Abstract
Oxygenic photosynthesis has historically been considered limited to be driven by the wavelengths of visible light. However, in the last few decades, various adaptations have been discovered that allow algae, cyanobacteria, and even plants to utilize longer wavelength light in the far-red spectral range. These adaptations provide distinct advantages to the species possessing them, allowing the effective utilization of shade light under highly filtered light environments. In prokaryotes, these adaptations include the production of far-red-absorbing chlorophylls d and f and the remodeling of phycobilisome antennas and reaction centers. Eukaryotes express specialized light-harvesting pigment–protein complexes that use interactions between pigments and their protein environment to spectrally tune the absorption of chlorophyll a. If these adaptations could be applied to crop plants, a potentially significant increase in photon utilization in lower shaded leaves could be realized, improving crop yields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
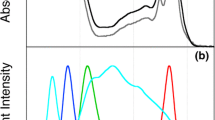
Oxygenic photosynthesis begins with the capture of light energy by pigment–protein antenna complexes (Blankenship 2014; Saer and Blankenship 2017). The spectrum of light absorbed by these complexes is controlled by the properties of the pigments and the interactions of the pigments with one another and with the protein environment in which they are located. In plants and most algae and cyanobacteria, the spectral range that can be utilized effectively for photosynthesis is primarily limited to the visible range, approximately 400 to 700 nm, which makes up only about half of the incident solar spectrum (Fig. 1a) (Zhu et al. 2010). However, specialized pigments or pigment–proteins are utilized in some species of algae and cyanobacteria to expand this spectral range into the far-red region, about 700–750 nm for these species (Fig. 1b, c) (Chen and Blankenship 2011; Blankenship and Chen 2013). This spectral expansion allows these specialized organisms to thrive in niches otherwise unavailable to their spectrally limited neighbors (Swingley et al. 2008; Duxbury et al. 2009; Mohr et al. 2010).
Solar spectrum at ground level and several examples of far-red antennas and systems. a Quantum solar output spectrum. Data derived from ASTM 1.5. b An example of far-red adaptation in Eukaryotes, showing whole cells of the far-red adapted Eustigmatophyte alga FP5 (black line) and the green alga Chlorella vulgaris grown under white light (red line). c Several examples of far-red absorption in prokaryotes, showing whole cells of Chl f-producing far-red adapted filamentous environmental isolate LSP65 (green line), the white light-grown Synechocystis sp. PCC 6803 (blue line), and Chl d-containing Acaryochloris marina, grown under white light (magenta line). On both panels, vertical lines indicate boundaries of classically-defined photosynthetically active radiation (PAR) (400 and 700 nm) and the far-red region primarily used by shade-adapted oxygenic phototrophs (700 and 750 nm). Scattering in absorbance spectra of whole cells has not been mitigated, and these spectra are normalized to unity
Far-red light is particularly enriched in shaded conditions. When a stratification exists in a forest canopy, water column, microbial mat, or other biological community, far-red light tends to penetrate the first few layers of phototrophs (Kasperbauer 1987; Gilbert et al. 2001). The top layers generally absorb most of the visible wavelengths of light. This leaves an enrichment of far-red light that passes through the canopy or upper layer and reaches lower strata. In a forest or crop canopy, this far-red light serves as a trigger for phytochromes in the leaves of shaded plants, causing them to initiate a shade-avoidance response that leads to stem stretching and leaning to escape the shade (Kasperbauer 1987). Additionally, Photosystem I (PSI) antennas in higher plants have been shown to utilize some far-red wavelengths, and this can be important to photosynthetic output in shaded leaves (Rivadossi et al. 1999).
In some specialized algae and cyanobacteria, these far-red wavelengths can be put to much more use for the organism (Chen and Blankenship 2011). To do this, several adaptations have been discovered. Chlorophylls may be modified, such that they can absorb far-red wavelengths. These pigments include chlorophylls d and f (Chl d and f) (Fig. 2), and have so far only been discovered in cyanobacteria (Miyashita et al. 1996; Chen et al. 2010; Chen 2019). Cyanobacteria may also modify their phycobilisome antennas to absorb far-red light when exposed to far-red conditions, and this is typically observed in conjunction with Chl f production (Ho et al. 2016b; Li et al. 2016). Finally, many algae, including many diatoms and some Eustigmatophytes, can absorb far-red light by shifting the absorption spectrum of chlorophyll a (Chl a) in their light-harvesting complex (LHC)-type antennas instead of utilizing a chemically distinct pigment (Koehne et al. 1999; Kotabová et al. 2014; Wolf et al. 2018). Evidence suggests that this may be due to an aggregation phenomenon in the antenna systems (Bína et al. 2014).
Several chlorophyll structures and their absorption spectra. a A chlorophyll macrocycle is shown, with labeled R groups substituted in various chlorophylls. b Identification of the various R groups in each of the four chlorophylls. c Spectra of each of these chlorophylls in pure methanol that have been purified using HPLC
By using one or more of these adaptations, these organisms are specifically evolved to effectively utilize wasted far-red light while living in the lower layers of photosynthetic strata. Many of these organisms are capable of turning their far-red light utilization system on and off as needed through complex regulatory mechanisms (Kotabová et al. 2014; Zhao et al. 2015). This allows them to survive highly varied light conditions.
While these specific far-red adaptations have not been observed in plants, they could potentially play an important role in crop improvements. Heavily shaded lower leaves in dense crop canopies could be made more productive by utilizing the transmitted far-red wavelengths that make it through the canopy leaves. In an idealized situation in which all photons of wavelengths between 700 and 750 nm could be utilized in a crop field, a 19% increase in absorbable photons could be realized (Chen and Blankenship 2011). In the field, this ideal situation is unlikely to be achieved, but even an increase of a few percent could have a significant impact.
Far-red light utilization and response in plants
In crop and forest canopies, light is filtered by the upper canopy leaves, which tend to preferentially absorb blue and red wavelengths of sunlight while transmitting or reflecting much of the far-red and a small fraction of the green (Kasperbauer 1987; Ort et al. 2015). Plants are much more effective at absorbing and utilizing visible light than far-red, which is a notable feature of any photosynthetic action spectrum. The perception that leaves are green is the result of a slightly lower absorption of green light than red and blue, although this difference is still much less significant than the difference of far-red versus visible light. Plants primarily use this differential absorption as a shade-sensing mechanism. Under shaded conditions, the spectrum of light is enriched in far-red wavelengths, and the ratio of red to far-red light is sensed by phytochromes (Gilbert et al. 2001; Ort et al. 2015).
Phytochromes are bilin-binding photosensors involved in various aspects of plant development, including germination, hypocotyl elongation, gene expression, and flowering, just to name a few (Reed et al. 1994; Furuya and Schäfer 1996; Nagy and Schäfer 2002; Rockwell and Lagarias 2010). These photosensors do not directly contribute to the photosynthetic capacity of the plant, but are crucial for the response to light conditions. In addition to plants, these important regulators are also found in fungi, algae, and bacteria (Karniol et al. 2005; Rockwell and Lagarias 2010; Fortunato et al. 2016). The reversible phytochromes can be switched from the far-red-absorbing to the red-absorbing form by a short pulse of far-red light (Furuya and Schäfer 1996). Phytochromes are used to sense photoperiod in many flowering plants, switching to the far-red-absorbing form as the day goes on (Reed et al. 1994). During the night, the far-red form will slowly revert if kept in darkness (Searle 1965). A short pulse of far-red light at the end of the day can have a strong effect on plants, as this will rapidly reset the photosensor back to its red-absorbing form (Kasperbauer 1987; Furuya and Schäfer 1996). The mechanism of red/far-red light sensing in phytochromes has been shown to involve photoisomerization of the 15/16 double bond on the bilin chromophore (Rockwell and Lagarias 2010 and references therein). Phytochromes may be knotted or knotless, and in the knotted phytochromes from Deinococcus radiodurans, a chromophore-binding pocket located at the knot structure in the protein has been shown through crystallographic studies (Wagner et al. 2005). When the bilin chromophore is photoexcited, a conformational change is propagated from this chromophore to the surrounding protein structure, which in turn alters interaction sites on the surface of the protein (Ulijasz et al. 2010).
Phytochromes play many important roles in agriculture. In greenhouses, it is common to use phytochrome-mediated responses to control flowering. In short-day plants (plants that flower when days are short), flowering initiation can be delayed using a night-interruption treatment consisting of a high red-to-far-red ratio (Craig and Runkle 2013). In crop canopies, the ratio of far-red to red light is an important determinant of plant morphology, with closer plant spacing leading to higher ratios of far-red to red and shade-avoidance responses (Kasperbauer 1987). Shade-avoidance responses include stem stretching, internode length, and branching patterns. The response to far-red light, therefore, of crop plants can be an important factor in determining minimum row spacing.
Despite the low absorption cross section beyond 700 nm, plants can effectively intercept and use a limited fraction of far-red light. Light as far to the red as 780 nm was able to drive some oxygen evolution in one report (Pettai et al. 2005). This Photosystem II (PSII) activity was suggested to be the result of possible low-energy Chl a dimers that might be present in LHCII. PSI antennas have been shown to utilize far-red light much more effectively owing to their efficient far-red antennas. In the lower leaves of forest canopies, far-red light may make up 40% of the total photon capture and usage (Rivadossi et al. 1999). In the antennas of PSI, far-red-absorbing Chl a molecules and some of the ligands interacting with them have been identified (Morosinotto et al. 2003, 2005; Wientjes and Croce 2011; Wientjes et al. 2012). The protein environment surrounding these pigments is essential to their red-shifted absorption, and specific ligand–pigment interactions and Chl a dimerization are responsible for the red-shifting of the absorption spectra of these antennas (Rivadossi et al. 1999; Morosinotto et al. 2003, 2005). In plants, these red-shifted forms of Chl a found within the light-harvesting antennas of PSI absorb light at wavelengths up to 738 nm, up to 6 kT lower than P700 (Croce et al. 1996). This indicates a necessity for thermally activated uphill energy transfer between low-energy antenna pigments and P700 (Jennings et al. 2003).
This efficient utilization of far-red wavelengths preferentially by PSI also presents a particular problem for plant leaves utilizing shade light. Under shaded conditions, a significant increase in LHCII is required to match the activities of PSI and PSII under shaded conditions (Rivadossi et al. 1999). Given the relatively low absorption cross sections under far-red conditions, to improve the utilization efficiency of PSII, particular modifications found in algae and cyanobacteria could be employed. These natural adaptations to deep shade light conditions will be discussed in more detail below.
Far-Red-Absorbing Chlorophyll d
A far-red light niche adaptation of particular interest is Chl d, a chlorophyll with a Qy absorption maximum of 696 nm in methanol (Fig. 2c) and significantly longer in vivo (Fig. 1c). Chl d was discovered by Manning and Strain in 1943 in pigment extractions from red macroalgae growing along the Northern California intertidal zones (Manning and Strain 1943). At the time, they logically attributed their newly discovered pigment to the macroalgae themselves, and they called this new pigment Chl d. However, as time went on, it was realized that the red macroalgae do not in fact contain any Chl d and for some time it was thought that Chl d was merely an artifact of Manning and Strain’s chlorophyll extraction procedure, given the observation that Chl a could be converted to Chl d in solvent (Holt and Morley 1959). However, in 1996, Miyashita et al. isolated and cultured an oxygenic photosynthetic prokaryote that contained Chl d as its primary photopigment from extracts of ascidia (Miyashita et al. 1996, 1997). Amazingly, the prokaryote they cultured contained only a few percent Chl a. This unique prokaryote was named Acaryochloris marina (Miyashita et al. 2003). The finding led to further research suggesting that the Chl d isolated by Manning and Strain probably came from epiphytes living on the surface of the red macroalgae, not an artifact of extraction (Murakami et al. 2004; Larkum and Kühl 2005).
Since this discovery, several other Acaryochloris strains have been identified. Acaryochloris is the only known genus containing Chl d as a primary photopigment, though many other far-red adapted prokaryotes contain traces of the pigment. In such cases, Chl d has been found in very low levels in PSII of these Chl f-containing species when grown under far-red light (Airs et al. 2014; Gan et al. 2014a, b). However, in Acaryochloris, Chl d makes up to 95% of the total chlorophylls found in the organism, implicating the pigment as a near-complete replacement for Chl a.
In PSII of Chl d-containing A. marina, PSII contains primarily Chl d, as well as two pheophytin a (Pheo a) molecules and a very small amount of Chl a (Chen et al. 2005; Tomo et al. 2007). Chl d is found to compose the primary donor of PSII, and in Acaryochloris, P680 is replaced by P725 (Itoh et al. 2007), although P713 has also been suggested (Tomo et al. 2007). This represents a significant red shift compared to the typical P680 found in Chl a-containing phototrophs. The Chl a that is found in each PSII reaction center (RC) is also of notable importance (Schlodder et al. 2007; Tomo et al. 2007; Ohashi et al. 2008; Renger and Schlodder 2008). However, some debate still exists as to its exact function and location in PSII. The special pair of PSII is often considered to be composed entirely of Chl d molecules (Tomo et al. 2007), though some have suggested that a Chl a molecule is also part of the special pair based on spectroscopic evidence (Renger and Schlodder 2008). While some argument still exists regarding the exact location and function of the Chl a in PSII, it is understood that Pheo a serves as the primary electron acceptor (Chen et al. 2005). The redox potential of the Pheo a has been shown to be lower in Chl d-containing organisms than in Chl a-containing ones (Allakhverdiev et al. 2010).
In A. marina, photochemistry of PSI is also driven by Chl d (Hu et al. 1998). The primary donor of PSI in Acaryochloris is P740, significantly red shifted compared to P700 in Chl a-containing species, and is made up of a special pair of Chl d molecules (Hu et al. 1998). Despite absorbing at a lower energy than P700, excited P740 (P740*) is still capable of generating a reducing power that is roughly equivalent to that of P700 (Hu et al. 1998).
Chl d differs from Chl a only in the addition of a formyl group in the place of a vinyl in the C3 position of the chlorin ring (Fig. 2a). The biosynthetic enzyme responsible for the formation of Chl d in vivo still has not been identified. 18O labeling experiments have shown that Chl a and molecular oxygen serve as precursors in the synthesis of Chl d in Acaryochloris (Schliep et al. 2010; Chen 2014). Given the precursors to Chl d, the multifunction oxidases known as cytochrome P450s have been implicated as possible Chl d synthase candidates (Chen and Blankenship 2011; Blankenship et al. 2013; Chen 2014). P450s are particularly strong candidates because of their ability to oxidize various compounds using molecular oxygen. However, to date, no cytochrome P450 has been shown to catalyze the catalysis of Chl d production in vivo. Another hypothesis would assume that chlorophyllide a (Chlide a) serves as a precursor, and would draw parallels between the synthesis of Chl d and a very common Chl molecule in plants, Chl b. The addition of a formyl at the C7 site instead of C3 would generate Chl b. The precursor to Chl b, Chlide b is synthesized from Chlide a using the enzyme Chlide a oxidoreductase (CAO) (Oster et al. 2000). The oxygen atom in the formyl group is derived from molecular oxygen (O2) (Porra et al. 1994). This seeming similarity suggests the possibility that CAO could be implicated in the biosynthesis of Chl d. However, the chemistry performed by CAO is to oxidize a methyl group to a formyl, whereas in the case of Chl d, a vinyl must be oxidized and a C–C double bond broken, so the chemistry is significantly different. In both of these cases, the need for molecular oxygen has been suggested to provide evidence to put the evolutionary origin of Chl d after the origin of oxygenic photosynthesis (Chen and Blankenship 2011).
A potential opportunity to investigate the synthesis process of Chl d has recently arisen with the discovery of a very unusual Acaryochloris (RCC1774) that does not produce any Chl d, but rather produces Chl b like the somewhat-related prochlorophytes (Partensky et al. 2018; King et al. unpublished observations). Given the unusual pigmentation in these otherwise very similar species, this may suggest that the biosynthetic processes of Chl d and b are evolutionarily related. Significantly, it may be possible to compare the new genome-sequencing data for both Chl d and Chl b-containing Acaryochloris to gain insights into the identity of the Chl d synthase.
Far-Red-Absorbing Chlorophyll f
While Chl d alone holds the position of the far-red pigment that can replace nearly every function of Chl a in the RC, another modified chlorophyll takes the prize for longest wavelength absorption. Chl f was discovered in 2010 in cyanobacterial isolates from stromatolites in Shark Bay, Australia (Chen et al. 2010). Chl f has the longest wavelength absorption of any known chlorophyll at 707 nm in methanol (Fig. 2c) and longer in vivo (Fig. 1c), which is the result of a formyl group in the C2 position on the chlorin ring instead of a methyl as in Chl a (Chen et al. 2010; Li et al. 2013). The first cyanobacterium discovered containing Chl f was the filamentous cyanobacterium Halomicronema hongdechloris, which contains only 12.5% Chl f when grown under far-red light, but no Chl f at all under white light (Chen et al. 2012; Li et al. 2014). When grown under far-red light conditions, H. hongdechloris responds by altering the localization of the phycobilisomes and Chl f (Majumder et al. 2017). H. hongdechloris does not limit its light-harvesting system alterations under various light conditions to chlorophyll pigmentation, and has been shown to dramatically alter its phycobilisome antennas in response to far-red light (Li et al. 2016). When grown under far-red light, isolated phycobilisomes not only exhibit a marked decrease in overall size and structure, but also an absorption shift to 712 nm, nearly 100 nm to the red of the absorption maximum of white light-grown cells (Li et al. 2016).
Following the discovery of Chl f in H. hongdechloris, a proverbial floodgate was opened and it was quickly discovered that Chl f-producing cyanobacteria are widely dispersed and quite common throughout the natural environment. More than 12 different Chl f strains have been reported, found in widely ranging filtered light environments besides stromatolites, including hot springs (Brown et al. 2010; Gan et al. 2014a, b). When grown under far-red light, these cyanobacteria photoacclimate through a process termed FaRLiP (Far-Red Light Photoacclimation) (Gan et al. 2014a). FaRLiP involves significant changes to the organisms, including the production of Chl f, as well as a small amount of Chl d, and alterations to PSI, PSII, phycobilisomes, and several light receptors, all of which are encoded on a common 21-gene cluster (Gan et al. 2014a, b; Gan and Bryant 2015; Zhao et al. 2015; Ho et al. 2016b, c). FaRLiP is regulated using a red/far-red knotless phytochrome RfpA, as well as RfpB and RfpC, which are involved in a signaling cascade that induces the expression of the FaRLiP gene cluster (Gan et al. 2014b; Zhao et al. 2015). In addition to these regulators, the FaRLiP cluster encodes alternative core subunits for PSI, PSII, and the phycobilisome (Gan et al. 2014b). While the altered cores of PSI and PSII under far-red light have been shown to contain Chl d and Chl f under these conditions (Nürnberg et al. 2018), the phycobilisomes also display a red-shifted absorption in addition to rearrangement of the core subunits under far-red light (Gan et al. 2014b; Ho et al. 2016c; Li et al. 2016). The rearrangements are due to changes in the linker subunits, and the red-shifting of the phycocyanobilin is due to the lack of a normal covalent bond to a cystine that would be expected under white light conditions (Gan et al. 2014b; Li et al. 2016). This results in an additional double bond, increasing conjugation in the pigment and red-shifting its absorption (Miao et al. 2016).
Under far-red light, FaRLiP-capable cyanobacteria make significant changes to their entire photosynthetic apparatus, but Chl f does not almost fully replace Chl a in the way that Chl d does. While its low abundance even in far-red light-adapted cells might suggest that it serves only as an accessory pigment, Chl f has been shown to play an integral part in photochemistry in cyanobacteria when FaRLiP responses are activated. In the Chl f-producing cyanobacterium Chroococcidiopsis thermalis, Chl f has been identified in PSI and PSII when the cells were grown under far-red light, where it participates in charge separation (Nürnberg et al. 2018). When grown under far-red light, P700 is replaced by P745 and P680 is replaced by P727, and both contain Chl f while P727 also contains a single molecule of Chl d (Nürnberg et al. 2018). Similar to the case with Chl d, these red-shifted primary donors are still capable of driving oxygenic photosynthesis, indicating that the red limit is further into the far-red region than originally thought.
The biosynthetic process of Chl f has been determined to involve a light-dependent “super-rogue” D1 protein, called ChlF or PsbA4 (Ho et al. 2016a). Using either Chl a or Chlide a as a precursor, the enzyme uses catalytically active chlorophylls and an absorbed photon to oxidize the C2 methyl to a formyl group. It is, however, unknown whether the oxygen source is for the oxidation. Ho et al. suggest a possible implication of the Chl f synthase as a precursor to the D1 protein in the early evolution of photosynthesis, rather than the opposite interpretation. This hypothesis would suggest that Chl f preceded oxygenic photosynthesis, and that the Mn4CaO5 cluster, essential to water oxidation in PSII, came later. However, the limited distribution of Chl f-producing species and the possibility of evolutionary tree reconstruction artifacts make this conclusion uncertain.
Because of the pervasiveness of Chl f in nature, additional species are frequently found in additional genera of cyanobacteria (Zhang et al. 2019). The ability to photoacclimate to far-red enriched conditions allows cyanobacteria with the FaRLiP adaptations to survive in stratified or otherwise shaded growing conditions. Given the effects of such adaptations, the FaRLiP gene cluster could potentially be transformed into plants to improve their shade light utilization.
Algal light-harvesting complexes that red shift the absorption of Chlorophyll a
Like plants, algae take a very different approach to far-red utilization than cyanobacteria. Some species of algae are very adept at far-red adaptation, but they do not produce specialized forms of chlorophyll for the purpose like cyanobacteria do. Instead, algae use a much more poorly understood system of LHC modifications that serve to shift the absorption spectrum of Chl a. Less is known about how algae utilize far-red light than cyanobacteria, possibly owing to their large genomes and the fact that the phenotypes are often not as clear-cut as the production of a novel chlorophyll. Here, we describe several systems that have been characterized to date, as well as some of the studies that serve as foundations to understand how these processes work.
Algal light harvesting begins with the absorption of light in a system of integral transmembrane LHCs (Blankenship 2014). For PSII, these antennas are mostly trimeric in structure, being composed of three units, each of which contains three transmembrane helices. The pigment composition of these pigment–proteins varies by species. Green algae (and land plants) contain Chl a and b, as well as several carotenoids including xanthophyll. These antenna complexes are designated LHCII, and they have been characterized in some detail, including through crystallography (Liu et al. 2004). A notable feature of LHCII antennas is their lateral mobility in the membrane. Through the process of state transitions, LHCII antennas can move through the thylakoid membrane to interact with PSI or PSII, helping to balance the excitation of the two types of RC (Rochaix 2014). Also, a great diversity exists in LHCIIs expressed in plants and algae.
In stramenopile algae, formerly called heterokonts, a different type of LHC is present, though it is related to the LHCII found in plants and green algae. Stramenopiles include brown algae, diatoms, eustigmatophytes, and others, and make up an important part of the plankton in the oceans. In diatoms, the antenna is a Fucoxanthin-Chlorophyll Protein, or FCP, and it contains Chl a, Chl c, and the carotenoid fucoxanthin (Gundermann and Büchel 2014; Büchel 2015). FCPs are found in trimeric form, like LHCIIs, but have not been characterized in the same level of detail. However, an important step in the characterization of these FCP systems was the determination of the crystal structure of Lhcf4 in the diatom Phaeodactylum tricornutum (Wang et al. 2019). The structure indicated key pigment locations important to understanding energy transfer and non-photochemical quenching in these diatom systems. There are separate FCP organizations and types for PSI and PSII (Gundermann and Büchel 2014). The antenna system is similar in Eustigmatophytes, which are free-living coccoid secondary endosymbiont algae of about 10 µm in size with a characteristic red lipidic body of unknown function (Eliáš et al. 2017). Eustigmatophyte antennas are Violaxanthin Chlorophyll Proteins, or VCPs. VCPs contain only Chl a and carotenoid, principally violaxanthin and vaucheriaxanthin (Sukenik et al. 2000; Litvín et al. 2016; Eliáš et al. 2017).
In diatoms and eustigmatophytes, a high level of gene diversity is present. In the eustigmatophyte Nannochloropsis oceanica, for instance, 17 different gene products associated with LHC-type proteins have been identified (Litvín et al. 2016). In both N. oceanica and the close relative Nannochloropsis gaditana, large PSII–LHC complexes were not observed due to its weak interaction, though PSI–LHC complexes were, and it is apparent that there are separate LHC pools for both PSI and PSII (Basso et al. 2014; Litvín et al. 2016). However, several LHC-type antennas did have some interaction with PSII even after purification (Litvín et al. 2016). It can therefore be concluded that the interaction of LHC antennas to PSI is stronger than to PSII in Nannochloropsis, though separate pools do exist for each RC.
While Nannochloropsis has not been shown to utilize far-red light effectively, several Eustigmatophyte algae have been shown to possess LHCs capable of absorbing far-red light to promote oxygenic photosynthesis (Wolf et al. 2018; Bína et al. 2019; Niedzwiedzki et al. 2019). One of these species, the Eustigmatophyte alga FP5, has been shown to survive entirely on far-red wavelengths of light using an antenna system that contains only Chl a and carotenoids (Fig. 1b) (Wolf et al. 2018). In FP5, the LHC appears to rely on an aggregation-based mechanism to red shift its Chl a to an absorption component of approximately 705 nm. This is sufficient to allow survival using light from a 740 nm LED or filtered shade light.
The green alga Ostreobium is a coral symbiont capable of growing in highly shaded and far-red light-enriched environments. This alga has a notable far-red fluorescence peak at 719 nm at 77 K in whole cells, and fluorescence excitation shows a strong contribution to the far-red fluorescence from a pigment absorbing at 702 nm, indicating the presence of low-energy pigments even when grown under white light (Fork and Larkum 1989). Ostreobium sp. has also been shown to possess a significant far-red absorption shoulder in whole cells. When compared with another species of Ostreobium lacking the far-red spectral features, a denaturing electrophoretic analysis identified a unique Lhca1 polypeptide, which was confirmed by N-terminal amino acid sequencing (Koehne et al. 1999). This same study showed evidence for up to three far-red pigments within whole membranes, but concluded that these are more likely caused by protein–pigment interactions than excitonic interactions between pigments. This hypothesis, combined with the fact that the system is not conserved among all species of Ostreobium is also interesting, and provides a possible means for future comparative biochemical studies.
The finding that the far-red spectral forms of chlorophyll in Ostreobium sp. are primarily associated with a PSI antenna provides a basis for some interesting biological questions. In a later study, it was shown that the far-red antenna system in Ostreobium sp. is capable of driving PSII under far-red illumination (Wilhelm and Jakob 2006). In this study, when Ostreobium sp. was grown under far-red light, it was capable of growth, and a photosynthetic action spectrum of oxygen evolution confirmed an extension of the red drop and PSII activity under wavelengths out to 740 nm. Whether this might represent a mobile Lhca protein or some sort of physical interaction in the form of a supercomplex of both RCs and the far-red antenna remains an open question.
A more recently characterized eukaryotic far-red antenna is that of the alveolate Chromera velia. The discovery of the coral reef symbiont was published in 2008, and has been shown to be a close relative of apicomplexan parasites, which contain a non-photosynthetic plastid (Moore et al. 2008). The light-harvesting systems of this unusual alga are of particular interest due to their evolutionary history. While their plastid is indeed photosynthetic, phylogenetically, they are more closely related to parasitic, non-photosynthetic apicomplexans than to the photosynthetic alveolates dinoflagellates (Moore et al. 2008; Weatherby and Carter 2013). The plastid is clearly divergent from that of the dinoflagellates, in that it lacks the peridinin and Chl c that they contain, but rather contains Chl a, violaxanthin, isofucoxanthin, and β,β-carotene (Moore et al. 2008; Quigg et al. 2012).
The retained photosynthetic plastid of Chromera has some interesting features, including an unusual antenna composition. While the photosynthetic apparatus of C. velia appears to be rather primitive, its efficiency is still high and the ability to respond to various light intensities is still adequate (Quigg et al. 2012). The plastid of the apicomplexans and Chromera seems to have arisen from the endosymbiosis of a red alga (secondary endosymbiosis) and the plastid appears to be closely related to that of heterokonts (stramenopiles) (Janouškovec et al. 2010). In a separate study, the 23 nuclear-encoded LHC genes were found to have varied evolutionary histories, including 17 related to the FCPs in diatoms and dinoflagellate PCP, but only 3 of red algal origin (Pan et al. 2012). This is interesting given that the Chromera light-harvesting (CLH) proteins lack Chl c, and given the red algal lineage of the plastid. Biochemical characterization of the antenna complexes in C. velia revealed the anticipated FCP-like LHCs, which were named Chromera Light Harvesting (CLH), and also PSI–LHCr complexes, which were red algal in origin (Tichy et al. 2013).
When grown under long wavelength light, this coral symbiont, like Ostreobium sp. described above, is capable of producing a far-red antenna system. When grown under red light, a notable 710 nm room temperature fluorescence was observed, which was accompanied by a red shoulder on the absorption spectrum (Kotabová et al. 2014). This was not observed under blue light, and was reversible. The LHC-type proteins responsible for this shift were named Red–CLH complexes, and were functionally linked to PSII (Kotabová et al. 2014). When the Red–CLH proteins were purified, it was found that they were made up of antenna multimers or aggregates that could be easily disrupted, leading to a complete loss of their far-red absorption and fluorescence properties (Bína et al. 2014). Given that this property has also been observed in a Eustigmatophyte alga (Wolf et al. 2018), which shares an ancestor with Chromera through the secondary endosymbiotic event (Janouškovec et al. 2010), it appears that such a multimerization red-shifting effect may be a widespread adaptation among secondary endosymbionts.
Conclusions
In nature, surviving the common niche of deep shade is a driving factor for far-red light adaptations. Whether the case of a shaded coral symbiont alga (Fork and Larkum 1989), cyanobacteria forming shaded layers within a stromatolite (Chen et al. 2010), or a shaded lifestyle on the bottom of an ascidian (Miyashita et al. 1996) or brown macroalgal frond (Murakami et al. 2004), organisms throughout the tree of life have evolved mechanisms to use the dimly transmitted wavelengths of marginal quality far-red light. In land plants, these adaptations do not seem to represent a large portion of the absorption spectrum, even under light-limited conditions, though far-red light is still an important signal. This has led to a great deal of speculation regarding the potential gains that could be realized in agriculture if such systems could be transformed into plants.
Because photosynthesis is a quantum process, it is not a significant disadvantage to use far-red light, as long as the energy of the absorbed photons is high enough to support photochemistry. The champion of this expansion is currently the Chl f-related adaptations, which have been shown to shift the primary donor of PSI all the way out to 745 nm and PSII to 727 nm (Nürnberg et al. 2018). If all of the photons between 700 and 750 nm could be used by crop plants, a 19% increase in the number of absorbable photons could be realized (Chen and Blankenship 2011). Of course, this provides an upper limit, but nevertheless, it serves to demonstrate the fact that a far-red utilizing crop plant could be of value in terms of yield enhancement. Control of such a system would rely on phytochromes, similar to the smart canopies that have been suggested previously using non-far-red adaptations (Ort et al. 2015). This would allow light-starved lower leaves to capture unused far-red light, which saturated upper leaves would not intercept.
Several approaches could be taken to engineering a far-red utilizing crop plant. One would be to use the genes from the 21-gene FaRLiP cluster (Gan et al. 2014a) to produce Chl f in a plant, along with all of the modified RC proteins. However, this presents several serious challenges, including differences in eukaryotic and prokaryotic RCs and the presence of LHC proteins in plants that may or may not properly incorporate Chl f in vivo. To circumvent some of these challenges, it might instead be possible to engineer plants with some of the eukaryotic far-red LHC proteins we have described, such as the Red–CLH proteins (Bína et al. 2014; Kotabová et al. 2014) or the red Lhca1-type light-harvesting complex from Ostreobium sp. (Koehne et al. 1999). While these do not possess a significant reach into the far-red as the modified pigments, this might be balanced by the potentially simpler and less costly path to engineering and subsequent commercialization. Far-red-utilizing oxygenic phototrophs offer a potential method to increase photosynthetic yields in crop plants worldwide. Continued research will be needed to discover new systems and engineer those already present in plants.
References
Airs RL, Temperton B, Sambles C et al (2014) Chlorophyll f and chlorophyll d are produced in the cyanobacterium Chlorogloeopsis fritschii when cultured under natural light and near-infrared radiation. FEBS Lett 588:3770–3777. https://doi.org/10.1016/j.febslet.2014.08.026
Allakhverdiev SI, Tomo T, Shimada Y et al (2010) Redox potential of pheophytin a in photosystem II of two cyanobacteria having the different special pair chlorophylls. Proc Natl Acad Sci USA 107:3924–3929. https://doi.org/10.1073/pnas.0913460107
Basso S, Simionato D, Gerotto C et al (2014) Characterization of the photosynthetic apparatus of the Eustigmatophycean Nannochloropsis gaditana: evidence of convergent evolution in the supramolecular organization of photosystem I. Biochim Biophys Acta Bioenerg 1837:306–314. https://doi.org/10.1016/j.bbabio.2013.11.019
Bína D, Gardian Z, Herbstová M et al (2014) Novel type of red-shifted chlorophyll a antenna complex from Chromera velia: II. Biochemistry and spectroscopy. Biochim Biophys Acta Bioenerg 1837:802–810. https://doi.org/10.1016/j.bbabio.2014.01.011
Bína D, Durchan M, Kuznetsova V, Litvín R (2019) Energy transfer dynamics in a red-shifted violaxanthin-chlorophyll a light-harvesting complex. Biochim Biophys Acta Bioenerg 1860:111–120. https://doi.org/10.1016/j.bbabio.2018.11.006
Blankenship RE (2014) Molecular mechanisms of photosynthesis, 2nd edn. Wiley, West Sussex
Blankenship RE, Chen M (2013) Spectral expansion and antenna reduction can enhance photosynthesis for energy production. Curr Opin Chem Biol 17:457–461. https://doi.org/10.1016/j.cbpa.2013.03.031
Blankenship RE, Chen M, Willows R (2013) Gene constructs comprising nucleic acids that modulate chlorophyll biosynthesis and uses thereof
Brown II, Bryant DA, Casamatta D et al (2010) Polyphasic characterization of a thermotolerant siderophilic filamentous cyanobacterium that produces intracellular iron deposits. Appl Environ Microbiol 76:6664–6672. https://doi.org/10.1128/aem.00662-10
Büchel C (2015) Evolution and function of light harvesting proteins. J Plant Physiol 172:62–75. https://doi.org/10.1016/j.jplph.2014.04.018
Chen M (2014) Chlorophyll modifications and their spectral extension in oxygenic photosynthesis. Annu Rev Biochem 83:317–340. https://doi.org/10.1146/annurev-biochem-072711-162943
Chen M (2019) Chlorophylls d and f: synthesis, occurrence, light-harvesting, and pigment organization in chlorophyll-binding protein complexes. In: Advances in botanical research, 1st edn. Elsevier Ltd. https://doi.org/10.1016/bs.abr.2019.03.006
Chen M, Blankenship RE (2011) Expanding the solar spectrum used by photosynthesis. Trends Plant Sci 16:427–431. https://doi.org/10.1016/j.tplants.2011.03.011
Chen M, Telfer A, Lin S et al (2005) The nature of the photosystem II reaction centre in the chlorophyll d-containing prokaryote, Acaryochloris marina. Photochem Photobiol Sci 4:1060–1064. https://doi.org/10.1039/b507057k
Chen M, Schliep M, Willows RD et al (2010) A red-shifted chlorophyll. Science 329:1318–1319. https://doi.org/10.1126/science.1191127
Chen M, Li Y, Birch D, Willows RD (2012) A cyanobacterium that contains chlorophyll f—a red-absorbing photopigment. FEBS Lett 586:3249–3254. https://doi.org/10.1016/j.febslet.2012.06.045
Craig DS, Runkle ES (2013) A moderate to high red to far-red light ratio from light-emitting diodes controls flowering of short-day plants. J Am Soc Hortic Sci 138:167–172. https://doi.org/10.21273/jashs.138.3.167
Croce R, Zucchelli G, Garlaschi FM et al (1996) Excited state equilibration in the photosystem I–light-harvesting I complex: P700 is almost isoenergetic with its antenna. Biochemistry 35:8572–8579. https://doi.org/10.1021/bi960214m
Duxbury Z, Schliep M, Ritchie RJ et al (2009) Chromatic photoacclimation extends utilisable photosynthetically active radiation in the chlorophyll d-containing cyanobacterium, Acaryochloris marina. Photosynth Res 101:69–75. https://doi.org/10.1007/s11120-009-9466-7
Eliáš M, Amaral R, Fawley KP et al (2017) Handbook of the protists. Springer, Berlin
Fork DC, Larkum AWD (1989) Light harvesting in the green alga Ostreobium sp., a coral symbiont adapted to extreme shade. Mar Biol 103:381–385. https://doi.org/10.1007/bf00397273
Fortunato AE, Jaubert M, Enomoto G et al (2016) Diatom phytochromes reveal the existence of far-red-light-based sensing in the ocean. Plant Cell 28:616–628. https://doi.org/10.1105/tpc.15.00928
Furuya M, Schäfer E (1996) Photoperception and signalling of induction reactions by different phytochromes. Trends Plant Sci 1:301–307. https://doi.org/10.1016/1360-1385(96)88176-3
Gan F, Bryant DA (2015) Adaptive and acclimative responses of cyanobacteria to far-red light. Environ Microbiol 17:3450–3465. https://doi.org/10.1111/1462-2920.12992
Gan F, Shen G, Bryant D (2014a) Occurrence of Far-Red Light Photoacclimation (FaRLiP) in diverse Cyanobacteria. Life 5:4–24. https://doi.org/10.3390/life5010004
Gan F, Zhang S, Rockwell NC et al (2014b) Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light. Science (80) 345:1–11. https://doi.org/10.1126/science.1256963
Gilbert IR, Jarvis PG, Smith H (2001) Proximity signal and shade avoidance differences between early and late successional trees. Nature 411:792–795. https://doi.org/10.1038/35081062
Gundermann K, Büchel C (2014) The structural basis of biological energy generation. Springer, Dordrecht, pp 21–37
Ho M, Shen G, Canniffe DP et al (2016a) Light-dependent chlorophyll f synthase is a highly divergent paralog of PsbA of photosystem II. 9178. https://doi.org/10.1126/science.aaf9178
Ho MY, Gan F, Shen G et al (2016b) Far-Red Light Photoacclimation (FaRLiP) in Synechococcus sp. PCC 7335: I. Regulation of FaRLiP gene expression. Photosynth Res 131:1–14. https://doi.org/10.1007/s11120-016-0309-z
Ho MY, Gan F, Shen G, Bryant DA (2016c) Far-Red Light Photoacclimation (FaRLiP) in Synechococcus sp. PCC 7335. II. Characterization of phycobiliproteins produced during acclimation to far-red light. Photosynth Res 131:1–16. https://doi.org/10.1007/s11120-016-0303-5
Holt AS, Morley HV (1959) A proposed structure for chlorophyll d. Can J Chem 37:507–514
Hu Q, Miyashita H, Iwasaki I et al (1998) A photosystem I reaction center driven by chlorophyll d in oxygenic photosynthesis. Proc Natl Acad Sci USA 95:13319–13323. https://doi.org/10.1073/pnas.95.22.13319
Itoh S, Mino H, Itoh K et al (2007) Function of chlorophyll d in reaction centers of photosystems I and II of the oxygenic photosynthesis of Acaryochloris marina. Biochemistry 46:12473–12481. https://doi.org/10.1021/bi7008085
Janouškovec J, Horák A, Oborník M et al (2010) A common red algal origin of the apicomplexan, dinoflagellate, and heterokont plastids. Proc Natl Acad Sci USA 107:10949–10954. https://doi.org/10.1073/pnas.1003335107
Jennings RC, Zucchelli G, Croce R, Garlaschi FM (2003) The photochemical trapping rate from red spectral states in PSI–LHCI is determined by thermal activation of energy transfer to bulk chlorophylls. Biochim Biophys Acta Bioenerg 1557:91–98. https://doi.org/10.1016/s0005-2728(02)00399-7
Karniol B, Wagner JR, Walker JM, Vierstra RD (2005) Phylogenetic analysis of the phytochrome superfamily reveals distinct microbial subfamilies of photoreceptors. Biochem J 392:103–116. https://doi.org/10.1042/bj20050826
Kasperbauer MJ (1987) Far-red light reflection from green leaves and effects on phytochrome-mediated assimilate partitioning under field conditions. Plant Physiol 85:350–354. https://doi.org/10.1104/pp.85.2.350
Koehne B, Elli G, Jennings RC et al (1999) Spectroscopic and molecular characterization of a long wavelength absorbing antenna of Ostreobium sp. Biochim Biophys Acta Bioenerg 1412:94–107. https://doi.org/10.1016/s0005-2728(99)00061-4
Kotabová E, Jarešová J, Kaňa R et al (2014) Novel type of red-shifted chlorophyll a antenna complex from Chromera velia. I. Physiological relevance and functional connection to photosystems. Biochim Biophys Acta Bioenerg 1837:734–743. https://doi.org/10.1016/j.bbabio.2014.01.012
Larkum AWD, Kühl M (2005) Chlorophyll d: the puzzle resolved. Trends Plant Sci 10:355–357. https://doi.org/10.1016/j.tplants.2005.06.005
Li Y, Cai ZL, Chen M (2013) Spectroscopic properties of chlorophyll f. J Phys Chem B 117:11309–11317. https://doi.org/10.1021/jp402413d
Li Y, Lin Y, Loughlin PC, Chen M (2014) Optimization and effects of different culture conditions on growth of Halomicronema hongdechloris—a filamentous cyanobacterium containing chlorophyll f. Front Plant Sci 5:67. https://doi.org/10.3389/fpls.2014.00067
Li Y, Lin Y, Garvey CJ et al (2016) Characterization of red-shifted phycobilisomes isolated from the chlorophyll f-containing cyanobacterium Halomicronema hongdechloris. Biochim Biophys Acta Bioenerg 1857:107–114. https://doi.org/10.1016/j.bbabio.2015.10.009
Litvín R, Bína D, Herbstová M, Gardian Z (2016) Architecture of the light-harvesting apparatus of the eustigmatophyte alga Nannochloropsis oceanica. Photosynth Res. https://doi.org/10.1007/s11120-016-0234-1
Liu Z, Yan H, Wang K et al (2004) Crystal structure of spinach major light-harvesting complex at 2.72 A resolution. Nature 428:287–292. https://doi.org/10.1038/nature02373
Majumder ELW, Wolf BM, Liu H et al (2017) Subcellular pigment distribution is altered under far-red light acclimation in cyanobacteria that contain chlorophyll f. Photosynth Res 134:183–192. https://doi.org/10.1007/s11120-017-0428-1
Manning WM, Strain HH (1943) Chlorophyll d, a green pigment of red algae. J Biol Chem 151:1–19
Miao D, Ding WL, Zhao BQ et al (2016) Adapting photosynthesis to the near-infrared: non-covalent binding of phycocyanobilin provides an extreme spectral red-shift to phycobilisome core-membrane linker from Synechococcus sp. PCC7335. Biochim Biophys Acta Bioenerg 1857:688–694. https://doi.org/10.1016/j.bbabio.2016.03.033
Miyashita H, Ikemoto H, Kurano N et al (1996) Chlorophyll d as a major pigment. Nature 383:402–402
Miyashita H, Adachi K, Kurano N et al (1997) Pigment composition of a novel oxygenic photosynthetic prokaryote containing chlorophyll d as the major chlorophyll. Plant Cell Physiol 38:274–281. https://doi.org/10.1093/oxfordjournals.pcp.a029163
Miyashita H, Ikemoto H, Kurano N et al (2003) Acaryochloris marina gen. et sp. nov. (Cyanobacteria), an oxygenic photosynthetic prokaryote containing Chl d as a major pigment. J Phycol 39:1247–1253. https://doi.org/10.1111/j.0022-3646.2003.03-158.x
Mohr R, Voss B, Schliep M et al (2010) A new chlorophyll d-containing cyanobacterium: evidence for niche adaptation in the genus Acaryochloris. ISME J 4:1456–1469. https://doi.org/10.1038/ismej.2010.67
Moore RB, Oborník M, Janouškovec J et al (2008) A photosynthetic alveolate closely related to apicomplexan parasites. Nature 451:959–963. https://doi.org/10.1038/nature06635
Morosinotto T, Breton J, Bassi R, Croce R (2003) The nature of a chlorophyll ligand in Lhca proteins determines the far red fluorescence emission typical of photosystem I. J Biol Chem 278:49223–49229. https://doi.org/10.1074/jbc.m309203200
Morosinotto T, Mozzo M, Bassi R, Croce R (2005) Pigment–pigment interactions in Lhca4 antenna complex of higher plants photosystem I. J Biol Chem 280:20612–20619. https://doi.org/10.1074/jbc.m500705200
Murakami A, Miyashita H, Iseki M et al (2004) Chlorophyll d in an epiphytic cyanobacterium of red algae. Science 303:1633. https://doi.org/10.1126/science.1095459
Nagy F, Schäfer E (2002) Phytochromes control photomorphogenesis by differentially regulated, interacting signalling pathways in higher plants. Annu Rev Plant Biol 53:329–355. https://doi.org/10.1146/annurev.arplant.53.100301.135302
Niedzwiedzki DM, Wolf BM, Blankenship RE (2019) Excitation energy transfer in the far-red absorbing violaxanthin/vaucheriaxanthin chlorophyll a complex from the eustigmatophyte alga FP5. Photosynth Res. https://doi.org/10.1007/s11120-019-00615-y
Nürnberg DJ, Morton J, Santabarbara S et al (2018) Photochemistry beyond the red limit in chlorophyll f-containing photosystems. Science (80) 360:1210–1213
Ohashi S, Miyashita H, Okada N et al (2008) Unique photosystems in Acaryochloris marina. Photosynth Res 98:141–149. https://doi.org/10.1007/s11120-008-9383-1
Ort DR, Merchant SS, Alric J et al (2015) Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc Natl Acad Sci USA 112:1–8. https://doi.org/10.1073/pnas.1424031112
Oster U, Tanaka R, Tanaka A, Rudiger W (2000) Cloning and functional expression of the gene encoding the key enzyme for chlorophyll b biosynthesis (CAO) from Arabidopsis thaliana. Plant J 21:305–310
Pan H, Šlapeta J, Carter D, Chen M (2012) Phylogenetic analysis of the light-harvesting system in Chromera velia. Photosynth Res 111:19–28. https://doi.org/10.1007/s11120-011-9710-9
Partensky F, Six C, Ratin M et al (2018) A novel species of the marine cyanobacterium Acaryochloris with a unique pigment content and lifestyle. Sci Rep. https://doi.org/10.1038/s41598-018-27542-7
Pettai H, Oja V, Freiberg A, Laisk A (2005) The long-wavelength limit of plant photosynthesis. FEBS Lett 579:4017–4019. https://doi.org/10.1016/j.febslet.2005.04.088
Porra RJ, Schäfer W, Cmiel E et al (1994) The derivation of the formyl-group oxygen of chlorophyll b in higher plants from molecular oxygen. Achievement of high enrichment of the 7-formyl-group oxygen from 18O2 in greening maize leaves. Eur J Biochem 219:671–679
Quigg A, Kotabová E, Jarešová J et al (2012) Photosynthesis in Chromera velia represents a simple system with high efficiency. PLoS ONE. https://doi.org/10.1371/journal.pone.0047036
Reed JW, Nagatani A, Elich TD et al (1994) Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol 104:1139–1149
Renger T, Schlodder E (2008) The primary electron donor of photosystem II of the cyanobacterium Acaryochloris marina is a chlorophyll d and the water oxidation is driven by a chlorophyll a/chlorophyll d heterodimer. J Phys Chem B 112:7351–7354. https://doi.org/10.1021/jp801900e
Rivadossi A, Zucchelli G, Garlaschi FM, Jennings RC (1999) The importance of PS I chlorophyll red forms in light-harvesting by leaves. Photosynth Res. https://doi.org/10.1023/a:1006236829711
Rochaix J-D (2014) Regulation and dynamics of the light-harvesting system. Annu Rev Plant Biol 65:287–309. https://doi.org/10.1146/annurev-arplant-050213-040226
Rockwell NC, Lagarias JC (2010) A brief history of phytochromes. ChemPhysChem 11:1172–1180. https://doi.org/10.1002/cphc.200900894
Saer RG, Blankenship RE (2017) Light harvesting in phototrophic bacteria: structure and function. Biochem J 474:2107–2131. https://doi.org/10.1042/bcj20160753
Schliep M, Crossett B, Willows RD, Chen M (2010) 18O labeling of chlorophyll d in Acaryochloris marina reveals that chlorophyll a and molecular oxygen are precursors. J Biol Chem 285:28450–28456. https://doi.org/10.1074/jbc.m110.146753
Schlodder E, Çetin M, Eckert HJ et al (2007) Both chlorophylls a and d are essential for the photochemistry in photosystem II of the cyanobacteria, Acaryochloris marina. Biochim Biophys Acta Bioenerg 1767:589–595. https://doi.org/10.1016/j.bbabio.2007.02.018
Searle NE (1965) Physiology of flowering. Annu Rev Plant Physiol 16:97–118
Sukenik A, Livne A, Apt KE, Grossman AR (2000) Characterization of a gene encoding the light-harvesting violaxanthin-chlorophyll protein of Nannochloropsis sp. (Eustigmatophyceae). J Phycol 36:563–570. https://doi.org/10.1046/j.1529-8817.2000.99115.x
Swingley WD, Chen M, Cheung PC et al (2008) Niche adaptation and genome expansion in the chlorophyll d-producing cyanobacterium Acaryochloris marina. Proc Natl Acad Sci USA 105:2005–2010. https://doi.org/10.1073/pnas.0709772105
Tichy J, Gardian Z, Bina D et al (2013) Light harvesting complexes of Chromera velia, photosynthetic relative of apicomplexan parasites. Biochim Biophys Acta Bioenerg 1827:723–729. https://doi.org/10.1016/j.bbabio.2013.02.002
Tomo T, Okubo T, Akimoto S et al (2007) Identification of the special pair of photosystem II in a chlorophyll d-dominated cyanobacterium. Proc Natl Acad Sci USA 104:7283–7288. https://doi.org/10.1073/pnas.0701847104
Ulijasz AT, Cornilescu G, Cornilescu CC et al (2010) Structural basis for the photoconversion of a phytochrome to the activated Pfr form. Nature 463:250–254. https://doi.org/10.1038/nature08671
Wagner JR, Brunzelle JS, Forest KT, Vierstra RD (2005) A light-sensing knot revealed by the structure of the chromophore-binding domain of phytochrome. Nature 438:325–331. https://doi.org/10.1038/nature04118
Wang W, Yu L-J, Xu C et al (2019) Structural basis for blue-green light harvesting and energy dissipation in diatoms. Science (80-) 363:1–8. https://doi.org/10.1126/science.aav0365
Weatherby K, Carter D (2013) Chromera velia. The missing link in the evolution of parasitism. Adv Appl Microbiol. https://doi.org/10.1016/B978-0-12-407672-3.00004-6
Wientjes E, Croce R (2011) The light-harvesting complexes of higher-plant Photosystem I: Lhca1/4 and Lhca2/3 form two red-emitting heterodimers. Biochem J 433:477–485. https://doi.org/10.1042/bj20101538
Wientjes E, Roest G, Croce R (2012) From red to blue to far-red in Lhca4: how does the protein modulate the spectral properties of the pigments? Biochim Biophys Acta Bioenerg 1817:711–717. https://doi.org/10.1016/j.bbabio.2012.02.030
Wilhelm C, Jakob T (2006) Uphill energy transfer from long-wavelength absorbing chlorophylls to PS II in Ostreobium sp. is functional in carbon assimilation. Photosynth Res 87:323–329. https://doi.org/10.1007/s11120-005-9002-3
Wolf BM, Niedzwiedzki DM, Magdaong NCM et al (2018) Characterization of a newly isolated freshwater Eustigmatophyte alga capable of utilizing far-red light as its sole light source. Photosynth Res 135:177–189. https://doi.org/10.1007/s11120-017-0401-z
Zhang Z-C, Li Z-K, Yin Y-C, Li Y, Jia Y, Chen M, Qiu B-S (2019) Widespread occurrence and unexpected diversity of red-shifted chlorophyll producing cyanobacteria in humid subtropical forest ecosystems. Environ Microbiol 21:1497–1510
Zhao C, Gan F, Shen G, Bryant DA (2015) RfpA, RfpB, and RfpC are the master control elements of Far-Red Light Photoacclimation (FaRLiP). Front Microbiol 6:1–13. https://doi.org/10.3389/fmicb.2015.01303
Zhu X-G, Long SP, Ort DR (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61:235–261. https://doi.org/10.1146/annurev-arplant-042809-112206
Acknowledgements
Funding for this work was from the Photosynthetic Antenna Research Center (PARC). PARC is a Department of Energy (DOE) Energy Frontier Research Center (EFRC) funded by Grant #DE-SC0001035. Benjamin Wolf was supported by the William H. Danforth Plant Science Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wolf, B.M., Blankenship, R.E. Far-red light acclimation in diverse oxygenic photosynthetic organisms. Photosynth Res 142, 349–359 (2019). https://doi.org/10.1007/s11120-019-00653-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-019-00653-6