Abstract
Chromatic photoacclimation and photosynthesis were examined in two strains of Acaryochloris marina (MBIC11017 and CCMEE5410) and in Synechococcus PCC7942. Acaryochloris contains Chl d, which has an absorption peak at ca 710 nm in vivo. Cultures were grown in one of the three wavelengths (525 nm, 625 nm and 720 nm) of light from narrow-band photodiodes to determine the effects on pigment composition, growth rate and photosynthesis: no growth occurred in 525 nm light. Synechococcus did not grow in 720 nm light because Chl a does not absorb effectively at this long wavelength. Acaryochloris did grow in 720 nm light, although strain MBIC11017 showed a decrease in phycobilins over time. Both Synechococcus and Acaryochloris MBIC11017 showed a dramatic increase in phycobilin content when grown in 625 nm light. Acaryochloris CCMEE5410, which lacks phycobilins, would not grow satisfactorily under 625 nm light. The cells adjusted their pigment composition in response to the light spectral conditions under which they were grown. Photoacclimation and the Q y peak of Chl d could be understood in terms of the ecological niche of Acaryochloris, i.e. habitats enriched in near infrared radiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photosynthesis, the conversion of the sun’s energy to organic carbon substrates, is the most widespread and biologically important reaction on the planet (Blankenship 2002). Chlorophyll (Chl) a is the primary photosynthetic pigment found in the majority of photosynthetic eukaryotes and has traditionally been considered the only Chl capable of participating directly in oxygenic photosynthesis (Blankenship 2002; Kiang et al. 2007; Björn et al. 2009).
Chl a has an in vitro absorbance maximum in the red region (Q y) at ~665 nm when extracted in acetone, methanol or ethanol and ~662 nm in diethyl ether (Scheer 1991; Ritchie 2006, 2008). Chl a has an in vivo absorbance of ~680 nm depending on the organism due to a red-shift that occurs when bound to the photosystem proteins (French et al. 1972). Absorbance above 700 nm is negligible in most bound Chl a (Kiang et al. 2007), although Photosystem I (PS I) can weakly absorb further into the near infra-red region up to about 730 nm (Gobets and van Grondelle 2001).
Cyanobacteria are oxygenic photosynthetic prokaryotes and typically contain Chl a as their only chlorophyll pigment, as well as carotenoids and phycobilins as accessory pigments (Stanier and Bazine 1977; Blankenship 2002). Phycocyanin and allophycocyanin are phycobilinproteins that absorb strongly in the orange-red part of the spectrum (560–640 nm) and are the major light harvesting pigments in many cyanobacteria. Carotenoids, present in PS I and PS II and in light harvesting complexes, are mainly involved in protection from photodamage. Recently, orange-carotenoid protein complexes have been found in cyanobacteria (Wu and Krogmann 1997). They play important roles related to the light-induced non-photosynthetic quenching processes (Kirilovsky 2007; Boulay et al. 2008). Phycobiliproteins in cyanobacteria are involved in this process too, through interaction with orange-carotenoid protein complexes (Wilson et al. 2006; Scott et al. 2006).
In 1996, a cyanobacterium, Acaryochloris marina, was found to contain Chl d as its primary photosynthetic pigment (Miyashita et al. 1996). Acaryochloris, which was discovered living commensally with a colonial ascidian, contains, in addition to Chl d, a comparatively small amount of Chl a, α-carotene, zeaxanthin and phycobiliproteins (Marquardt et al. 1997; Miyashita et al. 1997). Later it was established that Acaryochloris lives on the undersides of these didemnid ascidians, in an infrared-rich and visible light-depleted environment (Kühl and Larkum 2001; Kühl et al. 2005, 2007). It has also been found in a number of other habitats where, presumably near infrared light is enriched (for example the Salton Sea in California, USA is the source of Acaryochloris CCME5410) (Murakami et al. 2004; Miller et al. 2005; Ohkubo et al. 2006, Kashiyama et al. 2008).
Chl d has an in vitro Q y at ~697 nm in methanol ~692 nm in diethyl ether, and an in vivo Q y between 708 and 720 nm (Miyashita et al. 1996; Chen et al. 2002; Nieuwenburg et al. 2003; Larkum and Kühl 2005). It was originally discovered in 1943, but was later thought to be an artefact of extraction (Manning and Strain 1943; Holt and Morley 1959). This notion tended to discourage interest in Chl d. The longer wavelength Q y peak of Chl d allows Acaryochloris to utilise some of the near infrared (NIR) light that has first passed through a Chl a-containing Prochloron cosymbiont present on the upper side of the ascidian host (Miyashita et al. 2003; Kühl et al. 2005; Swingley et al. 2008). This demonstrates the extended spectrum made available for photosynthesis by Chl d.
Chromatic photoacclimation occurs in response to light intensity and spectral distribution (Perry et al. 1981; Palenik 2001; Grossman 2003; Kehoe and Gutu 2006). Photoacclimation can be a mechanism to prevent photodamage or to maximise the photosynthetically active radiation (PAR) utilised (Falkowski and Laroche 1991). Cyanobacteria have been shown to redistribute photosynthetic pigments depending on light conditions (Mullineaux 2001; Kehoe and Gutu 2006). Chan et al. (2007) investigated pigment adaptation of the Chl d-containing organism (Acaryochloris CCMEE5410) under different white light intensities, and Gloag et al. (2007) examined chromatic photoacclimation in Acaryochloris MBIC11017 by using coloured glass filters to control the wavelengths of light available to the cultures. The glass filters (red, yellow and blue) were cut-off filters rather than narrow-bandpass filters (Gloag et al. 2007). Light emitting diodes (LEDs), used in the present study, provide much narrower and accurate wavelength peaks than coloured glass filters, and can be obtained with very specific emitted wavelengths.
The aim of this research was to determine chromatic photoacclimation in Acaryochloris grown in specific light wavelengths. This was examined using two strains of Acaryochloris: Acaryochloris MBIC11017 (Miyashita et al. 1996), and Acaryochloris CCMEE5410, which lacks phycobilins (Miller et al. 2005; Chan et al. 2007). Synechococcus PCC7942 was used as a reference cyanobacterium that utilises Chl a as its primary chlorophyll and phycobilisomes (PBS) as its major light harvesting system.
Materials and methods
Culture conditions
The two Acaryochloris strains (MBIC11017 and CCMEE5410) were cultured in KES in seawater (Miyashita et al. 1996) and Synechococcus PCC7942 was grown in BG11 (Rippka et al. 1981). Controls were grown in white fluorescent lights (Sylvania Gro-Lux). Three light boxes were lit by internal LEDs and excluded all external light: 525 nm (green), 625 nm (orange-red) and 720 nm (near infra-red, NIR). The LEDs of 525 nm and 625 nm (Cat. ZD0174 and ZD0156, Jaycar Electronics, Australia), and 720 nm (Cat. L720-04AU, Epitex, Japan) were used to build the “light boxes” with light intensities of 10, 20–25 and 15 μmol m−2 s−1 (PAR), respectively. The light intensities in the LED boxes were measured with a Quantum LI-189 light meter (LiCor Corp, USA), but NIR region light intensity was measured with a SKP200 light meter (Skye Instruments, UK) which has a light sensitivity ranging from 500 to 750 nm. The specifications of the diodes were: 525 nm wavelength with 30 nm half width and a 15° viewing angle; 625 nm wavelength with 20 nm half width a 15° viewing angle; 720 nm wavelength with 20 nm half width and a 20° viewing angle.
Two replicates of each species per light box were inoculated in a 1:50 ratio with the medium in a 100 ml culture flask, and another two were inoculated in a 1:100 ratio. Cultures were grown for 2–5 weeks at 27°C on an orbital shaker (~100 rpm). Samples were taken for analysis in a dark room lit by a dim, green light, to avoid the influence of external light.
Growth studies
In vivo absorbance spectra were obtained for each sample over a four-week growth period using a spectrophotometer (Shimadzu UV-2550, Japan) with a Taylor-sphere attachment (ISR-240A, Shimadzu, Japan) (scan speed = fast, slit width = 0.5, sampling interval = auto) to account for scattering, and the computer programme UVPC Personal Spectroscopy Software v3.9. Curves obtained were smoothed with the same programme (δλ = 20). Curves were scaled to standardise their Q y peaks. The Soret band (also called B band) of absorption was not used as it was strongly masked by the absorption of accessory pigments (Haardt and Maske 1987).
Relative abundance of phycocyanin to Chl d, the PC index, was determined by using the maximum phycobilin absorbance (620 nm) to the maximum Chl d absorbance (708 nm) ratio; i.e. (A620 – A750)/(A708 – A750). The ratios of chlorophyll and carotenoids to Chl d were calculated based on the HPLC analysis.
Doubling time was obtained by comparing absorbance at maximal Q y region over three weeks.
Pigments analysis
Chl d/Chl a ratios were determined by high performance liquid chromatography (HPLC) analysis. The photopigments were extracted from harvested cells in 100% pre-chilled methanol on ice. HPLC analysis was performed on a Shimadzu VP series HPLC system with a reverse phase C18 column 250 mm × 4.6 mm (Phenomenex) at a flow rate of 1 ml/min. A gradient programme was developed for HPLC analysis. Solvent A contained 80% acetonitrile and 20% 0.1 M ammonium acetate; Solvent B was 100% methanol. The column was equilibrated using a mixture of solvent A and B (1:1). The HPLC was run for 0–5 min with the mixture of solvent A and solvent B (1:1); then linear gradient for 20 min from 50% solvent B to 100% solvent B; in the end 20 min in 100% solvent B. Calculation of ratio values of pigments was based on HPLC chromatogram peak areas using Shimadzu Class-VP 6.14 software. Each peak was evaluated individually at its published absorption maximum wavelength and normalised to its extinction coefficient at this wavelength (molar extinction coefficient of ε = 77.62 mol−1 cm−1 at 696 nm for Chl d (in methanol)); ε = 68.72 mol−1 cm−1 at 665 nm for Chl a (in methanol); ε = 133 mol−1 cm−1 at 452 nm for zeaxanthin (in methanol); ε = 145 mol−1 cm−1 at 448 nm for α-carotene (in methanol) (Jeffrey and Vesk 1997; Ritchie 2006).
Results
Growth studies
The absorption spectra of the cultures grown under white light, from which inoculations were made, are shown in Fig. 1. Synechococcus PCC7942 has a red absorption peak (Q y) at 677 nm due to the presence of Chl a. The peak at 625 nm represents the main absorption peak of phycobiliproteins (PB) and confirms that Synechococcus uses PBS as its major light harvesting system (Fig. 1). Two strains of Acaryochloris (MBIC11017 and CCMEE5410) demonstrated their major Chl d Q y peaks of 707–709 nm; a two nm red-shift in Q y peaks of Chl d in the cultures of MBIC11017 and CCMEE5410 was observed (Fig. 1). A shoulder around 740 nm was observed in Acaryochloris CCMEE5410, but was not seen in Acaryochloris MBIC11017 (Fig. 1). Three arrows in Fig. 1 indicate the wavelengths of LED light used in this study. Green light (525 nm) is minimally absorbed; and no species was able to grow in 525 nm green light (Data not shown).
In vivo absorption spectra. a In vivo absorption spectra of Synechococcus PCC7942, Acaryochloris strains MBIC11017 and CCMEE5410 grown in white light (~30 μmol photons m−2 s−1 PAR) at maximum population density. Spectra are standardised at their Q y peaks. Arrows indicate wavelengths emitted by LEDs in the light boxes. b The Q y peak of Synechococcus (Chl a) is shown at 677 nm, The Q y peaks of Chl d in Acaryochloris strains showed a 2 nm red-shifted from 707 nm in Acaryochloris MBIC11017 to 709 nm in Acaryochloris CCMEE5410
The composition of pigments were analysed using HPLC (Table 1). Under the 720 nm light cultural condition, Chl d/Chl a ratio was similar between the two Acaryochloris strains, MBIC11017 and CCMEE5410, although there was a slightly higher Chl d/Chl a ratio in Acaryochloris CCMEE5410 in the culture grown under white light. Acaryochloris MBIC11017 showed the highest Chl d/Chl a ratio of 61.8 (i.e. Chl a was less than 2% of total chlorophylls) when they were grown in the 625 nm light. The zeaxanthin, the major carotenoid in Acaryochloris, showed no changes in Acaryochloris CCMEE5410 grown under different light conditions (Table 1), but Acaryochloris MBIC11017 showed its maximum zeaxanthin ratio (to Chls) of 1.1 when grown in white light.
The light of 720 nm appears only to be absorbed by Chl d in Acaryochloris. Two strains of Acaryochloris (MBIC11017 and CCMEE5410) had very similar absorption spectra when grown in 720 nm light; Synechococcus PCC7942 did not grow in 720 nm light (Fig. 2a).
Spectral characteristic comparisons. In vivo absorption spectra of Synechococcus and Acaryochloris strains MBIC11017 and CCMEE5410, grown in 625 nm (about 10–25 μmol m−2 s−1 PAR), and 720 nm light (about 10–25 μmol photons m−2 s−1 Skye Instruments 500–750 nm). Spectra are standardised at their Q y peaks. Inset box is the PC index of Synechococcus grown under white light (Control) and the 625 nm light
All species grew in 625 nm light, but Acaryochloris CCMEE5410 showed a much slower growth rate. The latter only reached a density of 30% of the culture grown under the 720 nm condition (See supplementary figure). The reason for this is apparently the lack of phycobiliproteins in this strain (Fig. 2b) (Miller et al. 2005; Chan et al. 2007). CCMEE5410 had a slightly higher growth rate than Acaryochloris MBIC11017 under the 720 nm light condition (See supplementary figure).
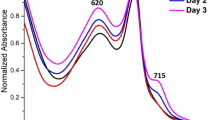
Comparing Synechococcus grown under white light conditions, the 625 nm light increased the PC index up to 1.4 times (Fig. 2b, inset). This phenomenon has been observed in Acaryochloris MBIC11017 grown under 625 nm light conditions, indicating an increase in phycobilins (PC index of 0.28) compared to the Acaryochloris MBIC11017 cells grown in white light (PC index of 0.24) or 720 nm light (PC index of 0.16). The PC index increased up to 1.9 times in 5 weeks (Fig. 3b). No changes were observed in Acaryochloris CCMEE5410 in its absorbance in the region of 550–650 nm (Fig. 3a). It is clear that the phycobiliproteins in Acaryochloris MBIC11017 are accumulated under 625 nm light (Fig. 3b), which is consistent with the profile of accumulation of phycobilisomes in Synechococcus PCC7942 (Fig. 2b), although the amount of phycobiliproteins in Acaryochloris MBIC11017 is much less than in Synechococcus.
In vivo absorption spectra of Acaryochloris spp. In vivo absorption spectra of Acaryochloris strains MBIC11017 and CCMEE5410 grown in 625 nm, and 720 nm light (~10–25 μmol photons m−2 s−1 Skye Instruments 500–750 nm). Spectra were standardised at their Q y peaks. The inset is PC index of Acaryochloris MBIC11017
When comparing two absorption spectra of Chl d-containing Acaryochloris strains, MBIC11017 and CCMEE5410, grown in 720 nm light over time (Fig. 4), the spectrum of Acaryochloris CCMEE5410 showed no changes in the region of 550–650 nm (phycobilin absorbance region), whilst Acaryochloris MBIC11017 did. Acaryochloris MBIC11017 began with quite high levels in the region of phycobilins (~620 nm region); however, it decreased over time and approached an absorbance similar to Acaryochloris CCMEE5410 when it was grown under 720 nm light for 5 weeks. The PC index dramatically decreased during this time in 720 nm light (Fig. 4, inset). The PC index (Fig. 4, inset) decreased from 0.3 of the original to 0.15 after 5 weeks in 720 nm light. This is direct evidence for pigment adaptation to the available light conditions. The 720 nm light is not absorbed by phycobiliproteins; therefore, after 5 weeks in the 720 nm light, Acaryochloris MBIC11017 had lost its phycobiliproteins.
Comparison of spectral profile in the region of 580 nm to 680 nm. Acaryochloris MBIC11017 and CCMEE5410 were grown in the 720 nm light (25 μmol photons m−2 s−1 Skye Instruments 500–750 nm) for 5 weeks. a compared spectra were recorded on 2 weeks old culture; b compared spectra were recorded on 5 weeks. Grey lines represent Acaryochloris strain CCMEE5410 and the black lines are Acaryochloris strain MBIC11017. PC indices (A620 – A750)/(A675 – A750) are indicated in inset against weeks of culturing in the 720 nm light. The grey columns are Acaryochloris strain CCMEE5410 and Black columns are Acaryochloris strain MBIC11017
Discussion
Chromatic photoadaptation is a light-dependent process that allows cyanobacteria to adapt themselves into their particular light environment (Mullineaux 2001; Montgomery 2008). The results obtained here are consistent with what we know of the pigment composition of the Acaryochloris and Synechococcus strains. The MBIC11017 strain of Acaryochloris has phycobiliproteins (Marquardt et al. 1997, Hu et al. 1999; Chan et al. 2007), whereas they have not been found in the Acaryochloris CCMEE5410 strain (Miller et al. 2005; Chan et al. 2007). Thus, in the MBIC11017 strain of Acaryochloris the absorption peaks at 625 nm, 676.5 nm and 706–709 nm can be attributed to phycocyanins, Chl a/allophycocyanins and Chl d, respectively (Blankenship 2002; Miyashita et al. 1997; Petrášek et al. 2005). Neither Acaryochloris strain displays a recognisable Chl a peak due to the low levels of Chl a, less than 5% of total chlorophylls, present in all Acaryochloris strains (Miyashita et al. 1997; Chan et al. 2007). The Q y absorption peak of Acaryochloris MBIC11017 and Acaryochloris CCMEE5410 strains were centred at 709 and 707 nm, respectively (Fig. 1). The difference of recorded Q y peaks (709 nm and 715 nm) between the current report and that of Chan et al. (2007) is caused by their use of an uncorrected baseline of the latter, although Chan et al. did not discuss any difference between the Q y peaks of these two strains. The Shimadzu Taylor-sphere, which was used in the current study, is able to collect all scattered transmitted light caused by light-scattering particles (cells in the cultural suspension), and hence the baseline of the absorption spectra is properly corrected. The Q y peaks were read after they were smoothed using the UVPC Personal Spectroscopy Software v3.9 (δλ = 20) that might cause a slight shift of Q y when the absorbances were low. The pigment–pigment interactions and small differences in conformation of pigment-binding protein complexes in vivo can cause small shifts in absorption (Knapp et al. 1985; Lapouge et al. 1999; Cinque et al. 2000; Croce et al. 2000). It is therefore most probable that this difference in Q y absorbance peaks between the two strains is either due to the presence of phycobilins in the Acaryochloris MBIC11017 strain and absence in the Acaryochloris CCMEE5410 strain, or due to small differences in the binding of Chl d to the pigment-binding proteins.
Chl a and the phycobilins have no absorbance peak at the region of 720 nm, so Synechococcus PCC7942 was unable to grow at this wavelength (Fig. 2a). The ability for growing in 720 nm light by both strains of Acaryochloris demonstrates the competitive advantage that Chl d provides in environments abundant in Chl a-containing organisms, i.e. Chl d allows those organisms that possess it to live in the habitats where chlorophyll a and other photopigments have “scrubbed” out the visible light. This hypothesis is strongly backed by previous studies that have shown Acaryochloris growing in habitats depleted of typical PAR but abundant in NIR light (Murakami et al. 2004; Kühl et al. 2005). Recently, Kashiyama et al. (2008) analysed the pigment compositions of surface sediments from diverse aquatic environments and suggested that Chl d-containing organisms could be ubiquitously distributed in aquatic environments (Kashiyama et al. 2008) but have not been systematically searched for until recently. The recently developed universal algorithms for estimations of chlorophylls a, b, c & d in mixed algal populations of oxygenic photoautotrophs will help in screening for such organisms (Ritchie 2008).
Synechococcus PCC7942 displayed strong photoacclimation when grown in the 625 nm light by increasing production of phycobilins (Fig. 3b). Studies of phycobilin abundances relative to Chl a are uncovering the strategies used by photosynthetic organisms during light acclimation from yellow to orange light and the means by which they maximise the efficiency of energy absorption to the restricted light, such as mono wavelength lights (Stomp et al. 2004, Six et al. 2005). Photoacclimation to 625 nm light is important because phycobilins, the major light harvesting pigments of Synechococcus PCC7942, absorb strongly in this region.
The chromatic photoacclimation displayed by the Acaryochloris MBIC11017 strain grown in 720 nm light by down-regulating expression of phycobiliproteins (Fig. 4) is an example of environmental phenotypic effects. Acaryochloris CCMEE5410 is unable to produce phycobilins and its ability to acclimate to environmental conditions is thus restricted. This is highlighted by its decreased growth rate in 625 nm light found in the present study. Acaryochloris is able to both adapt and acclimate to light conditions that would otherwise limit growth, increasing the size of its habitable niche (Swingley et al. 2008).
Conclusions
Acaryochloris marina MBIC11017 is able to chromatically photoacclimate by altering its pigment composition in response to its environment. Acaryochloris CCMEE5410 also displays this, but to a lesser degree due to a lack of phycobiliproteins. Further research is needed to determine the validity and understand the mechanisms behind the observed effects of restricted light wavelengths on photosynthesis.
Growth studies have demonstrated how Acaryochloris is uniquely adapted to its natural PAR deficient habitat by utilising far red radiation. The ability of Acaryochloris to alter pigment ratios also increases the potential environmental niches of this organism (Moore et al. 1995; MacIntyre et al. 2002). The use of many pigments allows an increase in PAR and has implications in understanding photosynthetic mechanisms and adaptive evolution.
Abbreviations
- Chl:
-
Chlorophyll
- LED:
-
Light emitting diode
- NIR:
-
The near infrared light
- PAR:
-
Photosynthetically active radiation
- PBS:
-
Phycobilisomes
- PS:
-
Photosystem
References
Björn LO, Papageorgiou GC, Blankenshp RE, Govindjee (2009) A viewpoint: why chlorophyll a? Photosynth Res 99:85–98
Blankenship RE (2002) Molecular mechanisms of photosynthesis. Blackwell, Oxford, UK
Boulay C, Abasova L, Six C, Vass I, Kirilovsky D (2008) Occurrence and function of the orange carotenoid protein in photoprotective mechanisms in various cyanobacteria. Biochim Biophys Acta. 1777:1344–1354
Chan YW, Nenninger A, Clokie SJH, Mann NH, Scanlan DJ, Whitworth AL, Clokie MRJ (2007) Pigment composition and adaptation in free-living and symbiotic strains of Acaryochloris marina. FEMS Microbiol Ecol 61:65–73
Chen M, Quinnell RG, Larkum AWD (2002) Chlorophyll d as the major photopigment in Acaryochloris marina. J Porphyrins Phthalocyanines 6:763–773
Cinque G, Croce R, Bassi R (2000) Absorption spectra of chlorophyll a and b in Lhcb protein environment. Photosynth Res 64:233–242
Croce R, Cinque G, Holzwarth AR, Bassi R (2000) The Soret absorption properties of carotenoids and chlorophylls in antenna complexes of higher plants. Photosynth Res 64:221–231
Falkowski PG, Laroche J (1991) Acclimation to spectral irradiance in algae. J Phycol 27:8–14
French CS, Brown JS, Lawrence MC (1972) Four universal forms of chlorophyll a. Plant Physiol 49:421–429
Gloag RS, Ritchie RJ, Chen M, Larkum AWD, Quinnell RG (2007) Chromatic photoacclimation, photosynthetic electron transport and oxygen evolution in the chlorophyll d-containing oxyphotobacterium Acaryochloris marina. Biochim Biophys Acta-Bioenergetics 1767:127–135
Gobets B, van Grondelle R (2001) Energy transfer and trapping in photosystem I. Biochim Biophys Acta 1507:80–99
Grossman AR (2003) A molecular understanding of complementary chromatic adaptation. Photosynth Res 76:207–215
Haardt H, Maske H (1987) Specific in vivo absorption-coefficient of chlorophyll a at 675 nm. Limnol Oceanogr 32:608–619
Holt AS, Morley HV (1959) A proposed structure for chlorophyll d. Can J Chem 37:507–514
Hu Q, Marquardt J, Iwasaki I, Miyashita H, Kurano N, Mörschel E, Miyachi S (1999) Molecular structure, localization and function of biliproteins in the chlorophyll a/d containing oxygenic photosynthetic prokaryote Acaryochloris marina. Biochim Biophys Acta-Bioenergetics 1412:250–261
Jeffrey SW, Vesk M (1997) Introduction to marine phytoplankton and their pigment signatures. In: Jeffrey SW, Mantoura RFC, Wright SW (eds) Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods. UNESCO Publ, Paris, pp 37–84
Kashiyama Y, Miyashita H, Ohkubo S, Ogawa NO, Chikaraishi Y, Takano Y, Suga H, Toyofuku T, Nomaki H, Kitazato H, Nagata T, Ohkouchi N (2008) Evidence of global Chlorophyll d. Science 321:658
Kehoe DM, Gutu A (2006) Responding to color: the regulation of complementary chromatic adaptation. Ann Rev Plant Biol 57:127–150
Kiang NY, Siefert J, Govindjee, Blankenship RE (2007) Spectral signatures of photosynthesis I. Rev Earth org Astrobiol 7:222–251
Kirilovsky D (2007) Photoprotection in cyanobacteria: the orange carotenoid protein (OCP)-related non-photochemical-quenching mechanism. Photosynth Res 93:7–16
Knapp EW, Fischer SF, Zinth W, Sander M, Kaiser W, Deisenhofer J, Michel H (1985) Analysis of optical-spectra from single-crystals of Rhodopseudomonas viridis reaction centers. Proc Natl Acad Sci USA 82:8463–8467
Kühl M, Larkum AWD (2001) The microenvironment and photosynthetic performance of Prochloron sp in symbiosis with didemnid ascidians. In: Seckbach J (ed) Symbiosis. Kluwer, Dordrecht, pp 273–290
Kühl M, Chen M, Ralph PJ, Schreiber U, Larkum AWD (2005) A niche for cyanobacteria containing chlorophyll d. Nature 433:820
Kühl M, Chen M, Larkum AWD (2007) Biology of the chlorophyll d-containing cyanobacterium Acaryochloris marina. In: Seckbach J (ed) Algae and cyanobacteria in extreme environments. Springer, Berlin, pp 101–123
Lapouge K, Naveke A, Gall A, Ivancich A, Seguin J, Scheer H, Sturgis JN, Mattioli TA, Robert B (1999) Conformation of bacteriochlorophyll molecules in photosynthetic proteins from purple bacteria. Biochem 38:11115–11121
Larkum AWD, Kühl M (2005) Chlorophyll d: the puzzle resolved. Trends Plant Sci 10:355–357
MacIntyre HL, Kana TM, Anning T, Geider RJ (2002) Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria. J Phycol 38:17–38
Manning WM, Strain HH (1943) Chlorophyll d, a green pigment of red algae. J Biol Chem 151:1–19
Marquardt J, Senger H, Miyashita H, Miyachi S, Morschel E (1997) Isolation and characterization of biliprotein aggregates from Acaryochloris marina, a Prochloron-like prokaryote containing mainly chlorophyll d. FEBS Lett 410:428–432
Miller SR, Augustine S, Olson TL, Blankenship RE, Selker J, Wood AM (2005) Discovery of a free-living chlorophyll d-producing cyanobacterium with a hybrid proteobacterial/cyanobactyerial small-subunit rRNA gene. Proc Natl Acad Sci USA 102:850–855
Miyashita H, Ikemoto H, Kurano N, Adachi K, Chihara M, Miyachi S (1996) Chlorophyll d as a major pigment. Nature 383:402
Miyashita H, Adachi K, Kurano N, Ikemoto H, Chihara M, Miyachi S (1997) Pigment composition of a novel oxygenic photosynthetic prokaryote containing chlorophyll d as the major chlorophyll. Plant Cell Physiol 38:274–281
Miyashita H, Ikemoto H, Kurano N, Miyachi S, Chihara M (2003) Acaryochloris marina gen. et sp nov (Cyanobacteria), an oxygenic photosynthetic prokaryote containing Chl d as a major pigment. J Phycol 39:1247–1253
Montgomery BL (2008) Shedding new light on the regulation of complementary chromatic adaptation. Cent Eur J Biol 3(4):351–358
Moore LR, Goericke R, Chisholm SW (1995) Comparative physiology of Synechococcus and Prochlorococcus - Influence of light and temperature on growth, pigments, fluorescence and absorptive properties. Marine Ecol Prog Ser 116:259–275
Mullineaux CW (2001) How do cyanobacteria sense and respond to light? Mol Microbiol 41:965–971
Murakami A, Miyashita H, Iseki M, Adachi K, Mimuro M (2004) Chlorophyll d in an epiphytic cyanobacterium of red algae. Science 303:1633
Nieuwenburg P, Clarke RJ, Cai Z-L, Chen M, Larkum AWD, Cabral NM, Ghiggino KP, Reimers JR (2003) Examination of the photophysical processes of chlorophyll d leading to a clarification of proposed uphill energy transfer processes in cells of Acaryochloris marina. Photochem Photobiol 77:628–637
Ohkubo S, Miyashita H, Murakami A, Takeyama H, Tsuchiya T, Mimuro M (2006) Molecular detection of epiphytic Acaryochloris spp. on marine macroalgae. Appl Environ Microbiol 72:7912–7915
Palenik B (2001) Chromatic adaptation in marine Synechococcus strains. App Environ Microbiol 67:991–994
Perry MJ, Talbot MC, Alberte RS (1981) Photoadaptation in marine-phytoplankton: response of the photosynthetic unit. Marine Biol 62:91–101
Petrášek Z, Schmitt FJ, Theiss C, Huyer J, Chen M, Larkum A, Eichler HJ, Kemnitz K, Eckert HJ (2005) Excitation energy transfer from phycobiliprotein to chlorophyll d in intact cells of Acaryochloris marina studied by time- and wavelength resolved fluorescence spectroscopy. Photochem Photobiol Sci 4:1016–1022
Rippka RJ, Waterbury B, Stanier RY (1981) Isolation and purification of cyanobacteria: some general principles. J General Microbiol 111:1–161
Ritchie RJ (2006) Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89:27–41
Ritchie RJ (2008) Universal chlorophyll equations for estimating chlorophylls a, b, c & d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol or ethanol solvents. Photosynthetica 46:115–126
Scheer H (ed) (1991) Chlorophylls. CRC Press, Boca Raton, USA
Scott M, McCollum C, Vasilev S, Crozier C, Espie GS, Krol M, Huner NPA, Bruce D (2006) Mechanism of the down regulation of photosynthesis by blue light in the cyanobacterium Synechocystis sp. Biochem 45:8952–8958
Six C, Thomas J-C, Thion L, Lemoine Y, Zal F, Partensky F (2005) Two novel phycoerythrin-associated linker proteins in the marine cyanobacterium Synechococcus sp. strain WH8102. J Bacteriol 187:1685–1694
Stanier RY, Bazine GC (1977) Phototrophic prokaryotes: the cyanobacteria. Ann Rev Microbiol 31:225–274
Stomp M, Huisman J, de Jongh F, Veraart AJ, Gerla D, Rijkeboer M, Ibelings BW, Wollenzien UIA, Stal LJ (2004) Adaptive divergence in pigment composition promotes phytoplankton biodiversity. Nature 432:104–107
Swingley WD, Chen M, Cheung PC, Conrad AL, Dejesa LC, Hao J, Honchak BM, Karbach LE, Kurdoglu A, Lahiri S, Mastrian SD, Miyashita H, Page L, Ramakrishna P, Satoh S, Sattley WM, Shimada Y, Taylor HL, Tomo T, Tsuchiya T, Wang ZT, Raymond J, Mimuro M, Blankenship RE, Touchman JW (2008) Niche adaptation and genome expansion in the chlorophyll d-producing cyanobacterium Acaryochloris marina. Proc Natl Acad Sci USA 105:2005–2010
Wilson A, Ajlani G, Verbavatz JM, Vass I, Kerfeld CA (2006) A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell 18:992–1007
Wu YP, Krogmann DW (1997) The orange carotenoid protein of Synechocystis PCC 6803. Biochim Biophys Acta 1322:1–7
Acknowledgement
MC holds an Australian Research Council (ARC) QEII fellow and wishes to thank ARC for the financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11120_2009_9466_MOESM1_ESM.jpg
Figure supplementary 1. Growth rate. Growing rate was determined by monitoring the A708 absorbance (Chl d Q y peak) and the fitting curve was modelled using an exponential curve equation (as in the figure). Grey dashed line, CCMEE5410 grown in 720 nm light; Black dashed line, MBIC11017 grown in 720 nm light; Black solid line, Acaryochloris MBIC11017 grown in 625 nm light and grey solid line, Acaryochloris CCMEE5410 grown in 625 nm. (JPG 326 kb)
Rights and permissions
About this article
Cite this article
Duxbury, Z., Schliep, M., Ritchie, R.J. et al. Chromatic photoacclimation extends utilisable photosynthetically active radiation in the chlorophyll d-containing cyanobacterium, Acaryochloris marina . Photosynth Res 101, 69–75 (2009). https://doi.org/10.1007/s11120-009-9466-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-009-9466-7