10(2):109–114
Dundar AN, Gocmen D (2013) Effects of autoclaving temperature and storing time on resistant starch formation and its functional and physicochemical properties. Carbohydr Polym 97(2):764–771. https://doi.org/10.1016/j.carbpol.2013.04.083
Ek KL, Brand-Miller J, Copeland L (2012) Glycemic effect of potatoes. Food Chem 133(4):1230–1240. https://doi.org/10.1016/j.foodchem.2011.09.004
Erbas M (2010) The effects of different debittering methods on the production of lupin bean snack from bitter Lupinus albus L. seeds. J Food Qual 33(6):742–757. https://doi.org/10.1111/j.1745-4557.2010.00347.x
Flores-Silva PC, Roldan-Cruz CA, Chavez-Esquivel G, Vernon-Carter EJ, Bello-Perez LA, Alvarez-Ramirez J (2017) In vitro digestibility of ultrasound-treated corn starch. Starch 69(9–10):1700040. https://doi.org/10.1002/star.201700040
Foster-Powell K, Holt SH, Brand-Miller JC (2002) International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 76(1):5–56. https://doi.org/10.1093/ajcn/76.1.5
Frei M, Siddhuraju P, Becker K (2003) Studies on the in vitro starch digestibility and the glycemic index of six different indigenous rice cultivars from the Philippines. Food Chem 83(3):395–402. https://doi.org/10.1016/S0308-8146(03)00101-8
Goñi I, Garcia-Alonso A, Saura-Calixto F (1997) A starch hydrolysis procedure to estimate glycemic index. Nutr Res 17(3):427–437. https://doi.org/10.1016/S0271-5317(97)00010-9
Gündoğdu Sertakan S (2006) Posibility of using triticale flour at producing biscuit and cracker. Ph.D. Thesis, Trakya University, Edirne
Hu X-P, Huang T-T, Mei J-Q, Jin Z-Y, Xu X-M, Chen H-Q (2015) Effects of continuous and intermittent retrogradation treatments on in vitro digestibility and structural properties of waxy wheat starch. Food Chem 174:31–36. https://doi.org/10.1016/j.foodchem.2014.11.026
Iftikhar SA, Dutta H (2019) Status of polymorphism, physicochemical properties and in vitro digestibility of dual retrogradation-annealing modified rice starches. Int J Biol Macromol 132:330–339. https://doi.org/10.1016/j.ijbiomac.2019.03.206
Kapelko-Żeberska M, Zięba T, Spychaj R, Gryszkin A (2015) Acetylated adipate of retrograded starch as RS 3/4 type resistant starch. Food Chem 188(Supplement C):365–369. https://doi.org/10.1016/j.foodchem.2015.05.018
Karaoğlu M, Aktaş HKN (2006) Effect of different modification techniques on the physicochemical and thermoanalytical properties of wheat and corn starch. J Tekirdag Agric Fac 6:83–91
Lertwanawatana P, Frazier RA, Niranjan K (2015) High pressure intensification of cassava resistant starch (RS3) yields. Food Chem 181:85–93. https://doi.org/10.1016/j.foodchem.2015.02.005
Li S, Ward R, Gao Q (2011) Effect of heat-moisture treatment on the formation and physicochemical properties of resistant starch from mung bean (Phaseolus radiatus) starch. Food Hydrocoll 25(7):1702–1709. https://doi.org/10.1016/j.foodhyd.2011.03.009
Liu Y, Chen W, Chen C, Zhang J (2015) Physicochemical property of starch-soluble dietary fiber conjugates and their resistance to enzymatic hydrolysis. Int J Food Prop 18(11):2457–2471. https://doi.org/10.1080/10942912.2014.968785
Majzoobi M, Beparva P (2014) Effects of acetic acid and lactic acid on physicochemical characteristics of native and cross-linked wheat starches. Food Chem 147:312–317. https://doi.org/10.1016/j.foodchem.2013.09.148
Moongngarm A, Srijinda P, Paseephol T, Samana S, Martphutorn N (2015) Effect of heat moisture treatment using different solvents and incubation times on resistant starch formation of rice flour. Am J Appl Sci 12(8):560. https://doi.org/10.3844/ajassp.2015.560.566
Mudgil D, Barak S (2013) Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: a review. Int J Biol Macromol 61:1–6. https://doi.org/10.1016/j.ijbiomac.2013.06.044
Olmedilla-Alonso B, Jiménez-Colmenero F, Sánchez-Muniz FJ (2013) Development and assessment of healthy properties of meat and meat products designed as functional foods. Meat Sci 95(4):919–930. https://doi.org/10.1016/j.meatsci.2013.03.030
Pinhero RG, Waduge RN, Liu Q, Sullivan JA, Tsao R, Bizimungu B, Yada RY (2016) Evaluation of nutritional profiles of starch and dry matter from early potato varieties and its estimated glycemic impact. Food Chem 203:356–366. https://doi.org/10.1016/j.foodchem.2016.02.040
Raigond P, Ezekiel R, Raigond B (2015) Resistant starch in food: a review. J Sci Food Agric 95(10):1968–1978. https://doi.org/10.1002/jsfa.6966
Ren X, Chen J, Molla MM, Wang C, Diao X, Shen Q (2016) In vitro starch digestibility and in vivo glycemic response of foxtail millet and its products. Food Funct 7(1):372–379. https://doi.org/10.1039/c5fo01074h
Schakel S, Schauer R, Himes J, Harnack L, Van Heel N (2008) Development of a glycemic index database for dietary assessment. J Food Compos Anal 21(Supplement):S50–S55. https://doi.org/10.1016/j.jfca.2007.02.004
Simsek S (2011) Production of resistant starch from taro (Colocasia esculenta L. schott) corm and determination of its effects on health by in vitro methods. Ph.D. Thesis, Ege University, İzmir
Tharanathan M, Tharanathan RN (2001) Resistant starch in wheat-based products: isolation and characterisation. J Cereal Sci 34(1):73–84. https://doi.org/10.1006/jcrs.2000.0383
Wang L, Li S, Gao Q (2014) Effect of resistant starch as dietary fiber substitute on cookies quality evaluation. Food Sci Technol Res 20(2):263–272. https://doi.org/10.3136/fstr.20.263
Wasserman L, Signorelli M, Schiraldi A, Yuryev V, Boggini G, Bertini S, Fessas D (2007) Preparation of wheat resistant starch. J Therm Anal Calorim 87(1):153–157. https://doi.org/10.1007/s10973-006-8209-7
Yeo LL, Seib PA (2009) White pan bread and sugar-snap cookies containing wheat starch phosphate, a cross-linked resistant starch 1. Cereal Chem 86(2):210–220. https://doi.org/10.1094/CCHEM-86-2-0210
Zeng S, Wu X, Lin S, Zeng H, Lu X, Zhang Y, Zheng B (2015) Structural characteristics and physicochemical properties of lotus seed resistant starch prepared by different methods. Food Chem 186:213–222. https://doi.org/10.1016/j.foodchem.2015.03.143
Zhang H, Jin Z (2011) Preparation of products rich in resistant starch from maize starch by an enzymatic method. Carbohydr Polym 86(4):1610–1614. https://doi.org/10.1016/j.carbpol.2011.06.070
Acknowledgements
The authors would like to thank Akdeniz University for equipment support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Candal, C., Erbas, M. The effects of different processes on enzyme resistant starch content and glycemic index value of wheat flour and using this flour in biscuit production. J Food Sci Technol 56, 4110–4120 (2019). https://doi.org/10.1007/s13197-019-03880-w
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03880-w